Abstract
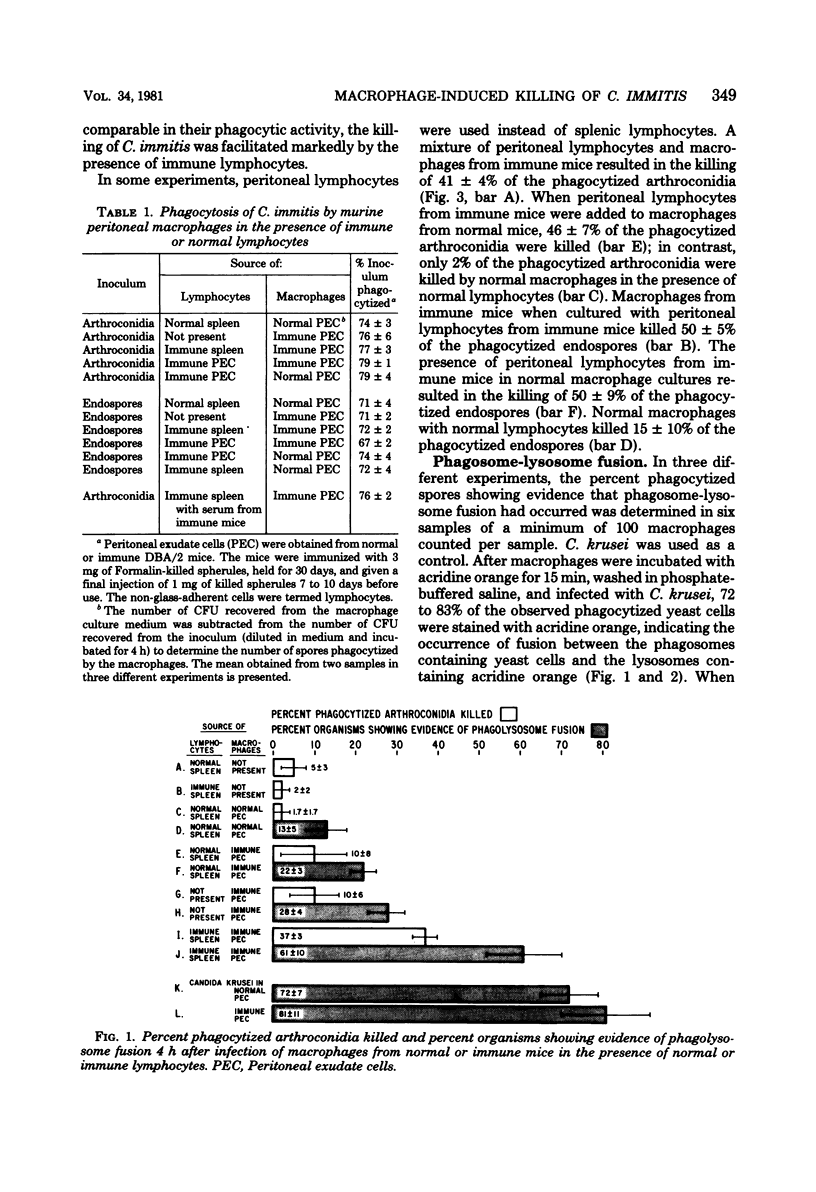
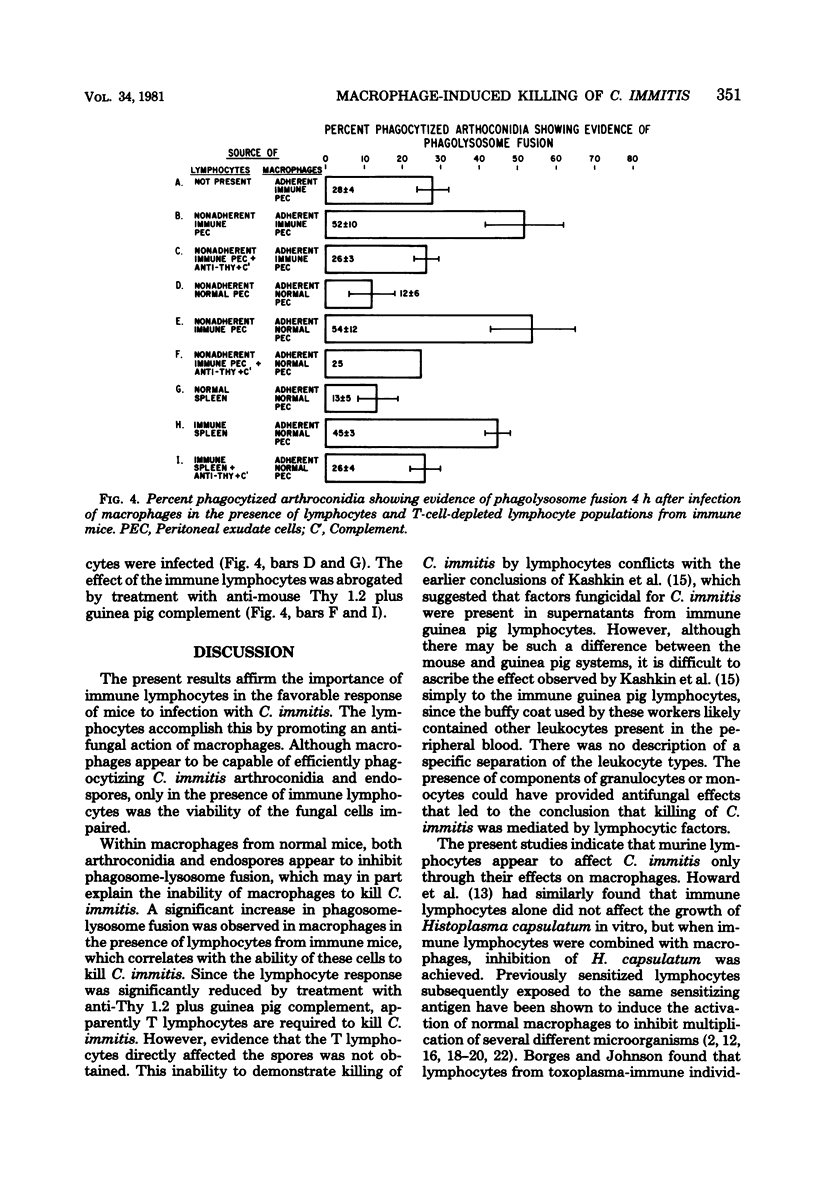
Peritoneal macrophages from normal mice phagocytized arthroconidia and endospores of Coccidioides immitis without affecting the viability of the spores within 4 h after infection. In contrast, macrophages, when infected in the presence of lymphocytes from immune mice, significantly reduced the viability of phagocytized endospores and arthroconidia. The inability of macrophages from normal mice to kill C. immitis may in part be explained by the observation that C. immitis appeared to inhibit fusion of the phagosomes containing fungal spores with the lysosomes within the macrophages. However, fusion of phagosomes containing spores and lysosomes was observed in macrophages infected in the presence of lymphocytes from immune mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Holmberg C. A. In vitro response of alveolar macrophages to infection with Coccidioides immitis. Infect Immun. 1980 May;28(2):594–600. doi: 10.1128/iai.28.2.594-600.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Pappagianis D., Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977 Sep;17(3):580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges J. S., Johnson W. D., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J Exp Med. 1975 Feb 1;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Collins M., Pappagianis D. Effects of lysozyme and chitinase on the spherules of Coccidioides immitis in vitro. Infect Immun. 1973 May;7(5):817–822. doi: 10.1128/iai.7.5.817-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of alveolar macrophages with Nocardia asteroides: immunological enhancement of phagocytosis, phagosome-lysosome fusion, and microbicidal activity. Infect Immun. 1980 Nov;30(2):578–587. doi: 10.1128/iai.30.2.578-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs D. J., Jerrells T. R. In vitro evaluation of immunity to Coxiella burnetii. J Immunol. 1976 Sep;117(3):996–1003. [PubMed] [Google Scholar]
- Howard D. H., Otto V., Gupta R. K. Lymphocyte-mediated cellular immunity in histoplasmosis. Infect Immun. 1971 Nov;4(5):605–610. doi: 10.1128/iai.4.5.605-610.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkin K. P., Likholetov S. M., Lipnitsky A. V. Studies on mediators of cellular immunity in experimental coccidioidomycosis. Sabouraudia. 1977 Mar;15(1):59–68. doi: 10.1080/00362177785190111. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect Immun. 1971 Oct;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Madin S. H. Cellular responses in lungs of immunized mice to intranasal infection with Coccidioides immitis. Sabouraudia. 1968 Feb;6(2):94–102. doi: 10.1080/00362176885190181. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]


