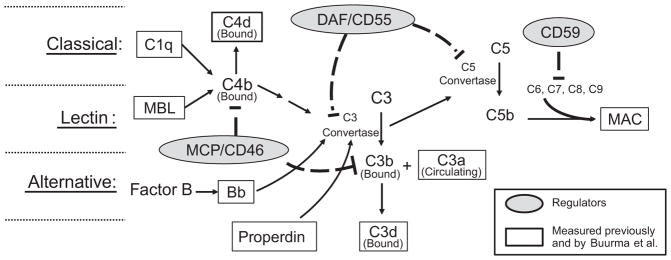
Figure 1.
Simplistic schematic of complement system activation depicting regulators and complement components measured by Buurma and others. The complement system is activated via three pathways: Classical, lectin and alternative. All three pathways result in formation of C3 convertase to cleave C3 into a covalently bound C3b fragment and a fluid phase C3a fragment. C1q is indicative of classical pathway involvement, and mannose binding lectin (MBL) indicates lectin pathway involvement. With activation of either the classical or lectin pathway, C4b covalently binds locally to invader or self surfaces. C4b either participates in formation of C3 convertase, or is degraded to bound C4d. With alternative pathway activation, Factor B is cleaved to form Bb which participates in formation of the alternative pathway C3 convertase. Properdin stabilizes C3 convertase formed with alternative pathway activation. C3b can either participate as part of the C5 convertase complex to cleave C5 or be degraded to bound C3d. C5b, C6, C7, C8 and C9 assemble in cell membranes to form membrane attack complex (MAC) which deteriorates membrane potential and induces cell lysis. Regulators present on the trophoblast membrane as well as numerous cells in mother and fetus include MCP (CD46), DAF (CD55), and CD59. MCP, or membrane cofactor protein, facilitates degradation of C3b and C4b so they do not continue as part of the C3 convertase. DAF, or decay-accelerating factor, facilitates decay of convertases assembled from activation of any of the pathways, and CD59 hampers assembly of MAC.