Abstract
Spatial and temporal organization of signal transduction is coordinated through the segregation of signaling enzymes in selected cellular compartments. This highly evolved regulatory mechanism ensures the activation of selected enzymes only in the vicinity of their target proteins. In this context, cAMP-responsive triggering of protein kinase A is modulated by a family of scaffold proteins referred to as A-kinase anchoring proteins. A-kinase anchoring proteins form the core of multiprotein complexes and enable simultaneous but segregated cAMP signaling events to occur in defined cellular compartments. In this review we will focus on the description of A-kinase anchoring protein function in the regulation of cardiac physiopathology.
Keywords: AKAP, PKA, cAMP, cardiac disease
Spatial and temporal control of signal transduction events is frequently achieved by compartmentalization of intracellular effectors through adaptors or anchoring proteins. In particular, elements of the cAMP signaling cascade are localized in the cell via scaffold proteins referred to as A-kinase anchoring proteins (AKAPs).1 cAMP is a second messenger involved in the regulation of different cellular events that occur in response to extracellular stimuli. Binding of an extracellular stimulus to a selective G-protein coupled receptor (GPCR) triggers the activation of a heterotrimeric Gs protein and its effector, the adenylyl cyclase (AC), which generates the second messenger cAMP. In turn, cAMP exerts its effects through the activation of 3 effectors: protein kinase A (PKA), the exchange protein directly activated by cAMP and the cyclic nucleotide-gated ion channels.2,3 The primary effector of cAMP in the heart is PKA, a tetramer formed by 2 catalytic subunits that are inactivated by the binding of the 2 regulatory subunits. Binding of cAMP to the regulatory subunits induces the dissociation and the activation of the catalytic subunits, resulting in the phosphorylation of local substrates.4
Several studies have demonstrated that cAMP is not uniformly distributed throughout the cell.5,6 Indeed, numerous imaging studies have shown that cAMP levels rise selectively in a specific cellular compartment in a stimulus-specific manner and do not diffuse from one compartment to the other, allowing fidelity of the response.7 Spatially restricted activation of PKA is guaranteed by the binding of this kinase with AKAPs, a family of functionally related proteins that interact with the regulatory subunits of the PKA holoenzyme. The molecular feature of AKAPs is to possess a structurally conserved PKA anchoring domain, consisting of an amphipatic helix of 14 to 18 residues that selectively binds the dimerization and docking domain at the N-terminus of the PKA regulatory subunit dimer.8 –10 Although the vast majority of AKAPs bind the type II regulatory subunit of PKA, several AKAPs are referred to as dual-function anchoring proteins because they bind both the type I (RI) and the type II (RII) regulatory subunits of PKA.11 More recently, type I PKA specific anchoring proteins have been described.12–14 Several evidences have demonstrated that PKA-RI and PKA-RII isoforms are indeed anchored to specific subcellular sites via binding to these different AKAPs.7
AKAPs do not only position PKA inside the cell but they also ensure that this kinase is coupled to its upstream activators, including membrane receptors and ACs, and to signal termination enzymes, such as phosphodiesterases (PDE) and phosphatases.15–17 In this way, AKAPs help to establish intracellular cAMP gradients, generated via activation of a specific GPCR and uniquely modulated by different subsets of PDEs, resulting in stimulus-specific activation and action of PKA.7 AKAPs also coordinate signaling enzymes such as other kinases, GTPases, and regulatory proteins into multivalent transduction signalosomes. Thus, AKAPs provide the structural integrity for multiprotein complexes that often represent hubs for processing of multiple signals. A further layer of specificity proceeds through protein- or lipid-targeting domains on AKAPs that direct AKAP signaling complexes to intracellular membranes.18 A concerted research effort over the past 20 years has identified more than 50 genes encoding distinct anchoring proteins.19 Furthermore, numerous splice variants are transcribed from each gene in a cell type and tissue-specific manner.
To narrow the focus of this article, we will restrict our discussion to the actions of compartmentalized cAMP signaling and AKAP function in the cardiovascular system. In the heart, several AKAPs play a critical role in modulating multiple signaling pathways at the basis of cardiac physiopathology (Table).20 This review will especially focus on the importance of anchored-PKA in the regulation of cardiac cAMP compartmentation.
Table.
AKAPs in the Heart
| Gene Name | Alternative Name | Function | Intracellular Localization | Signaling Partners |
|---|---|---|---|---|
| AKAP1 | D-AKAP1, s-AKAP84, AKAP121, AKAP149 | Hypertrophy | Mitochondria, nuclear envelope, endoplasmic reticulum | PKA RI, PKA RII, PKCα, Src, PP1, PP2A, PP2B, PTPD1, PDE7A, AMY-1, Lfc, RSK1 |
| AKAP5 | AKAP75, AKAP79, AKAP150 | Contractility | Plasma membrane, T tubules | PKA RII, PKC, PP2B, LTCC, KCNQ2, β-AR, AC5, AC6, CAV3, SAP97 |
| AKAP6 | mAKAP | Hypertrophy, contractility, hypoxia | Nuclear envelope | PKA RII, PDE4D3, AC5, RyR2, CaNAβ, PP2A, NFATc, ERK5, MEK5, Epac1, Rap1, HIF1α, VHL, Siah2, PDK1, RSK3, NCX1, nesprin-1α, myopodin |
| AKAP7 | AKAP15, AKAP18 | Contractility | Plasma membrane, endoplasmic reticulum | PKA RII, LTCC, PLB, PP1, inhibitor1 |
| AKAP9 | Yotiao, AKAP350, AKAP450, CG-NAP, Hyperion | Cardiac repolarization | Plasma membrane, golgi, centrosome | PKA RII, PP1, PP2A, PKCε, PKN1, casein kinase 1, AC, PDE4D3, IP3-R, KCNQ1, CLIC |
| AKAP10 | D-AKAP2 | Cardiac rhythm | Mitochondria | PKA RI, PKA RII, PDZK1, Rab4, Rab11 |
| AKAP12 | Gravin, AKAP250, SSeCKS | β-AR signaling | Plasma membrane | PKA RII, β-AR, PKC, PDE4D, Src, PP2B |
| AKAP13 | AKAP-Lbc, Ht31, BRX | Hypertrophy and development | Cytoskeleton | PKA RII, Gα12/13, RhoA, actin, 14-3-3, PKC, PKD, KSR1, Raf, MEK1/2, ERK1/2, PKNα |
| PDE4DIP | Myomegalin, MMGL, CMYA2 | Contractility | Sarcomere | PKA, PDE4D |
| PIK3CG | p110γ | β-AR downregulation | Membrane | PKA RII, p101, p84/87, Ras, PDE3B, Bcr |
| SYNM | Synemin | Cytoskeletal organization | Plasma membrane, sarcomere | PKA RII, desmin, zyxin, talin, vinculin, vimentin, dystrobrevin, desmuslin, utrophin, α-actinin |
| TNNT2 | Troponin T | Contractility | Sarcomere | PKA RII, troponin I, troponin C, actin |
AKAP indicates A-kinase anchoring protein; PKA RI, type I regulatory subunit of protein kinase A; PP, protein phosphatase; PKA RII, type II regulatory subunit of protein kinase A; PDE, phosphodiesterases; LTCC, L-type Ca2+ channels; β-AR, β-adrenergic receptor; AC, adenylyl cyclase; CAV3, caveolin 3; RyR2, ryanodine receptor 2; NFAT, nuclear factor of activated T cells; Epac, exchange protein directly activated by cAMP; HIF1α, hypoxia-inducible factor-1; VHL, von Hippel-Lindau; Siah2, Seven in Absentia Homolog 2; PKN, protein kinase N; PTPD1, protein tyrosine phosphatase D1; AMY-1, associate of Myc-1; RSK, ribosomal S6 kinase; SAP97, synapse-associated protein 97; NCX1, sodium-calcium E changer-1; PLB, phospholamban; KSR1, kinase suppressor of Ras1.
Cardiac AKAPs
Cardiac Development
The heart is the first organ to form during embryogenesis and all subsequent events in the life of the organism are dependent on its function. Cardiac organogenesis is characterized by the precise temporal and region-specific regulation of cell proliferation, migration, death, and differentiation.21,22 All these processes are finely tuned by a variety of signal transduction pathways. Among these, anchored cAMP signaling is essential for cardiomyocyte differentiation and heart morphogenesis. AKAP-Lbc (also referred to as AKAP13 or BRX) is a key regulator of these events and the deletion of this AKAP in the mouse results in a thin and enlarged myocardium that leads to an arrest in cardiac development and subsequent embryonic lethality.23 This failure in cardiac formation is consistent with decreased activity of the small GTPase Rho, a direct target of AKAP-Lbc.24 Reduced Rho function in turn correlates with a repressed activity of the myocyte enhancer factor-2, a transcription factor important for the proper regulation of cardiac gene expression.25 AKAP-Lbc is thus a platform that links Rho signaling to an essential transcription program that drives cardiomyocyte development.26
Contractility
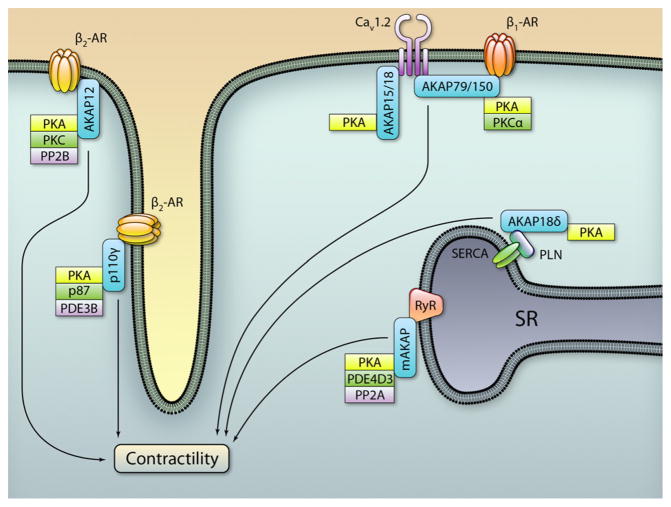
In the adult heart, cardiac contractility and relaxation are mediated by rapid changes in cytoplasmic Ca2+ concentration following the electric stimulation of the myocardium. During the excitation-contraction coupling, brief openings of sarcolemmal voltage-gated L-type Ca2+ channels (LTCCs) in response to an action potential generate local elevations in intracellular Ca2+. This highly localized Ca2+ rise in turn activates closely apposed ryanodine receptors (RyRs) in the sarcoplasmic reticulum (SR), via a mechanism referred to as Ca2+-induced Ca2+ release. This results in a substantial release of Ca2+ form the SR, thereby inducing a global increase in Ca2+ concentration that activates cardiac contractile proteins. LTCCs and RyRs are rapidly inactivated by Ca2+-dependent mechanisms and allow the cardiac sarcoplasmic reticular Ca2+-adenosine triphosphatase (SERCA2) pump to recover the released Ca2+ in the SR before the next heart beat.27 Tight regulation of Ca2+ handling is thus required for proper force and rate of contraction of the heart. The sympathetic nervous system (SNS) is one of the major regulators of heart rate in response to exercise or emotional stress. SNS controls cardiac electric activity through the activation of β-adrenergic receptors (β-ARs) that modulate the function of selected ion channels via phosphorylation by PKA.28 These Ca2+-related signaling events are regulated by different combinations of AKAPs that finely modulate the PKA-dependent signaling (Figure 1). Displacement of PKA from AKAPs by PKA anchoring disruptor peptides results in altered phosphorylation of key players in excitation-contraction coupling, thus leading to compromised cardiac contractility.29–32 Under this scenario, a pivotal role in regulating cAMP and Ca2+ transients is played by multiple AKAPs, including AKAP79/150, gravin, AKAP15/ 18, mAKAP, AKAP18δ and a group of sarcomeric AKAPs that have just recently been identified.
Figure 1. Regulation of cardiac contractility by A-kinase anchoring protein (AKAPs).
Intracellular distribution of AKAP complexes involved in myocardial contractility modulation. PKA indicates protein kinase A; PP2B, protein phosphatase 2B; PDE, phosphodiesterase; SERCA PLN, sarcoplasmic reticular Ca2+-adenosine triphosphatase phospholamban; SR, sarcoplasmic reticulum; AR, adrenergic receptor. (Illustration: Ben Smith.)
AKAP-βAR Complexes
Beta-adrenergic receptors (β-ARs) impact Ca2+ handling by increasing the force of contraction and by accelerating the rate of relaxation.28 The effect of catecholamines on the heart is mainly mediated by β1-ARs and β2-ARs. Although both receptors are very similar in structure, they perform different functions. Whereas β1-ARs couple only to Gs, agonist-bound β2-ARs undergo sequential coupling to both Gs and Gi.33 Functional differences between β1-ARs and β2-ARs can also be attributed to subtype-specific targeting to different cellular compartments.34,35 Compartmentalization of β-ARs in different plasma membrane microdomains can explain subtype-specific signaling.36 –38 Likewise, different AKAPs organize distinct β-AR-containing signalosomes. Of note, AKAP79/ 150 (also referred to as AKAP5) is bound to the plasma membrane through a N-terminal polybasic targeting domain that binds phospholipids and a palmitoylation domain that specifically targets AKAP79/150 to lipid rafts, at the level of the synaptic junction.39,40 The functional consequence of this targeting event is to confine PKA within lipid rafts.41 In this compartment, AKAP79/150 organizes a complex containing PKA, β1-AR, AC5/6, PP2B, Cav1.2, and caveolin 3 (CAV3) and controls a β1-AR-stimulated microdomain of cAMP that impacts on Ca2+ transients. Accordingly, in cells lacking AKAP79/150, β-AR activation does not modulate intracellular Ca2+ signaling.42 On the other hand, β2-ARs bind both AKAP79/150 and another anchoring protein called gravin (also referred to as AKAP12 or AKAP250 or SSeCKS).43 AKAP79/150 appears to function in switching signaling pathways of the receptor from AC to activation of the mitogen-activated protein kinase cascade. In contrast, gravin targets the receptor to the plasma membrane of cardiomyocyte-like H9c2 cells.44,45 Within this context, gravin is bound to PKA, β2-AR and PKC.44,46–48 Perturbation of this signaling complex leads to disruption of β-AR internalization and resensitization, critical events in G-protein coupled receptors regulation.47,49 Furthermore, although AKAP79/150 is essential to mediate the activation of the MAP kinase cascade on catecholamine stimulation, gravin is required for the ability of cells to recover from agonist-induced desensitization and recycling.50 Collectively, these findings offer a compelling argument for the spatial activation and segregation of different adrenergic receptors by selective AKAP signaling complexes.
AKAP-LTCC Complexes
LTCCs are the primary source of Ca2+ influx to initiate excitation-contraction coupling.28 From a molecular point of view, cardiac LTCCs include the pore-forming α1C subunit (also referred to as Cav1.2) and three auxiliary subunits (β, α2δ and γ) that are involved in trafficking Cav1.2 to the sarcolemma and in modulating the voltage dependence of channel gating.51 Alterations in LTCC density or function have been implicated in a variety of cardiovascular diseases, including atrial fibrillation, ischemic heart disease and heart failure.52 For these reasons, in cardiac physiology, LTCCs are regulated by a variety of neurotransmitters, hormones and cytokines. Of note, β-adrenergic system is a crucial regulator of LTCC-mediated Ca2+ homeostasis.53 During the “fight or flight” response, stimulation of β-ARs increases LTCC currents through PKA-mediated phosphorylation of the channel itself (Cav1.2 or β subunit) or of its associated proteins.54 –56 The increase in Ca2+ currents induced by PKA activation is due to an enhancement of the open-state probability of the channel, resulting from a shift in gating mode.57 Regulation of LTCCs requires PKA targeting to the distal C terminus (DCT) of the channel. Truncation of Cav1.2 DCT abolishes the regulation of LTCCs by the β-AR/PKA pathway,58 consistently with the finding of PKA phosphorylation sites at the distal C terminus of Cav1.2.59 – 61 Several lines of evidence have emphasized the importance of AKAPs in targeting PKA in the vicinity of LTCCs.62 In skeletal muscle and in cardiomyocytes, a low molecular weight AKAP, AKAP15/18 (also known as AKAP18α or AKAP7) has been identified as the anchoring protein that targets PKA to Cav1.2.53,62– 64 In higher detail, AKAP15/18 targets PKA to the C terminus of Cav1.2 through a modified leucine zipper motif located in its C-terminal region. Disruption of this interaction inhibits PKA-dependent enhancement of LTCC activity, both in skeletal muscle cells and in rat ventricular cardiomyocytes.64,65 The C terminus of Cav1.2 undergoes proteolytic processing in vivo, giving rise to two isoforms that differ by truncation of the C terminus. The proteolytically cleaved DCT acts as a regulatory domain of LTCC normal function, by binding to the truncated channel and inhibiting its function.66 Accordingly, mice expressing only truncated Cav1.2 develop severe cardiac hypertrophy and die perinatally. Deletion of the DCT disrupts the expression and localization of the AKAP15/18-PKA complex, resulting in an impaired regulation of LTCC function.58
Ca2+ signaling is regulated not only by AKAP15/18-PKA-Cav1.2 complex at the cell surface but also at the level of the sarcoplasmic reticulum. In this respect, two different AKAPs are involved: mAKAP and AKAP18δ.
mAKAP-RyR Complex
The muscle specific AKAP (mAKAP) is prominently expressed in cardiomyocytes and it is localized both at the sarcoplasmic reticulum, where it regulates Ca2+-induced Ca2+ release,67 and at the nuclear envelope, where it assembles a macromolecular complex integrating cAMP and Ca2+ signals.68 Accordingly, it has been shown that a mAKAP–PKA–RyR complex is strategically located within the cell to modulate both SR-dependent cytoplasmic Ca2+ rise and the perinuclear Ca2+ fluxes.67 mAKAP functions as a scaffold for a wide range of proteins including type II PKA,68 PDE4D3,16 AC5,69 protein phosphatase 2A,67 the MAP kinases MEK5 and ERK5, the small GTPase Rap1, and the cAMP-activated Rap1 exchange factor Epac1.70 Within this macromolecular complex, mAKAP-mediated PKA phosphorylation of the RyR is considered to promote opening of this channel and to increase cardiac function.71 Within this context, cAMP may increase Ca2+ fluxes via PKA-dependent phosphorylation of the RyR, in a manner tightly controlled by the PKA-activated PDE4D3 and protein phosphatase 2A-mediated dephosphorylation. Alternatively, recent studies report that PKA/PDE4D3-mediated control of RyR phosphorylation is irrelevant to normal cardiac function and sympathetic stimulation of the heart.72–74
AKAP18δ-SERCA2 Complex
SERCA2 controls Ca2+ reuptake into the sarcoplasmic reticulum, a rate-limiting step for cardiac relaxation. SERCA2 activity is regulated by numerous factors, including the cytoplasmic/SR Ca2+ gradient, the protein concentration of SERCA2, and the SR inhibitory protein phospholamban (PLN). Dephosphorylated PLN binds to SERCA2 and suppresses its activity, whereas phosphorylation of PLN on Ser16 by PKA dissociates PLN from SERCA2, increasing the Ca2+ reuptake into the SR. This PKA-mediated phosphorylation of PLN is strictly dependent on the function of AKAP18δ, a long splice variant of the AKAP18 gene.75,76 Both the displacement of AKAP18δ from PLN or the silencing of AKAP18δ significantly reduce the PKA-dependent PLN phosphorylation after β-adrenergic stimulation, resulting in a decrease in Ca2+ reuptake into the sarcoplasmic reticulum. Alterations in the function of PLN-SERCA2 complex are linked to cardiac dysfunction.77 Because AKAP18δ mediates PLN phosphorylation and subsequent increase in SERCA2 activity, modulation of AKAP18δ could represent a novel pharmacological target in the treatment of heart failure.78
Sarcomeric AKAPs
Several actin-associated (ezrin, gravin, WAVE-1, and AKAP79/ 150) and microtubule-associated (MAP2, AKAP350/450, hAKAP220, pericentrin, flagellar radial spoke protein 3) AKAPs have been described in different tissues.79 In the heart, multiple evidences have demonstrated the crucial role of AKAPs in targeting PKA at the sarcomere.80 In particular, 3 different AKAPs are involved in mediating PKA-dependent phosphorylation of sarcomeric proteins, crucial regulators of myocardial contractile function.
Synemin is the first intermediate filament protein shown to bind PKA RII and to localize a pool of PKA, allowing local substrate phosphorylation within the myocyte cytoskeleton. Intermediate filament-targeted PKA could phosphorylate substrates found at the Z-line or regulate intermediate filament structure. Synemin is overexpressed in failing hearts: this correlates with an increase in PKA targeting to sites undergoing molecular remodeling.81
Cardiac troponin T has been recently characterized as a novel dual-specificity AKAP able to dock PKA at the thin filaments in proximity of its main sarcomeric substrates.82 Within the myocardial contraction machinery, PKA phosphorylates cardiac myosin binding protein C and this event results in enhanced cardiac contractility due to the rearrangement of the myosin crossbridges and thick filament structure.83 This configuration ensures that PKA is tethered near its substrate thanks to the recently characterized dual AKAP myomegalin (MMGL). Myomegalin is a PDE4D-interacting protein84 involved in assembling a cAMP/PKA/PDE signaling module at the sarcomere.85 The translocation of myomegalin to the sarcomere is therefore compatible with a mechanism that would lead to increased β-adrenergic-stimulated phosphorylation of cardiac myosin binding protein C and cTnI, thus enhancing cardiac contraction as well as cardioprotection.86,87
Cardiac Rhythm and Arrhythmias
Cardiac contractility and rhythm respond rapidly to physical activity and emotional stress to meet the changes in the metabolic needs of the organism. The sympathetic nervous system is the main player of this response and acts by enhancing the current amplitude of the slowly activating delayed rectifier IKs potassium channel (also referred to as HERG).88 IKs channel is composed by the pore-forming α-subunit KCNQ1 that conducts the ionic current and the auxiliary β-subunit KCNE1 that controls the biophysical properties of the channel.89,90 IKs channels are regulated by the sympathetic nervous system via the β-AR/cAMP/PKA pathway. High cAMP levels cause an increase in the IKs amplitude and a slowdown in the current decay during deactivation.88 This cAMP-mediated regulation of the channel is controlled by the scaffold protein Yotiao (also referred to as AKAP9), which recruits PKA and the protein phosphatase 1 to the C-terminal domain of the KCNQ1 subunit.91,92 PKA-dependent functional regulation of IKs channels is lost when the binding site for Yotiao on the KCNQ1 subunit is mutated (KCNQ1-G589D).91,93 Mutations in both subunits of the IKs channel are associated with at least 2 heritable arrhythmic syndromes, referred to as catecholaminergic polymorphic ventricular tachycardia94 and long-QT syndrome.95 Variants of long-QT syndrome have been shown to be caused by mutations in both the IKs channel α (KCNQ1, LQT1) and β (KCNE1, LQT5) subunits.96,97 Recently, a cohort of patients with genotype-negative long-QT syndrome have been described to carry a missense mutation in Yotiao (S1570L). The S1570L mutation is in the binding domain of Yotiao for KCNQ1. Disruption of the Yotiao/KCNQ1 interaction reduces the PKA-mediated phosphorylation on KCNQ1 amino terminus (Ser27) and eliminates the functional response of IKs channel to cAMP.98 The interaction between Yotiao and KCNQ1 is thus essentially required for the maintenance of a normal heart rhythm.
Recent evidences suggest that AKAP79/150 is also involved in heart rhythm regulation. In physiological conditions, AKAP79/150 coordinates the binding of PKA and PKCα to Cav1.2 and facilitates the coordinated opening and closing of the channel.99,100 A gain of function mutation (G406R) in a cytoplasmic loop of Cav1.2 correlates with an abnormal coupling with AKAP79/150, eventually leading to LQT8, a disease also known as Timothy syndrome.101 This occurs through a mechanism whereby AKAP79/150 functions like a subunit of Cav1.2 that stabilizes the open conformation of the channel. Ablation of this anchoring protein restores normal gating of Cav1.2 and protects the heart from arrhythmias.101
Besides Yotiao and AKAP79/150, heart rhythm modulation involves D-AKAP2 (also referred to as AKAP10). D-AKAP2 controls the sensitivity of pacemaker cells to cholinergic stimulation, both in mouse embryonic stem cell-derived cardiomyocytes and in vivo, in mouse hearts. Accordingly, D-AKAP2–deficient mice display heart rhythm abnormalities, eventually leading to premature death from arrhythmia.102 Interestingly, a human polymorphism (I646V) affecting the affinity of D-AKAP2 for the regulatory subunit RI of PKA has been described. This variant correlates with increased basal heart rate and decreased heart rate variability, 2 events that are indicative of high risk of sudden cardiac death.102 Thus, heart rhythm regulation relies on the coordinated action of Yotiao, AKAP79/150, and D-AKAP2. Furthermore, a growing body of evidence indicates that arrhythmogenesis can also be linked to mitochondrial function.103
Oxidative Stress (Mitochondria, Hypoxia)
Mitochondria constitute a major generator of cellular energy and their activity is controlled by normal cellular homeostasis. A key aspect of mitochondrial function is the dynamic balance of fusion and fission, events that alter mitochondrial morphology and activity.104 Control of mitochondrial dynamics is evolutionary conserved and its deregulation is implicated in pathological conditions, including cardiovascular disorders such as dilated cardiomyopathy, myocardial infarction, and heart failure.105–107 The cAMP/PKA pathway has been recently found to regulate mitochondrial respiration, dynamics, and cellular apoptosis.108 Localization of PKA in proximity to mitochondrial substrates ensures efficient propagation of cAMP signals from the plasma membrane to this target organelle. cAMP signals are carried to mitochondria by a set of mitochondrial AKAPs that regulate mitochondrial function through the organization of signalosomes in this cellular compartment.109
mAKAP
Reduced oxygen levels, referred to as hypoxia, affect mitochondrial function by increasing glycolysis and lactate production. At the molecular level, hypoxia stabilizes hypoxia-inducible factor 1α (HIF-1α), which controls transcription of a wide range of genes, including factors implicated in the regulation of mitochondrial energy metabolism.110,111 Under normoxic conditions, the levels of HIF-1α are kept low through its ubiquitin-mediated proteasomal degradation.112 This multiprotein signaling complex is compartmentalized inside the cell by mAKAP. mAKAP sequesters HIF-1α at the perinuclear membrane, thereby minimizing the translocation distance to its site of action in the nucleus. Furthermore, mAKAP assembles and compartmentalizes components of the protein ubiquitin machinery that determine the bidirectional control of HIF-1α stability.113 During normoxia, mAKAP clusters HIF-1α with negative regulatory factors, like prolyl hydroxylase domains and Von Hippel Lindau, that enhance the efficiency of its degradation. Under hypoxic conditions, positive regulatory factors, including the ubiquitin E3 ligase seven in absentia homolog 2, bind to mAKAP and favor the stabilization of HIF-1α. The expression of HIF-1α target genes protects the heart from oxygen-deprivation injury that occurs under pathological stresses, and, in this condition, mAKAP favors the enhancement of the hypoxic response. Displacement of mAKAP from perinuclear membranes of cardiomyocytes alters the stability of HIF-1α and the transcription of genes associated with hypoxia.
AKAP121
Under hypoxic conditions, HIF-1α availability is controlled by the ubiquitin E3 ligase seven in absentia homolog 2.114 Seven in absentia homolog 2 is normally bound to mitochondrial AKAP121 and the expression levels of this ubiquitin E3 ligase are induced during hypoxia, thereby causing a degradation of AKAP121 and an attenuation of the cAMP/PKA signaling at the mitochondria.115 In normal physiology, AKAP121 regulates mitochondrial morphology by serving as a docking site for PKA at the mitochondrial membrane. Within this compartment, PKA phosphorylates and inhibits the mechanoenzyme dynamin-related protein 1, resulting in an inhibition of mitochondrial fission. Deregulation of AKAP121, that occurs on increased seven in absentia homolog 2 expression in ischemia-induced cardiomyocyte cell death, alleviates dynamin-related protein 1 inhibition, resulting in mitochondria fission.116
AKAP-dependent activation of the cAMP-PKA signaling at the mitochondria also controls oxidative stress, mainly caused by reactive oxygen species (ROS) production. These cAMP-mediated effects are mainly associated with PKA-dependent phosphorylation of complex I subunits117 that results in an enhanced functional capacity of the mitochondrial respiratory chain and in a reduced ROS production.118 In cardiac physiology, AKAP121 tethers PKA at the mitochondria and is thus involved in the control of ROS production, thereby protecting the cardiomyocyte from oxidative stress. Deregulated ROS production within the cardiomyocyte may contribute to the development of cardiac dysfunction. Indeed, displacement of AKAP121 from mitochondria by competitive peptides increases ROS levels and promotes cardiomyocyte death. Furthermore, in response to pressure-overload, AKAP121 protein expression is downregulated, thus resulting in mitochondrial stress, increased ROS production and cell death.119 All these evidences suggest that deregulated mitochondrial cAMP signaling could contribute to the development of cardiac dysfunction.
Hypertrophy
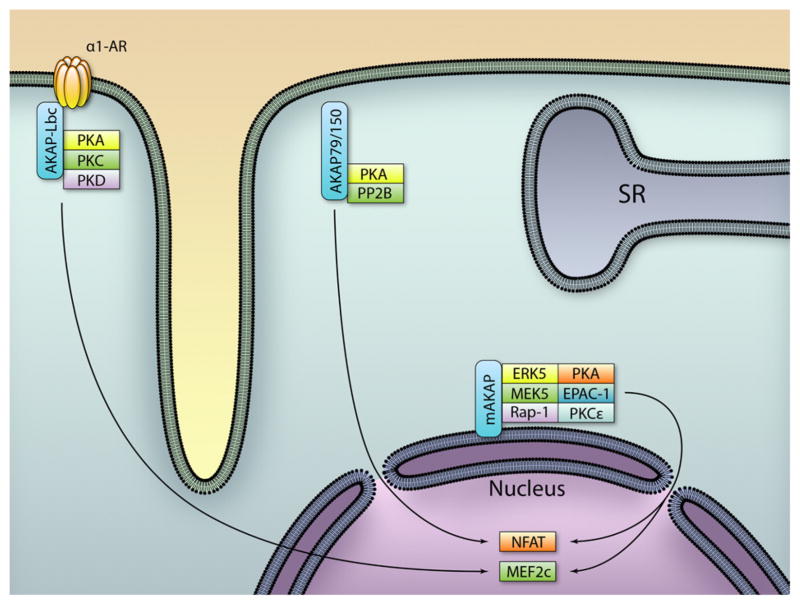
Cardiac hypertrophy is an adaptive remodeling process of the myocardium that occurs in response to various cardiac stresses. It is associated with an increase in cardiomyocyte size, a qualitative and quantitative change in the expression levels of contractile proteins and an activation of fetal cardiac genes.120,121 Because hypertrophy can ultimately progress to ventricular dilation, contractile dysfunction, and heart failure, significant efforts have been made to investigate the molecular players at the basis of this pathological process. Cardiomyocyte hypertrophy is controlled by membrane receptors that trigger multiple networks of intracellular mediators, which in turn transmit the hypertrophic signal to the nucleus.122 An emerging concept in the field of signal transduction is the existence of hubs where multiple signaling pathways converge and share common molecules, thereby facilitating crosstalk between pathways. In this respect, mAKAP, AKAP-Lbc, and AKAP79/150 are attractive candidates that could coordinate hypertrophic signals elicited from multiple stress stimuli (Figure 2).
Figure 2. Regulation of cardiac hypertrophy by A-kinase anchoring protein (AKAPs).
Intracellular localization of AKAP complexes controlling hypertrophic signaling pathways. PKA indicates protein kinase A; PKD, protein kinase D; PP2B, protein phosphatase 2B; SR, sarcoplasmic reticulum; NFAT, nuclear factor of activated T cells; MEF2, myocyte enhancer factor-2. (Illustration: Ben Smith.)
mAKAP
In addition to its role in regulating cardiac contractility and oxidative stress, mAKAP is also implicated in cardiac hypertrophy. Within this scenario, mAKAP assembles a perinuclear macromolecular complex that regulates gene transcription in response to multiple hypertrophic stimuli. This mAKAP complex includes at least 3 enzymes that are involved in the hypertrophic responses: the mitogen activated kinase ERK5,70 the Ca2+/calmodulin-dependent protein phosphatase calcineurin Aβ123 and the epsilon isoform of PLC.124 cAMP-dependent-triggering of the MAP kinase signaling activates the prohypertrophic transcription factor myocyte enhancer factor-2c and its regulated genes.70 On the other hand, Ca2+-induced activation of the mAKAP-associated calcineurin Aβ results in a dephosphorylation and in a nuclear translocation of the transcription factor nuclear factor of activated T cell (NFATc) that promotes the transcription of hypertrophic genes.125,126 The control of hypertrophic gene expression by the epsilon isoform of PLC implicates both the myocyte enhancer factor- and NFAT-dependent transcription.124 Whereas ERK-mediated hypertrophy is triggered by cytokine receptors70 and calcineurin Aβ is activated through the β-AR/cAMP/PKA/RyR2 mediated Ca2+ release,123 the epsilon isoform of PLC integrates multiple upstream signaling pathways that regulate hypertrophy, including endothelin-, norepinephrine-, insulin-like growth factor-1- and isoproterenol-activated signaling.124 The crucial role of mAKAP in the hypertrophic process has been further demonstrated by the reduction of cardiac hypertrophy on the peptide-mediated displacement of mAKAP from the nuclear envelope.70,124
AKAP-Lbc
Several lines of evidence indicate that α-adrenergic transmission, through the activation of heterotrimeric G proteins Gq and G12/13, triggers the GTPase RhoA and its signaling cascade that controls the transcription of genes involved in cardiomyocyte hypertrophy.127 At the cellular level, the activation of small GTPases is controlled by guanine nucleotide exchange factors that facilitate GDP-GTP exchange and the activation of the enzyme. Recent works have identified AKAP-Lbc not only as an AKAP that scaffolds PKA, PKC, and PKD,128 but also as a guanine nucleotide exchange factor for the small GTPase RhoA.24 AKAP-Lbc is activated in response to agonists that stimulate the α1-AR-G12/13 signaling pathway129 and is inactivated via anchored PKA-mediated phosphorylation and subsequent recruitment of the regulatory protein 14-3-3, which prevents AKAP-Lbc from being able to activate Rho.130 Thus, suppression of the Rho-specific exchange factor AKAP-Lbc correlates with a negative modulation of the hypertrophic signaling in response to GPCR-Gq/ G12/13 stimulation. Furthermore, prolonged α-adrenergic stimulation results in an upregulation of AKAP-Lbc protein levels, thereby directing the hypertrophic signal to the transcriptional machinery.26 In more detail, AKAP-Lbc facilitates the activation of PKD that inactivates the histone deacetylase HDAC5, thereby favoring myocyte enhancer factor-2– dependent transcription and the onset of cardiac hypertrophy.131 Therefore, AKAP-Lbc may provide a platform for crosstalk between PKD and Rho signaling pathways, in the context of cardiac hypertrophy.
AKAP79/150
Cardiac hypertrophy is also controlled by the calcium dependent Ser/Thr phosphatase calcineurin (CaN or PP2B) and the downstream transcriptional effectors, including NFAT. Indeed, hyperactivation of the CaN/NFAT pathway in cardiomyocytes of transgenic mice results in profound hypertrophy that rapidly progresses to heart failure.132,133 Several studies have demonstrated the positive effect of the inhibition of the CaN/NFAT signaling pathway in the treatment of cardiac hypertrophy.134 –136 AKAP79/150 has a CaN-binding domain and is one of the endogenous inhibitors of CaN in the brain.15 Cardiac-restricted transgenic mice overexpressing the CaN inhibitory domain of AKAP79/150 display inhibited CaN activity that is associated with attenuated cardiac hypertrophy in response to catecholamine stimulation and pressure overload.137 These findings suggest a primary role for AKAP-mediated control of CaN in the hypertrophic response, even if the precise role of AKAP79/150 in this context still remains to be fully understood.
Heart Failure
Heart failure is a complex and multifactorial disease, characterized by the inability of the heart to pump sufficient blood to meet the metabolic needs of the body and represents a leading cause of mortality worldwide. Heart failure can result from aberrant signaling events that normally regulate myocardial function. In addition, altered gene expression is a peculiar feature of the failing heart. Gene expression profiles have pointed out a large scale of rearrangement in the AKAP-PKA signaling modules during end-stage heart failure. For instance, the expression of AKAP-Lbc, AKAP18δ, AKAP2, and SPHKAP was found upregulated, whereas AKAP121 levels were diminished in the failing human heart.138,139 An example of altered cAMP compartmentation in the failing human myocardium is given by increased protein levels of AKAP18δ. The enhanced association of PKA RIIα with AKAP18δ may result in abnormal calcium reabsorption in the sarcoplasmic reticulum, ultimately leading to altered myocardial contractility.78
Another distinctive feature of failing hearts is a chronic activation of the β-AR signaling pathway that initially compensates for contractile dysfunction but then progresses to deterioration of cardiac structure and function. At the molecular level, β-ARs are downregulated and desensitized through the action of a complex signaling module that includes PKA, G protein-coupled receptor kinase 2 (GRK2), and β-arrestin.140 Tight control of cellular cAMP levels is thus required for normal myocardial contractility. The catalytic subunit of phosphoinositide-3 kinase gamma (p110γ) is an AKAP that controls cAMP levels.141 p110γ tethers PKA in the vicinity of its negative modulator PDE3B, thereby constituting a feedback module that negatively controls cardiac contractility. Moreover, in physiological conditions, the β-AR pathway activates PKA, which in turn phosphorylates and inhibits the lipid kinase activity of the PKA-bound p110γ. In pressure overload-induced heart failure, p110γ is upregulated and escapes PKA-mediated inhibition. Activated p110γ reduces cell surface expression of β-ARs, thereby contributing to the development of heart failure.142 Genetic and pharmacological inhibition of p110γ activity renormalizes β-AR density and improves contractility in failing hearts, thus establishing p110γ as a potential target for the treatment of heart failure.141 Importantly, the spatial localization of β-ARs also plays a critical role in cardiac physiology and in the development of heart failure.140 Indeed, redistribution of β2-AR signaling from the T-tubules to the cell crest in failing cardiomyocytes results in uncoupling of the β2-AR from the localized pools of PKA that are responsible for the compartmentation of the β2-AR–cAMP signaling.143 This results in a cell-wide cAMP propagation on β2-AR activation in failing cells that is similar to the patterns observed for β1-ARs, thus contributing to the heart failure phenotype. These findings, together with a still required more complete analysis of the AKAP function in failing cardiomyocytes, will provide a deeper understanding of this cardiac disease and will facilitate the development of new therapeutic strategies.
Conclusion
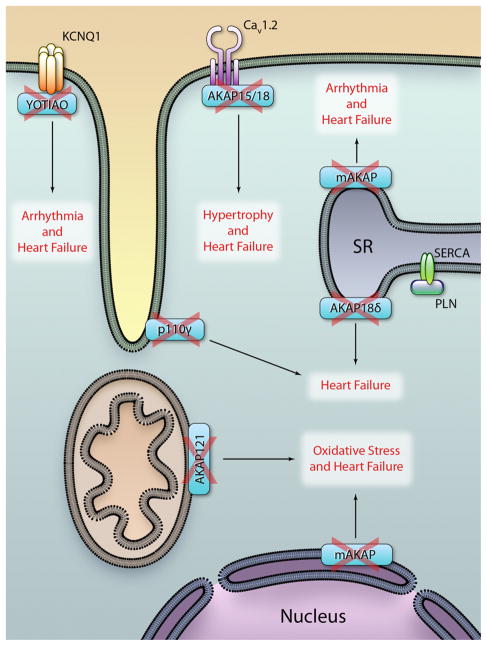
Evidence accumulated in decades of studies on AKAP and their partners clearly indicate that alteration of such complexes represents a key contributing factor for cardiac diseases (Figure 3). Manipulation of protein–protein interaction at AKAPs is thus emerging as a promising therapeutic strategy. Proof of concept studies show that small molecules can in principle act to pharmacologically modulate AKAP-based signaling complexes.29 However, the limited number of such attempts has only scratched the surface of a vast potential of pharmacological intervention. Complexes at cardiac anchoring proteins can encompass 10, 20, or more components where each interaction is in principle amenable to pharmacological modulation. Our knowledge of the biological and chemical properties of these protein–protein complexes is only at its infancy. To better define targets of therapeutic interest, future work has to focus on the biochemical details and the pathophysiological meaning of such protein–protein interactions. First, 3-dimensional structures of protein–protein interactions are necessary to define how and where these interactions occur.9,10 Native mass spectroscopy, small angle X ray scattering and cryoelectron microscopy have recently proven to be valuable tools suitable to tackle this issue.144,145 Second, the role of such interactions in relevant disease conditions needs a detailed validation. Genetic modeling of the disruption of selected AKAP complexes in knock-in mice will likely provide conclusive proofs for the therapeutic value of such interventions. Third, new biochemical assays that simplify the search for disrupting moieties are required to select small molecules of pharmacological interest. Finally, it is tempting to speculate that the identification of small molecules that act on spatial and temporal restricted signaling will eventually prove to be more effective treatments for different aspects of heart failure, especially because our current therapeutic arsenal of drugs is still inadequate to combat this global health problem.
Figure 3. Disruption of A-kinase anchoring protein (AKAP)-protein kinase A (PKA) signaling underlies different cardiac diseases that finally lead to heart failure.
Broken AKAPs correspond to disruption of the scaffolding function. SR, sarcoplasmic reticulum; PLN, phospholamban; SERCA, sarcoplasmic reticular Ca2+-adenosine triphosphatase. PKA indicates protein kinase A. (Illustration: Ben Smith.)
Acknowledgments
Sources of Funding
This work was supported by grants from Fondation Leducq 06CDV02 (E.H., J.D.S.) and 09CVD01 (E.H.), AIRC (E.H.), Fondazione Cariplo-Ricerca Biomedica 2009 (E.H.), Regione Piemonte (E.H.), and NIH grant HL08836 (J.D.S.).
Non-standard Abbreviations and Acronyms
- β-AR
β-adrenergic receptor
- AC
adenylyl cyclase
- AKAP
A-kinase anchoring protein
- cAMP
cyclic AMP
- GPCR
G protein-coupled receptor
- GRK-2
G protein-coupled receptor kinase 2
- HIF-1α
hypoxia-inducible factor 1α
- LTCC
voltage-gated L-type Ca2+ channel
- NFAT
nuclear factor of activated T cells
- PDE
phosphodiesterase
- PKA
protein kinase A
- PLN
phospholamban
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
sarcoplasmic reticular Ca2+-adenosine triphosphatase
- SR
sarcoplasmic reticulum
Footnotes
Disclosures
None.
References
- 1.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 2.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680– 686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Dremier S, Kopperud R, Doskeland SO, Dumont JE, Maenhaut C. Search for new cyclic AMP-binding proteins. FEBS Lett. 2003;546:103–107. doi: 10.1016/s0014-5793(03)00561-1. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SS, Stafford PH. Characterization of adenosine 3′:5′-monophosphate-dependent protein kinase and its dissociated subunits from porcine skeletal muscle. J Biol Chem. 1978;253:2284–2287. [PubMed] [Google Scholar]
- 5.Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci U S A. 1996;93:295–299. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 7.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836– 844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 8.Carr DW, Stofko-Hahn RE, Fraser ID, Bishop SM, Acott TS, Brennan RG, Scott JD. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J Biol Chem. 1991;266:14188–14192. [PubMed] [Google Scholar]
- 9.Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Tasken K, Carlson CR, Scott JD, Barford D. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–395. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kinderman FS, Kim C, von Daake S, Ma Y, Pham BQ, Spraggon G, Xuong NH, Jennings PA, Taylor SS. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell. 2006;24:397– 408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem. 1997;272:8057– 8064. doi: 10.1074/jbc.272.12.8057. [DOI] [PubMed] [Google Scholar]
- 12.Kovanich D, van der Heyden MA, Aye TT, van Veen TA, Heck AJ, Scholten A. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type I cAMP-dependent protein kinase. Chembiochem. 2010;11:963–971. doi: 10.1002/cbic.201000058. [DOI] [PubMed] [Google Scholar]
- 13.Means CK, Lygren B, Langeberg LK, Jain A, Dixon RE, Vega AL, Gold MG, Petrosyan S, Taylor SS, Murphy AN, Ha T, Santana LF, Tasken K, Scott JD. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc Natl Acad Sci U S A. 2011;108:E1227–E1235. doi: 10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruppelt A, Mosenden R, Gronholm M, Aandahl EM, Tobin D, Carlson CR, Abrahamsen H, Herberg FW, Carpen O, Tasken K. Inhibition of T cell activation by cyclic adenosine 5′-monophosphate requires lipid raft targeting of protein kinase A type I by the A-kinase anchoring protein ezrin. J Immunol. 2007;179:5159–5168. doi: 10.4049/jimmunol.179.8.5159. [DOI] [PubMed] [Google Scholar]
- 15.Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 16.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasken KA, Collas P, Kemmner WA, Witczak O, Conti M, Tasken K. Phosphodiesterase 4D and protein kinase a type II constitute a signaling unit in the centrosomal area. J Biol Chem. 2001;276:21999–22002. doi: 10.1074/jbc.C000911200. [DOI] [PubMed] [Google Scholar]
- 18.Skroblin P, Grossmann S, Schafer G, Rosenthal W, Klussmann E. Mechanisms of protein kinase A anchoring. Int Rev Cell Mol Biol. 2010;283:235–330. doi: 10.1016/S1937-6448(10)83005-9. [DOI] [PubMed] [Google Scholar]
- 19.Pidoux G, Tasken K. Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins. J Mol Endocrinol. 2010;44:271–284. doi: 10.1677/JME-10-0010. [DOI] [PubMed] [Google Scholar]
- 20.Diviani D, Dodge-Kafka KL, Li J, Kapiloff MS. A-kinase anchoring proteins: scaffolding proteins in the heart. Am J Physiol Heart Circ Physiol. 2011;301:H1742–H1753. doi: 10.1152/ajpheart.00569.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishman MC, Chien KR. Fashioning the vertebrate heart: earliest embryonic decisions. Development. 1997;124:2099–2117. doi: 10.1242/dev.124.11.2099. [DOI] [PubMed] [Google Scholar]
- 22.Sucov HM. Molecular insights into cardiac development. Annu Rev Physiol. 1998;60:287–308. doi: 10.1146/annurev.physiol.60.1.287. [DOI] [PubMed] [Google Scholar]
- 23.Mayers CM, Wadell J, McLean K, et al. The Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J Biol Chem. 2010;285:12344–12354. doi: 10.1074/jbc.M110.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276:44247– 44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 25.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23– 49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 28.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 29.Christian F, Szaszak M, Friedl S, et al. Small molecule AKAP-protein kinase A (PKA) interaction disruptors that activate PKA interfere with compartmentalized cAMP signaling in cardiac myocytes. J Biol Chem. 2011;286:9079–9096. doi: 10.1074/jbc.M110.160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fink MA, Zakhary DR, Mackey JA, Desnoyer RW, Apperson-Hansen C, Damron DS, Bond M. AKAP-mediated targeting of protein kinase a regulates contractility in cardiac myocytes. Circ Res. 2001;88:291–297. doi: 10.1161/01.res.88.3.291. [DOI] [PubMed] [Google Scholar]
- 31.McConnell BK, Popovic Z, Mal N, Lee K, Bautista J, Forudi F, Schwartzman R, Jin JP, Penn M, Bond M. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J Biol Chem. 2009;284:1583–1592. doi: 10.1074/jbc.M806321200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel HH, Hamuro LL, Chun BJ, et al. Disruption of protein kinase A localization using a trans-activator of transcription (TAT)-conjugated A-kinase-anchoring peptide reduces cardiac function. J Biol Chem. 2010;285:27632–27640. doi: 10.1074/jbc.M110.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 34.Xiang Y, Devic E, Kobilka B. The PDZ binding motif of the beta 1 adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J Biol Chem. 2002;277:33783–33790. doi: 10.1074/jbc.M204136200. [DOI] [PubMed] [Google Scholar]
- 35.Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 36.Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res. 2002;91:672– 680. doi: 10.1161/01.res.0000037000.74258.03. [DOI] [PubMed] [Google Scholar]
- 37.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- 38.Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Dell’Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4, 5-bisphosphate. EMBO J. 1998;17:2246–2260. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shcherbakova OG, Hurt CM, Xiang Y, Dell’Acqua ML, Zhang Q, Tsien RW, Kobilka BK. Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol. 2007;176:521–533. doi: 10.1083/jcb.200604167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delint-Ramirez I, Willoughby D, Hammond GV, Ayling LJ, Cooper DM. Palmitoylation targets AKAP79 protein to lipid rafts and promotes its regulation of calcium-sensitive adenylyl cyclase type 8. J Biol Chem. 2011;286:32962–32975. doi: 10.1074/jbc.M111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HY, Tao J, Shumay E, Malbon CC. G-protein– coupled receptor-associated A-kinase anchoring proteins: AKAP79 and AKAP250 (gravin) Eur J Cell Biol. 2006;85:643– 650. doi: 10.1016/j.ejcb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Fan G, Shumay E, Malbon CC, Wang H. c-Src tyrosine kinase binds the beta 2-adrenergic receptor via phospho-Tyr-350, phosphorylates G-protein-linked receptor kinase 2, and mediates agonist-induced receptor desensitization. J Biol Chem. 2001;276:13240–13247. doi: 10.1074/jbc.M011578200. [DOI] [PubMed] [Google Scholar]
- 45.Valentine CD, Haggie PM. Confinement of beta(1)- and beta(2)-adrenergic receptors in the plasma membrane of cardiomyocyte-like H9c2 cells is mediated by selective interactions with PDZ domain and A-kinase anchoring proteins but not caveolae. Mol Biol Cell. 2011;22:2970–2982. doi: 10.1091/mbc.E11-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nauert JB, Klauck TM, Langeberg LK, Scott JD. Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr Biol. 1997;7:52– 62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- 47.Shih M, Lin F, Scott JD, Wang HY, Malbon CC. Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem. 1999;274:1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- 48.Tao J, Wang HY, Malbon CC. Protein kinase A regulates AKAP250 (gravin) scaffold binding to the beta2-adrenergic receptor. EMBO J. 2003;22:6419– 6429. doi: 10.1093/emboj/cdg628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin F, Wang H, Malbon CC. Gravin-mediated formation of signaling complexes in beta 2-adrenergic receptor desensitization and resensitization. J Biol Chem. 2000;275:19025–19034. doi: 10.1074/jbc.275.25.19025. [DOI] [PubMed] [Google Scholar]
- 50.Tao J, Malbon CC. G-protein-coupled receptor-associated A-kinase anchoring proteins AKAP5 and AKAP12: differential signaling to MAPK and GPCR recycling. J Mol Signal. 2008;3:19. doi: 10.1186/1750-2187-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benitah JP, Alvarez JL, Gomez AM. L-type Ca(2+) current in ventricular cardiomyocytes. J Mol Cell Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Goonasekera SA, Hammer K, Auger-Messier M, et al. Decreased cardiac L-type Ca(2) channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122:280–290. doi: 10.1172/JCI58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray PC, Scott JD, Catterall WA. Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr Opin Neurobiol. 1998;8:330–334. doi: 10.1016/s0959-4388(98)80057-3. [DOI] [PubMed] [Google Scholar]
- 54.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 56.Osterrieder W, Brum G, Hescheler J, Trautwein W, Flockerzi V, Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982;298:576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- 57.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 58.Fu Y, Westenbroek RE, Yu FH, Clark JP, III, Marshall MR, Scheuer T, Catterall WA. Deletion of the distal C terminus of CaV1.2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J Biol Chem. 2011;286:12617–12626. doi: 10.1074/jbc.M110.175307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 60.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hell JW, Yokoyama CT, Wong ST, Warner C, Snutch TP, Catterall WA. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel alpha 1 subunit. J Biol Chem. 1993;268:19451–19457. [PubMed] [Google Scholar]
- 62.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297– 6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 64.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci U S A. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem. 2002;277:4079– 4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 66.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Auto-inhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci. 2001;114:3167–3176. doi: 10.1242/jcs.114.17.3167. [DOI] [PubMed] [Google Scholar]
- 68.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci. 1999;112:2725–2736. doi: 10.1242/jcs.112.16.2725. [DOI] [PubMed] [Google Scholar]
- 69.Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem. 2009;284:23540–23546. doi: 10.1074/jbc.M109.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, Makarewich CA, Kubo H, Wang W, Duran JM, Li Y, Berretta RM, Koch WJ, Chen X, Gao E, Valdivia HH, Houser SR. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ Res. 2012;110:831– 840. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beca S, Helli PB, Simpson JA, et al. Phosphodiesterase 4D regulates baseline sarcoplasmic reticulum Ca2+ release and cardiac contractility, independently of L-type Ca2+ current. Circ Res. 2011;109:1024–1030. doi: 10.1161/CIRCRESAHA.111.250464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ Res. 2012;110:796–799. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- 75.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, Tasken K, Klussmann E. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henn V, Edemir B, Stefan E, et al. Identification of a novel A-kinase anchoring protein 18 isoform and evidence for its role in the vasopressin-induced aquaporin-2 shuttle in renal principal cells. J Biol Chem. 2004;279:26654–26665. doi: 10.1074/jbc.M312835200. [DOI] [PubMed] [Google Scholar]
- 77.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 78.Lygren B, Tasken K. The potential use of AKAP18delta as a drug target in heart failure patients. Expert Opin Biol Ther. 2008;8:1099–1108. doi: 10.1517/14712598.8.8.1099. [DOI] [PubMed] [Google Scholar]
- 79.Diviani D, Scott JD. AKAP signaling complexes at the cytoskeleton. J Cell Sci. 2001;114:1431–1437. doi: 10.1242/jcs.114.8.1431. [DOI] [PubMed] [Google Scholar]
- 80.Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Remedios CD, Duncker DJ, Stienen GJ, van der Velden J. Sarcomeric dysfunction in heart failure. Cardiovasc Res. 2008;77:649– 658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 81.Russell MA, Lund LM, Haber R, McKeegan K, Cianciola N, Bond M. The intermediate filament protein, synemin, is an AKAP in the heart. Arch Biochem Biophys. 2006;456:204–215. doi: 10.1016/j.abb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Sumandea CA, Garcia-Cazarin ML, Bozio CH, Sievert GA, Balke CW, Sumandea MP. Cardiac troponin T, a sarcomeric AKAP, tethers protein kinase A at the myofilaments. J Biol Chem. 2011;286:530–541. doi: 10.1074/jbc.M110.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weisberg A, Winegrad S. Alteration of myosin cross bridges by phosphorylation of myosin-binding protein C in cardiac muscle. Proc Natl Acad Sci U S A. 1996;93:8999–9003. doi: 10.1073/pnas.93.17.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer J, Jin SL, Conti M. Myomegalin is a novel protein of the golgi/ centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem. 2001;276:11189–11198. doi: 10.1074/jbc.M006546200. [DOI] [PubMed] [Google Scholar]
- 85.Uys GM, Ramburan A, Loos B, Kinnear CJ, Korkie LJ, Mouton J, Riedemann J, Moolman-Smook JC. Myomegalin is a novel A-kinase anchoring protein involved in the phosphorylation of cardiac myosin binding protein C. BMC Cell Biol. 2011;12:18. doi: 10.1186/1471-2121-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chandra M, Dong WJ, Pan BS, Cheung HC, Solaro RJ. Effects of protein kinase A phosphorylation on signaling between cardiac troponin I and the N-terminal domain of cardiac troponin C. Biochemistry. 1997;36:13305–13311. doi: 10.1021/bi9710129. [DOI] [PubMed] [Google Scholar]
- 87.Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48:866– 875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67– 69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- 89.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78– 80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 90.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80– 83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 91.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496– 499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 92.Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 94.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 95.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Q, Curran ME, Splawski I, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 97.Abbott GW, Goldstein SA. Disease-associated mutations in KCNE potassium channel subunits (MiRPs) reveal promiscuous disruption of multiple currents and conservation of mechanism. FASEB J. 2002;16:390– 400. doi: 10.1096/fj.01-0520hyp. [DOI] [PubMed] [Google Scholar]
- 98.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1– e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 100.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng EP, Yuan C, Navedo MF, Dixon RE, Nieves-Cintron M, Scott JD, Santana LF. Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circ Res. 2011;109:255–261. doi: 10.1161/CIRCRESAHA.111.248252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tingley WG, Pawlikowska L, Zaroff JG, Kim T, Nguyen T, Young SG, Vranizan K, Kwok PY, Whooley MA, Conklin BR. Gene-trapped mouse embryonic stem cell-derived cardiac myocytes and human genetics implicate AKAP10 in heart rhythm regulation. Proc Natl Acad Sci U S A. 2007;104:8461– 8466. doi: 10.1073/pnas.0610393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown DA, O’Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc Res. 2010;88:241–249. doi: 10.1093/cvr/cvq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 105.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/ reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 107.Schaper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83:504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- 108.Carlucci A, Lignitto L, Feliciello A. Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends Cell Biol. 2008;18:604– 613. doi: 10.1016/j.tcb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 109.Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17:279–287. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 112.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423– 427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 113.Wong W, Goehring AS, Kapiloff MS, Langeberg LK, Scott JD. mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci Signal. 2008;1:ra18. doi: 10.1126/scisignal.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument-Bromage H, Tempst P, Frappell PB, Bowtell DD, Ronai Z. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 115.Carlucci A, Adornetto A, Scorziello A, Viggiano D, Foca M, Cuomo O, Annunziato L, Gottesman M, Feliciello A. Proteolysis of AKAP121 regulates mitochondrial activity during cellular hypoxia and brain ischaemia. EMBO J. 2008;27:1073–1084. doi: 10.1038/emboj.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim H, Scimia MC, Wilkinson D, Trelles RD, Wood MR, Bowtell D, Dillin A, Mercola M, Ronai ZA. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol Cell. 2011;44:532–544. doi: 10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scacco S, Vergari R, Scarpulla RC, Technikova-Dobrova Z, Sardanelli A, Lambo R, Lorusso V, Papa S. cAMP-dependent phosphorylation of the nuclear encoded 18-kDa (IP) subunit of respiratory complex I and activation of the complex in serum-starved mouse fibroblast cultures. J Biol Chem. 2000;275:17578–17582. doi: 10.1074/jbc.M001174200. [DOI] [PubMed] [Google Scholar]
- 118.Piccoli C, Scacco S, Bellomo F, Signorile A, Iuso A, Boffoli D, Scrima R, Capitanio N, Papa S. cAMP controls oxygen metabolism in mammalian cells. FEBS Lett. 2006;580:4539– 4543. doi: 10.1016/j.febslet.2006.06.085. [DOI] [PubMed] [Google Scholar]
- 119.Perrino C, Feliciello A, Schiattarella GG, et al. AKAP121 downregulation impairs protective cAMP signals, promotes mitochondrial dysfunction, and increases oxidative stress. Cardiovasc Res. 2010;88:101–110. doi: 10.1093/cvr/cvq155. [DOI] [PubMed] [Google Scholar]
- 120.Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 121.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 122.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 123.Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci. 2005;118:5637–5646. doi: 10.1242/jcs.02675. [DOI] [PubMed] [Google Scholar]
- 124.Zhang L, Malik S, Kelley GG, Kapiloff MS, Smrcka AV. Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPbeta) and integrates multiple hypertrophic stimuli in cardiac myocytes. J Biol Chem. 2011;286:23012–23021. doi: 10.1074/jbc.M111.231993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 126.Li J, Wu F, Zhang H, Fu F, Ji L, Dong L, Li Q, Liu W, Zhang Y, Lv A, Wang H, Ren J, Gao F. Insulin inhibits leukocyte-endothelium adherence via an Akt-NO-dependent mechanism in myocardial ischemia/reperfusion. J Mol Cell Cardiol. 2009;47:512–519. doi: 10.1016/j.yjmcc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 127.Maruyama Y, Nishida M, Sugimoto Y, Tanabe S, Turner JH, Kozasa T, Wada T, Nagao T, Kurose H. Galpha(12/13) mediates alpha(1)-adrenergic receptor-induced cardiac hypertrophy. Circ Res. 2002;91:961–969. doi: 10.1161/01.res.0000043282.39776.7c. [DOI] [PubMed] [Google Scholar]
- 128.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol Cell. 2004;15:889– 899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 129.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2007;104:10140–10145. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 2004;23:2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374– 8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, II, Kitsis RN, Molkentin JD. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circ Res. 2000;86:255–263. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- 134.Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 135.Meguro T, Hong C, Asai K, Takagi G, McKinsey TA, Olson EN, Vatner SF. Cyclosporine attenuates pressure-overload hypertrophy in mice while enhancing susceptibility to decompensation and heart failure. Circ Res. 1999;84:735–740. doi: 10.1161/01.res.84.6.735. [DOI] [PubMed] [Google Scholar]
- 136.Shimoyama M, Hayashi D, Takimoto E, Zou Y, Oka T, Uozumi H, Kudoh S, Shibasaki F, Yazaki Y, Nagai R, Komuro I. Calcineurin plays a critical role in pressure overload-induced cardiac hypertrophy. Circulation. 1999;100:2449–2454. doi: 10.1161/01.cir.100.24.2449. [DOI] [PubMed] [Google Scholar]
- 137.De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, Glascock BJ, Kimball TF, del Monte F, Hajjar RJ, Molkentin JD. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2001;98:3322–3327. doi: 10.1073/pnas.031371998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ruehr ML, Russell MA, Bond M. A-kinase anchoring protein targeting of protein kinase A in the heart. J Mol Cell Cardiol. 2004;37:653– 665. doi: 10.1016/j.yjmcc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 139.Aye TT, Soni S, van Veen TA, van der Heyden MA, Cappadona S, Varro A, de Weger RA, de Jonge N, Vos MA, Heck AJ, Scholten A. Reorganized PKA-AKAP associations in the failing human heart. J Mol Cell Cardiol. 2012;52:511–518. doi: 10.1016/j.yjmcc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 140.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 141.Perino A, Ghigo A, Ferrero E, et al. Integrating cardiac PIP3 and cAMP signaling through a PKA anchoring function of p110gamma. Mol Cell. 2011;42:84–95. doi: 10.1016/j.molcel.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perrino C, Schroder JN, Lima B, Villamizar N, Nienaber JJ, Milano CA, Naga Prasad SV. Dynamic regulation of phosphoinositide 3-kinase– gamma activity and beta-adrenergic receptor trafficking in end-stage human heart failure. Circulation. 2007;116:2571–2579. doi: 10.1161/CIRCULATIONAHA.107.706515. [DOI] [PubMed] [Google Scholar]
- 143.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 144.Gold MG, Stengel F, Nygren PJ, Weisbrod CR, Bruce JE, Robinson CV, Barford D, Scott JD. Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex. Proc Natl Acad Sci U S A. 2011;108:6426– 6431. doi: 10.1073/pnas.1014400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang QX, Gonen T. The influence of lipids on voltage-gated ion channels. Curr Opin Struct Biol. doi: 10.1016/j.sbi.2012.03.009. Epub ahead of print April 5, 2012. http://www.sciencedirect.com/science/article/pii/S0959440X12000450. [DOI] [PMC free article] [PubMed]