Abstract
Recognizing and avoiding aversive situations are central aspects of mammalian cognition. These abilities are essential for health and survival and are expected to have a prominent genetic basis. We modeled these abilities in eight common mouse inbred strains covering ~75% of the species’ natural variation and in gene-trap mice (>2000 mice), using an unsupervised, automated assay with an instrumented home cage (PhenoTyper) containing a shelter with two entrances. Mice visited this shelter for 20–1200 times/24 h and 71% of all mice developed a significant and often strong preference for one entrance. Subsequently, a mild aversive stimulus (shelter illumination) was automatically delivered when mice used their preferred entrance. Different genotypes developed different coping strategies. Firstly, the number of entries via the preferred entrance decreased in DBA/2J, C57BL/6J and 129S1/SvImJ, indicating that these genotypes associated one specific entrance with the aversive stimulus. Secondly, mice started sleeping outside (C57BL/6J, DBA/2J), indicating they associated the shelter, in general, with the aversive stimulus. Some mice showed no evidence for an association between the entrance and the aversive light, but did show markedly shorter shelter residence times in response to illumination, indicating they did perceive illumination as aversive. Finally, using this assay, we screened 43 different mutants, which yielded a novel gene, specc1/cytospinB. This mutant showed profound and specific delay in avoidance learning. Together, these data suggest that different genotypes express distinct learning and/or memory of associations between shelter entrance and aversive stimuli, and that specc1/cytospinB is involved in this aspect of cognition.
Keywords: Avoidance learning, behavioral flexibility, common inbred, high-throughput behavioral screening, home cage, SPECC1
Recognizing and coping with aversive situations are essential abilities for health and survival in mammals. Successful avoidance strategies require distinct cognitive abilities: learning and remembering stimuli that predict aversive situations or harm, and also the flexibility to suppress or alter routine- or preferred behaviors. Aberrancies in those behaviors are core symptoms of many affective disorders (Dziegielewski 2010) and are associated with many psychiatric disorders such as schizophrenia [(Szöke et al. 2005), for a meta-analysis: (Lesh et al. 2011)] and ADHD [for a meta-analysis: (Willcutt et al. 2005)], and with substance (ab)use (Kreek et al. 2005; Wills et al. 1994). Twin studies indicate that variation in harm avoidance and behavioral flexibility have a prominent genetic basis, with heritability estimates of 39–50%, (Finkel & McGue 1997; Gagne & Saudino 2010; Groot et al. 2004; Polderman et al. 2009; Stins et al. 2004; Yamagata et al. 2005). The long allele of the serotonin transporter gene has been implicated as a causal variant in harm avoidance, but evidence for non-involvement is currently prevailing (Munafò et al. 2009; Schinka 2005; Wray et al. 2009). Genome-wide association studies have not yet produced convincing alternatives (Verweij et al. 2010). Hence, to date we know little about which genetic variation is involved in these avoidance traits.
To unravel the mechanisms that drive avoidance behavior and to identify potential genetic factors that modulate these, mice are a suitable resource. Classical behavioral tests, such as passive avoidance (Baarendse et al. 2008; Crawley et al. 1997; McGaugh 1966), have been used to describe avoidance responses to an electric foot shock. Different studies in avoidance responses suggest that natural genetic variation substantially influences this type of behavior (Crawley et al. 1997). In addition, mice can also establish multiple, distinct associations with an aversive stimulus [as for instance in a fear conditioning (Stiedl et al. 1999)]. Using such paradigms, several features of avoidance learning and behavioral flexibility have been elucidated. However, classical stand-alone tests typically employ rather intense learning stimuli (i.e. electric shock) or require animals to be motivated by food deprivation. Unspecific stress levels are further enhanced by animal handling when placing mice in the test system, thereby potentially impacting on test results (Hurst & West 2010).
One prominent approach to avoid these limitations is to test animals in their home cage environment without human interference. Moreover, we expect that in a home cage environment even subtle stimuli are salient enough to drive avoidance learning. Innovations in automated detection of rodent behavior allow studying various aspects of spontaneous behavior (de Visser et al. 2005, 2006; Goulding et al. 2008; Jhuang et al. 2010; Voikar et al. 2010) and have paved the way for systematic (high-throughput) analysis of mouse behavior, also tapping in on the fast expansion of available genetically modified mice. Such automated, unbiased approaches to natural behaviors can also be applied to study cognitive traits, like learning and memory, but novel methods are required to automate analysis of these traits.
Toward this goal, we characterized the complex behavioral response indicative of avoidance learning in mice using an unsupervised, automated high-throughput system. We adopted an experimental procedure developed by de Heer et al. (2008) in an instrumented home cage, the PhenoTyper, containing a shelter with two entrances. This assay exploits the natural tendency of mice to develop a preference for one of two shelter entrances, and applies automated detection of shelter entrances. Automation can now be used to sanction specifically visits to the preferred entrance by applying a mild aversive stimulus (illumination of the shelter with bright light). This learning paradigm, which is not confounded by human interference and/or highly stressful stimuli, specifically addresses cognitive aspects of avoidance behavior and produces a wealth of information on other aspects of behavior. Using this assay, we screened >2000 mice and obtained evidence for specific associations between the aversive stimulus and the preferred shelter entrance, and more generalized associations between the stimulus and the shelter in general. Finally, as proof of principle for high-throughput gene function analysis, we screened a panel of 43 different mutants, and identified a novel gene, specc1/cytospinB, involved in avoidance learning.
Materials and methods
Eight inbred strains
129S1/SvImJ, A/J, BALB/C, C3H/HeJ, C57BL/6J, DBA/2J, FVB/N and NOD/LtJ. All male, aged between 7 and 18 weeks old, were obtained from Harlan (Horst, The Netherlands) or bred in house, housed under 12-h dark-light cycle with access to water and food ad libitum. After acclimation to the facility for at least 1 week, each mouse was individually housed in one of the 48 PhenoTyper® cages 6.5 days. All experimental procedures were approved by the national animal research committee and complied with the European Council Directive.
Sleeping Beauty transposon mice
Transposon seeder mice were obtained by crossing transposon concatamer GT3A/tTA mice, with SB10 mice expressing the transposase (Geurts et al. 2006). Both GT3A/tTA and SB10 mice had been back-crossed to C57BL/6 mice (Harlan) for >12 generations before crossing. Seeder mice were mated with C57BL/6J mice to generate mutant offspring. Integration of the transposon was detected by linker-mediated PCR. Two microgram of tail DNA was digested with NlaIII overnight at 37°C, cleaned up using Qiagen’s Qiaquick PCR purification kit (Venlo, Netherlands) and eluted in 50 µl water. Linker-oligonucleotide mix was prepared by mixing 50 µl linker+ (25 µm), 50 µl linker− (25 µm), and 1 µl 5 m NaCl, heated in a boiling bath for 5 min and slowly cooled to room temperature. Sequence of the linker oligonucleotides was:
linker+: 5′-TAATACGACTCACTATAGGGCTCCGCTTAAGGGACCATG-3′
linker−: 5′Phos-GTCCCTTAAGCGGAG-3′NH2
Digested genomic DNA (400 ng) and 6 µl linker-oligonucleotide mix were used for overnight ligation with T4 ligation kit (MRC Holland, Amsterdam, The Netherlands). The template was amplified in a 50 µl primary PCR reaction supplemented with primers New long IR/DR(R) (100 mm), linker primer (100 mm), 200 µm dNTPs, 2 mm MgCl2 and 1 unit of platinum Taq polymerase (Invitrogen). The PCR machine was programmed for touchdown PCR at 94°C for 2′, 25 cycles of 94°C for 15″, 60°C for 30″ (−0.5°C per cycle), 72°C for 1′30. The primary reaction was diluted 1:50 and 2 µl used in a nested PCR under the same exact conditions, except supplemented with 0.25 µm of each primer IR/DR(R) KJC1 (0.25 µm) and linker nested primer (0.25 µm). Sequences of the oligonucleotides were:
New long IR/DR(R): 5′-GTTATGCTAGATGGCCAGATCTAGCTTGTGG AAGG-3′
linker primer: 5′-GTAATACGACTCACTATAGGGC-3′
IR/DR(R) KJC1: 5′-CCACTGGGAATGTGATGAAAGAAATAAAAGC-3′
linker nested primer: 5′-AGGGCTCCGCTTAAGG GAC-3′
Secondary PCR products were separated by electrophoresis and cleaned by Invisorb spin DNA extraction kit (Invitek, Hayward, CA, USA) and eluted in 25 µl water. Three microliter was ligated in pGEM-T easy cloning kit (Promega, Fitchburg, WI, USA), transformed into DH5α Escherichia coli bacteria, plated on LB-AMP-X-gal plates and incubated overnight at 37°C. Colonies were inoculated and cells were grown overnight at 37°C. Plasmid were isolated and sequenced with BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA) and T7 and SP6 oligonucleotides. Sequence of the oligonucleotides was:
T7 primer: 5′-GTAATACGA CTCACTATAGGGC-3′
SP6 primer: 5′-ATTTAGGTGACACTATAG-3′
New mutants, where the gene trap had landed in an active gene, were bred to lose the transposase and the transposon donor site, which was confirmed by genomic PCR using oligonucleotides Specc1 F and Specc1 R or Specc1 R and IR/DR(R) KJC1, DMSO (10%), BSA (1×), dNTPs (400 µm) and 1 unit Taq DNA polymerase (New England Biolabs), resulting in respectively a wild-type amplified DNA product of 522 bp, and a mutant product of 632 bp. PCR cycle: 95°C for 5′, 35 cycles of 95°C for 30″, 55°C for 30″ 65°C for 1′ and 65°C for 5′. The sequences of the oligonucleotides were:
Specc1 F: 5′- CTGGGTAGCAGAATGTACGTCC-3′
Specc1 R: 5′- GCCTAGTAATGCCCTGATCTTC-3′
IR/DR(R) KJC1: 5′-CCACTGGGAATGTGATGAAAGAAATAAAAGC-3′
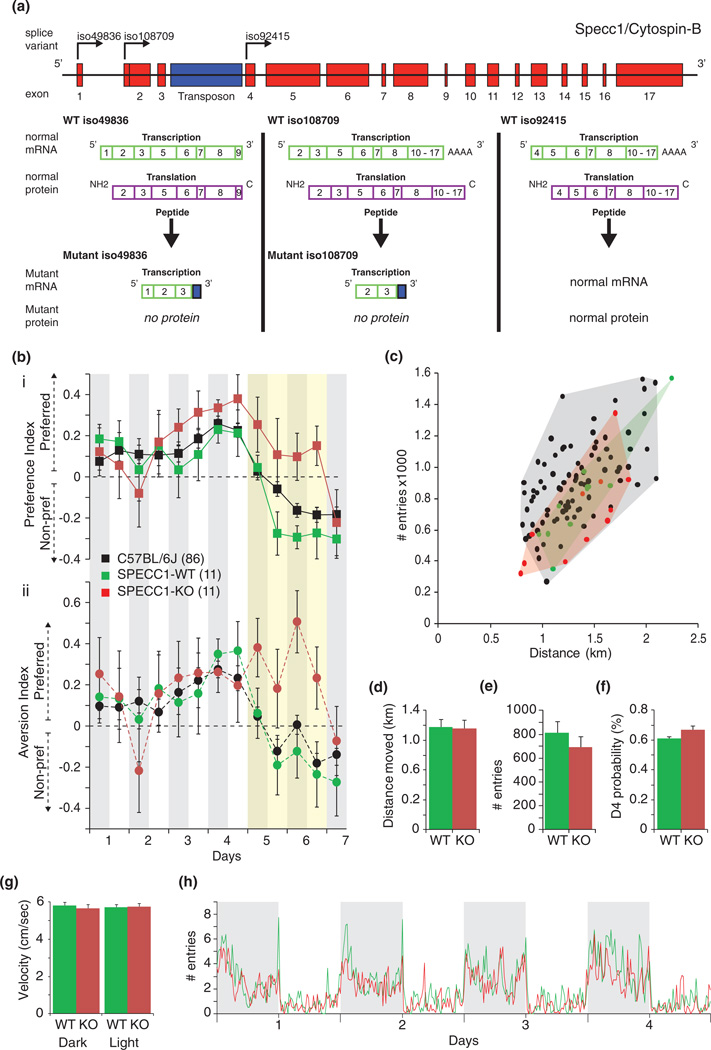
Specc1 male mutant mice, bearing a gene trap cassette in the third intron of the Specc1 gene deleting expression of two splice variants of the Specc1 gene, as outlined in Fig. 4a. Mice were analyzed in the PhenoTyper at the age of 8 weeks and compared to their wild-type littermates.
Figure 4. Comparison of SPECC1 –deficient (KO) and wild-type (WT) controls.
(a) SPECC1 gene-trap construction and different splice variants. (b/i) Preference index for every 12 h. (b/ii) Aversion index. (c) Relation between the distance moved and the frequency of entrance use. (d) Total distance moved in the first 4 days (km). (e) Total number of entries in the first 4 days. (f) Preferred entrance probability on day 4. (g) Mean velocity in the dark and light phases (cm/second). (h) Circadian rhythm of the total entries in 15 min bins.
Instrumented home cage
Activity in the home cage was automatically recorded by video-tracking in specially designed cages (PhenoTyper model 3000, Noldus Information Technology, www.noldus.com/phenotyper). Each cage contains a top unit with built-in hardware for video-tracking, that is, an infrared-sensitive video camera. The latter provide constant and even illumination of the cage. An infrared filter placed in front of the camera prevents interference with room illumination. This method allows continuous behavioral recordings in both dark and light periods. EthoVision was used as video tracking and trial control software. For the purpose of the high-throughput screen, Noldus Information Technology developed a special version of the software (EthoVision HTP 2.1.2.0) based on EthoVision XT 4.1 (Noldus et al. 2001). Forty eight PhenoTyper cages were connected in a specially designed computer network. Four cages were connected to a PC running video tracking and trial control software. The 12 data collection PCs were connected to a central PC (running software for experiment design and progress monitoring) and a data storage server (for storage and analysis of track files). The cages (L = 30 × W = 30 × H = 35 cm) were made of transparent Perspex walls with an opaque Perspex floor covered with bedding based on cellulose. A feeding station and a water bottle were attached on to two adjacent walls outside of the cage. A shelter (height: 10 cm, diameter: 9 cm; non-transparent material) was fixed in one of the corners. Two bright white LED, producing no heat, were mounted on two cage walls, shining inward through the Perspex wall into the shelter from two angles to provide the aversive light stimulus. In EthoVision, the shelter was defined as a ‘hidden zone’; the program distinguished the parameters ‘in shelter’ and ‘on shelter’. Video-tracking was performed at a rate of 15 samples/second. The following parameters were used for analysis: frequency of entries in each entrance of the shelter, the distance moved, and the time spent in the shelter, in total for the 6.5 days (for more information on the algorithms used by the program see the Ethovision XT 4.1 manual). During the first 4 days, the mouse could enter freely in the shelter. On day 4, the preferred entrance was defined by the system as the most used entrance. On days 5 and 6, each time the mouse used its preferred entrance, bright light (500 l×) was automatically switched on in the shelter as long as the mouse stayed inside. As soon as it left the shelter through either entrance the light was turned off. The light did not turn on when the mouse entered through the non-preferred entrance. The shelter light flashed once whenever the mouse moved into a 3 cm area around the preferred entrance of the shelter area on days 5 and 6 to signal the aversive learning trial.
Data-analysis
Raw track files from the PhenoTyper were processed using AHCODA analysis software (Synaptologics BV, Amsterdam, The Netherlands; www.synaptologics.com). Time bin of the shelter visit detection was selected to be 5 min unless stated otherwise. MatLab© was used to create a database to group all the data needed for the analysis.
Statistical analysis
Before any analysis was performed, data were examined for outliers (>3 times the SD from the strain mean) (see Table S12 for details). A small number of mice were define as outside sleeper because they were sleeping outside more than 25% of their time outside and less than 50% in their shelter (n = 22; 4 A/J; 3 BALB; 5 C57; 7 FVB and 3 NOD). Mice with no preference on day 4 (exactly 50% for each entrance) were exclude for the analysis. The difference between strains in the total number of entrances across the first 4 days was determined using generalized estimating equations (GEE) (Liang & Zeger 1986). The binomial test was used to determine preference on day 4, testing whether the probability to take a specific entrance was significantly higher than 50% (FDR < 0.014). The differences between the genotypes in the probability to use the preferred entrance on day 4 were tested using tests of equal proportions.
The preference index was determined as follows: [(number of entries through the preferred entrance) – (number of entries through non-preferred entrance)]/(total number of entries). The aversion index was defined as follows: [(time spent in the illuminated shelter after entering through the sanctioned entrance) – (time spent in the dark shelter after entering through the non-sanctioned entrance)]/(total time spent in shelter).
Comparisons between groups were made for multiple variables using a test of equal proportion. For a given level of analysis, statistical analysis was based on estimated FDR (Verhoeven et al. 2005), P-values were corrected by the minimum positive FDR with a threshold set at 5%. Kruskal–Wallis and Mann–Whitney U test were used for non-continuous data, like the onset and variability. GEE were used to model the frequency of the use of the entrance in the shelter, to model the change in preference resulting from the manipulation and the difference between strains in this change. Repeated measures ANOVA was used to discriminate the change of time spent in the shelter using one or the other entrance. All statistical analyses were performed in PASW Statistics© 17.0.2 and R© version 2.11.0.
Results
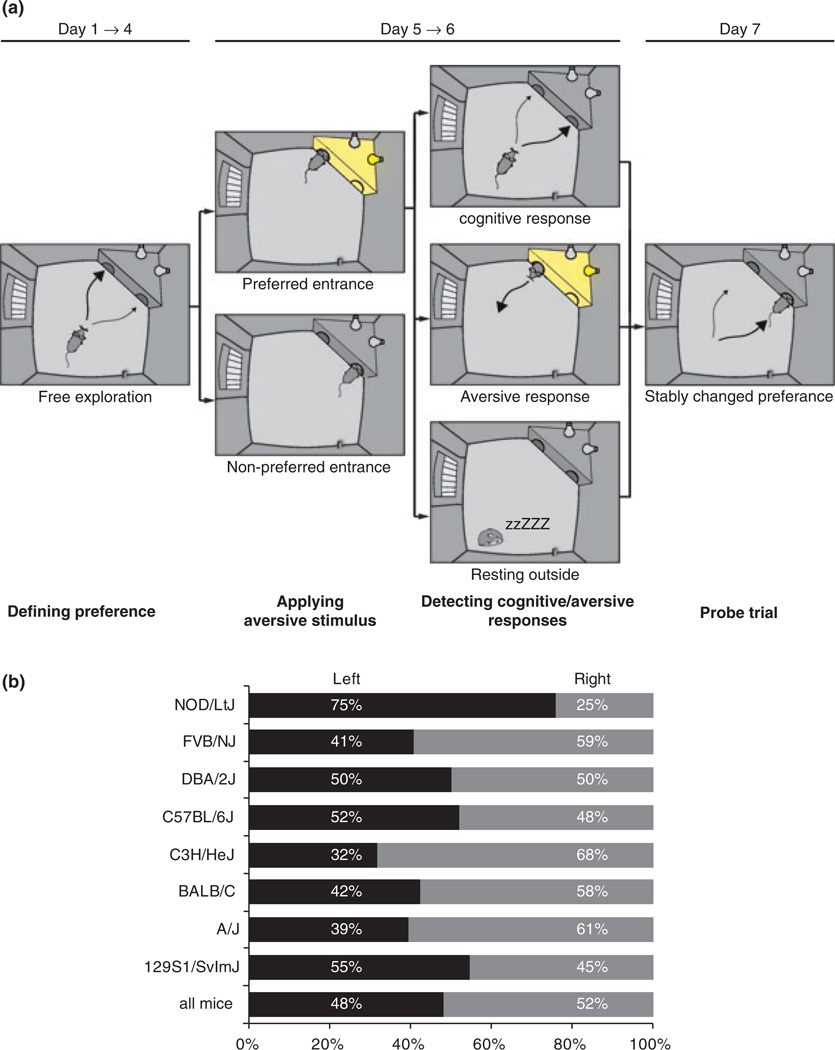
To analyze avoidance learning, mice were tracked in instrumented home cages (PhenoTyper) that contained a shelter with two entrances. Mice were left in these cages for 7 days without human interference and normal 12-h dark-light cycle. During the first 4 days, the animals developed a natural preference for one of the two shelter entrances. The PhenoTyper software automatically detected the preferred entrance on day 4 (Fig. 1a). During days 5 and 6, the use of this preferred entrance was sanctioned during each entry by a bright light that illuminated the shelter and remained on until the animal left the shelter again. The use of the other entrance was not sanctioned. On the last half day (dark phase of day 7), the aversive stimulus was no longer applied (probe trial, Fig. 1a). Our test set up is described in Fig. S1.
Figure 1. Protocol and data analysis.
(a) The protocol is divided in two phases. First the habituation phase when the mouse can freely explore the cage and the computer detects the preferred entrance. Second, the avoidance learning phase when the mouse experiences the aversive stimulus whenever it uses its preferred entrance. This potentially results in a modification of the behavior, typically, a decrease in the frequency of entering the shelter through the preferred entrance and in the time spent in the illuminated shelter. (b) Percentage of mice having a preference for the left or the right entrance. All strains show equal proportion to prefer the right or the left entrance except C3H and NOD that have a preference for the right (68%) and the left (75%) entrance, respectively.
We first studied basic sheltering behavior and development of a preferred entrance, and subsequently tested response strategies to an aversive stimulus in eight common inbred strains (129S1/SvImJ, n = 53; A/J, n = 33; BALB/C, n = 33; C3H/HeJ, n = 22; C57BL/6J, n = 87; DBA/2J, n = 36; FVB/N, n = 27; NOD/LtJ, n = 29). These strains together cover ˜75% of the natural genetic variation in mice (Roberts et al. 2007). Across the entire cohort, the two entrances were equally often preferred (52% vs. 48%; Fig. 1b), ruling out a general entrance bias due to the layout of the arena. Subsequently, we screened collections of gene-trap mice using the Sleeping Beauty transposon/transposase system (Geurts et al. 2006), and null mutant strains, in total 42 strains, typically n = 12 homozygous male mutants (in case of severe mutations: heterozygous) vs. 12 wild-type littermates (see Table S1 for genotypes and group sizes).
Genotypic differences in shelter visit pattern and preference
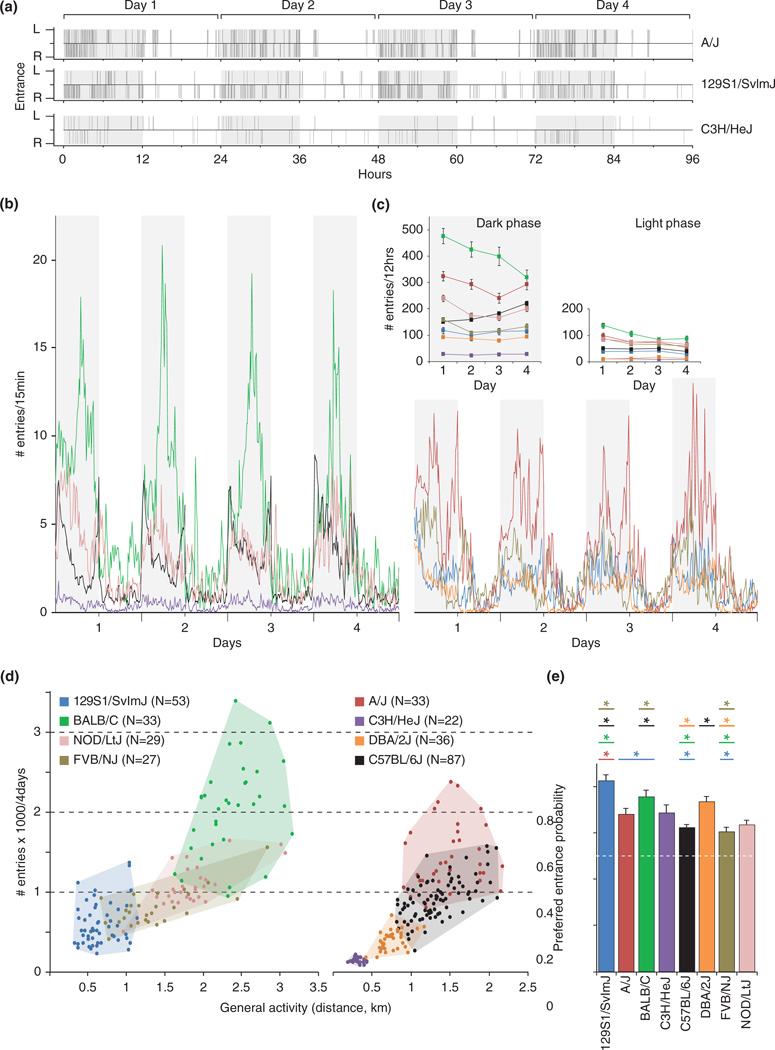
During the first 4 days without interference, mice developed a characteristic pattern of shelter visits, with frequent entries, often with short durations, during the dark phase, and fewer entries during the light phase (Fig. 2a). Among the inbred lines, substantial differences were observed in the number of visits (GEE, χ2 = 4024, df = 8, P < 0.001; Fig. 2a,b; see also Tables S2 and S3). The number of entries averaged over the total light and dark phases (12 h each, Fig. 2c) confirms the differences between the genotypes in visit frequencies (dark phase: GEE, Genotype × day: χ2 = 224, df = 21, P < 0.001; light phase: GEE, Genotype × day: χ2 = 114, df = 21, P < 0.001), and differences between light and dark phase for each genotype (Table S4, Supporting Information). Differences between genotypes were also similar in light and dark phases (Fig. 2c; similar strain ranking). Differences in shelter visit frequency among the genotypes correlated well with differences in general activity (Fig. 2d, Pearson correlation coefficient: 0.79, P < 0.0001, n = 320; see also Table S5).
Figure 2. Spontaneous use of the shelter entries.
(a) Entries through either the left (L) or right (R) entrance during the first 4 days for three mice of three different genotypes. (b) Total number of entries for every 15 min for eight inbred strains. (c) Comparison of the total number of entries in dark and light phases for each strain. (d) Relation between the number of entries and the distance moved accumulated on the first 4 days. Cloud borders encompass the extreme individuals of each strain. (e) Average probability (± SEM) of each strain to enter the shelter through the preferred entrance on day 4. The white dashed line represents the 50% chance level to take one entrance over the other. A test of equal proportion was used to compare the mean number of entries between strains (FDR=0.015). Grey backgrounds in the different panels represent dark phases. Data shows mean ± SEM.
After 4 days, 129S1/SvImJ (129S1) mice developed the strongest preference (group average: 83 ± 2% of entries via their preferred entrance), followed by BALB/C (BALB) and DBA/2J (DBA) (76 ± 3% and 73 ± 2%, Fig. 2e). Only 36% of the C3H/HeJ (C3H) mice developed a significant preference (see Table S6), although the group average probability to enter the preferred entrance is rather high (69 ± 3%; Fig. 2e). This is explained by the low number of entries of C3H, and thus, low statistical power (see also Figs S2 and S3). The total time spent outside decreased during the first 3 days in some strains, indicating a habitation effect. The time spent outside the shelter had stabilized by day 4 (Fig. S4).
Genotypic differences in avoidance behavior upon a mild aversive stimulus
Avoidance learning was studied by automatically applying a mild aversive stimulus (shelter illumination with bright light) during days 5 and 6 each time mice entered the shelter using their preferred entrance, but not the other (see Fig. 1). Prior to the analysis of the effect of the aversive stimulus, we discarded two different categories of mice that were not suitable for the experiment. The first category consisted of all mice presenting no shelter preference [exactly 50:50 between the two entries, three mice in total of three different strains; C57BL6/J (C57), DBA and NOD/LtJ (NOD)]. The second category comprised mice that did not visit the non-sanctioned entrance during the first 12 h of day 5 (the beginning of the test phase; Fig. S5 and Table S7). These mice had not experienced that only the preferred entrance was sanctioned and therefore had no incentive to change their preference.
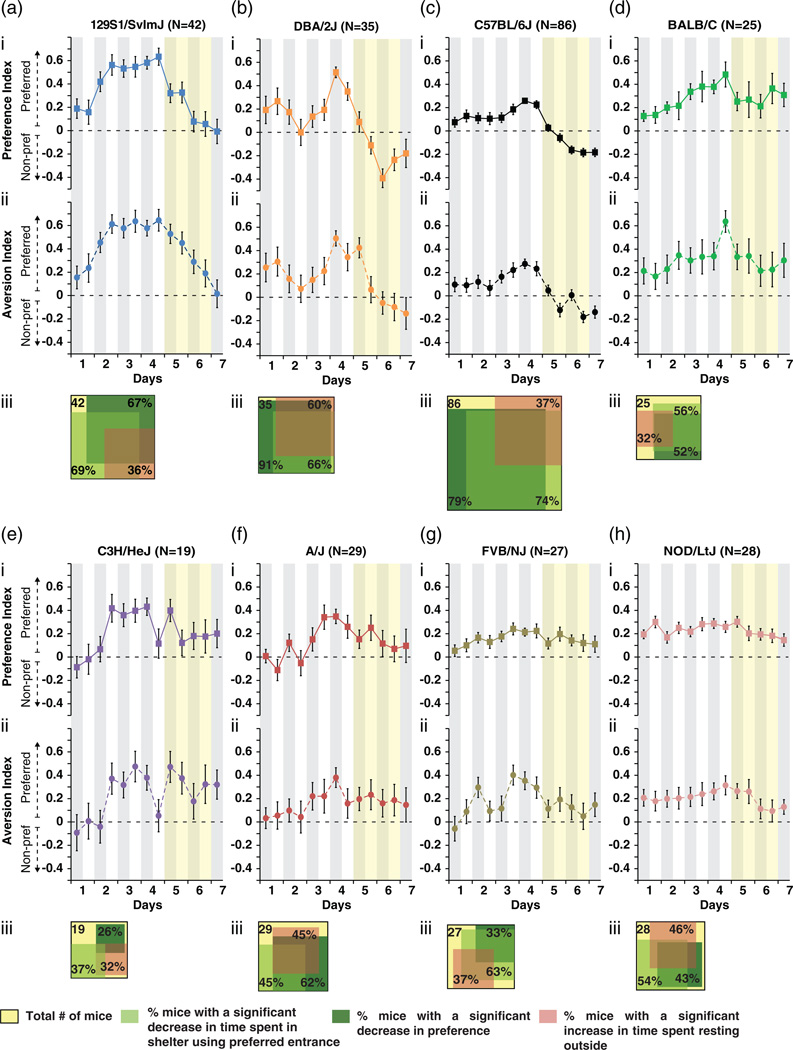
Upon introduction of the aversive stimulus, the number of entries via the preferred entrance decreased for most genotypes (Fig. S3). However, this response is a combination of specific aspects, recognizing and actively avoiding the sanctioned (wrong) entrance, and more general aspects, making fewer entries via either entrance (see Fig. S3) and/or avoiding the shelter altogether (see below). Therefore, we used a more specific measure of the cognitive aspects: the fraction of entries via the preferred (and later sanctioned) entrance over the total number of entries, referred to as the ‘preference index’. This also allows comparisons between light and dark phases (when substantially different absolute numbers of entrances are made, Fig. 2a–c). A decrease of this index indicates that a mouse has established a specific association between its preferred entrance and the aversive stimulus and is a direct consequence of a decision process that occurred before an entry was made. Therefore, we consider changes in the preference index as a specific cognitive response. 129S1, DBA and C57 mice showed the strongest decrease of this index, that is, the strongest cognitive response (Fig. 3a–c/i). The group average for DBA mice went from 0.51 (on day 4) to −0.4 (on day 6) over the 2 days that the preferred entry was sanctioned (Fig. 3a/i). After these 2 days, 91% of DBA and 79% of C57 mice showed significant cognitive responses [false discovery rate (FDR) < 0.01; Fig. 3a,c/iii]. 129S1 also showed a strong cognitive response, but the preference index did not decrease under 0 (Fig. 3b/i). The cognitive response was highly significant for these three genotypes on both days 5 and 6 (as compared to day 4, see Table S8, GEE P < 0.001). The cognitive response of BALB, NOD and A/J mice was slower and only reached significance by day 6 (Fig. 3d,h,f/i; Tables S8 and S9). The other two genotypes (FVB and C3H) showed no significant cognitive response (Fig. 3g,e/i), although several individuals of these strains did (Venn diagrams in Fig. 3g,e/iii). The cognitive response of all genotypes is highly comparable during light and dark phases (Fig. 3/i).
Figure 3. Effect of the aversive stimulus on shelter entrance.
(a–h/i) Preference index for every 12 h, using a GEE model to define the effect of the manipulation. (a–h/ii) The aversion index. (a–h/iii) Venn diagrams show the percentage of mice significantly changing their behavioral response from days 4 to 6, using a test of equal proportion to define significance of increase or decrease (the yellow square represents the whole group; the dark green: the decrease in preference FDR = 0.011; the light green: the decrease of time in shelter using the sanctioned entrance FDR = 0.023; in red the increase of time resting outside FDR = 0.05). The overlap of the squares represents the population that shows two or three of the behaviors between days 4 and 6. The squares are proportional to the size of the group (for more details on the groups see Table S10).
Mice that show weak/no cognitive responses might do so because they do not experience shelter illumination as aversive. To address this possibility, we measured the time a mouse spent in the shelter after using the sanctioned/wrong entrance (i.e. the time spent in the now illuminated shelter). A change in the time spent in the now illuminated shelter is a measure for how aversive the light stimulus is. This might be different for individual mice and is driven by multiple individual factors such as anxiety, visual sensitivity and bright light aversion. To visualize this measure, we calculated the aversion index (see methods). A decrease in this parameter (‘aversion index’) indicates that the mouse experiences shelter illumination as aversive. Indeed, the aversion index was significantly decreased for all genotypes, except for C3H and FVB, two visually impaired strains (Wong & Brown 2006) and for A/J (Fig. 3e,g,f/ii; see Table S10). This indicates that most genotypes did experience illumination as aversive, but that for three strains, on average, shelter illumination is probably not sufficiently aversive to evoke avoidance responses, although several individuals of these strains showed an aversion to the light (Venn diagrams in Fig. 3e,g,f/iii). During the 2 days that the aversive stimulus was applied, mice might also have habituated to illumination, limiting changes in the aversion index and explaining why this index levels off between days 5 and 7 (Fig. 3/ii).
To reveal the full potential of this avoidance test and visualize the complex response of each strain, we plotted these two indexes (12 h bins, dark and light phase) against each other, in one graphic (Fig. S6). This graphic shows the different strategies displayed by the different strain to cope with the aversive stimulus.
In addition to the two principal responses (cognitive and aversive responses), we observed that some mice showed an alternative response, avoiding the shelter altogether and resting (sleeping) outside the shelter. These mice apparently established a general association between the aversive stimulus and the entire shelter, rather than a specific association between the stimulus and one specific entrance, as outlined above. This generalized association was most prominent for DBA mice. On day 6, 60% of these mice showed a significant increase in the time spent resting outside (Fig. 3b/iii). A/J and NOD also showed prominent increases in outside resting (45 and 46%, respectively Fig. 3f,h/iii). For other genotypes, a smaller percentage of individuals started resting outside, but this response was observed for some individuals of all genotypes (Venn diagrams in Fig. 3/iii).
Specc1/cytospinB gene-trap mice show substantially delayed avoidance learning
Finally, we searched for novel genes involved in avoidance learning and tested whether our newly developed automated assay could be used for this. For this, we applied the avoidance learning assay to a collection of gene-trap mice, which were generated using the Sleeping Beauty transposon (Geurts et al. 2006). This approach yields random inactivation of typically single genes, unique for every individual new integrant, without flanking gene complications known to confound behavioral assessment of knockout mice (Wolfer et al. 2002). Out of 43 screened single gene mutants, we identified one integrant that showed a specific defect in avoidance behavior. Integration of the transposon was localized (see experimental procedures) to the third intron of specc1/cytospinB a gene with no functional annotation yet (Fig. 4a). The location of the trap is predicted to prevent expression from two of three transcription initiation sites, including the most widely expressed transcripts (canonical sequence, Fig. 4a). Loss of transcripts was confirmed by qPCR on whole brain mRNA according to the strategy outlined in Fig. 4a. In the brain, the gene was mainly expressed in the hippocampus (Allen brain atlas).
Homozygous specc1/cytospinB mutant mice (n = 11) were viable and showed no morphological abnormalities. The mutants had normal sensory-motor development and responded normally to visual cues. However, they exhibited a remarkable delayed response to the aversive stimulus and changed neither their preference (cognitive response) (see Fig. 4b/i; Tables S8 and S9) nor the time spent in the shelter after entering via the preferred entrance (aversive response) during the 2 days that the aversive stimulus was applied (Fig. 4b/ii). During these 2 days, control littermates (n = 11) showed cognitive- and aversive responses very similar to the responses previously observed for the founder strain (C57, Figs 3c and 4b; GEE and RM anova; see Tables S8 and S9). Only during the last day, day 7, specc1/cytospinB mutant mice showed a cognitive and aversion response which normal mice show 2 days earlier (Fig. 4b).
While avoidance learning was drastically delayed in the specc1/cytospinB mutant mice, other aspects of their behavior were normal. The overall activity pattern (Fig. 4c,d,f), their moving velocity (Fig. 4g), the pattern of entering the shelter, also relative to the day-night cycle (Fig. 4h), the total number of entries on days 1–4, that is, until the introduction of the aversive stimulus (Fig. 4e), as well as the strength of their preference for one of the two entrances (Fig. 4f) were all normal.
Discussion
In this study, we investigated aversive learning in mice using a fully automated assay that relies on mouse-triggered sanctioning of shelter entrance with a mild aversive stimulus. Our data show that the complex adaptive behavioral response of mice can efficiently and successfully be detected, analyzed and visualized even in large cohorts of (mutant) mice. Different genotypes and single gene mutants exhibit marked and quantitative differences in distinct aspects of this behavioral response.
The current data show that the natural preference of mice to reside in a dark/sheltered compartment can be exploited, as part of the natural behavioral repertoire (Kas et al. 2008), to generate high-content behavioral information on cognitive traits, behavioral flexibility and anxiety. In addition, the current data also revealed several striking new features of sheltering behavior, such as marked, genotype-dependent differences in visit frequencies, the existence of sharp peaks in shelter visit activity and genotype-dependent timing of these peaks relative to the light/dark cycle. This circadian variation in sheltering behavior may contribute to inconsistencies in anxiety tests among strains when performed at different circadian time (Brunner et al. 2002; Jones & King 2001).
Our assay produced three principal features that could be firmly established for all genotypes except two visually impaired genotypes, FVB and C3H (Wong & Brown 2006), and for transposon gene trap mice (in C57 background): (1) specific association between one shelter entrance and the aversive stimulus, that remained after conditioning was discontinued on day 7, was observed in most individuals of eight inbred strains and gene trap mice, (2) generalized associations, observed in all genotypes defined by the apparent association between the shelter in general and the aversive stimulus, without discriminating between the two entrances and (3) aversion responses (staying shorter in the shelter when it is illuminated), observed in most mice of all genotypes. As 65% of visually normal mouse strains showed significantly reduced shelter residence time after using the sanctioned (now illuminated) preferred entrance, it can be concluded that the mild aversive stimulus can be used successfully for those strains, and genetic resources derived thereof.
The avoidance response patterns observed in this study are a composite response consisting of aversion to the bright light inside the shelter, cognitive aspects (specific recognition of the sanctioned entrance and avoiding it) and behavioral flexibility aspects (suppression of the tendency to display learned behavior and switching to an alternative). Interestingly, some strains with high aversion response to light in the light–dark box test proved to be poor performers in our paradigm (i.e. A/J) and, conversely, low responders were good performers here [i.e. C57 (de Mooij-van Malsen et al. 2009)]. This indicates that the cognitive response is not merely a read-out of differences in aversion to the light that could be the result of factors such as light-induced anxiety and visual capabilities. Similarly, higher anxiety in DBA than in C57 mice is not a predictor of good fear learning (Baarendse et al. 2008; Stiedl et al. 1999). Instead, it is conceivable that the three genotypes whose cognitive response was most pronounced (i.e. 129S1, DBA and C57) have superior learning performance and/or behavioral adjustability, which make them suitable for future drug screening programs identifying cognition modifying compounds. The similar performance of C57 and DBA mice is of particular interest as DBA mice are generally inferior to C57 in aversively motivated tasks, have been reported to be hippocampus impaired, while under appetitive conditions they perform similar to C57 mice in spatial learning [see (Youn et al. 2012)]. This underscores the importance of avoiding unspecific stressors to assess cognitive function in mice for determining its heritable underpinnings.
The change in preference induced by the aversive light stimulus was still observed at day 7, when the aversive stimulus was no longer applied. This implicates an adaptive change in behavior in response to the light stimulus, which contributes face validity to the cognitive aspect of this task. Panels of inbred mouse strains have been characterized in different assays of learning and memory, such as contextual fear conditioning task (Bolivar et al. 2001; Bothe et al. 2004) and spatial navigation in Morris water maze (Lad et al. 2010) and Barnes maze (O’Leary et al. 2010). Different experimental conditions, anxiety and stressors such as water in the Morris water maze, complicate the comparisons between tests. Not surprisingly, strain rankings vary among these tasks, consistent with the idea that no single task reveals the full richness of learning and memory phenotypes in a wide range of genotypes (Wahlsten et al. 2005). Therefore, the present paradigm is an important new addition to existing paradigms, since it uses a mild aversive stimulus, runs without human interference and derives the cognitive response from the ratio of preferred entries excluding confounding effects of general activity.
To our knowledge, no systematic assessment of behavioral adjustability has been performed in the same panel of inbred strain used here for comparison. Generalized responses, that is, avoiding the shelter altogether and sleeping outside, might be a mechanistically distinct response from the specific association. It can be considered as a more direct response to an aversive situation and might be similar to generalized fear responses with reduced flexibility/discrimination. Interestingly, home cage recording revealed difference in activity level for some strain compare to classical behavioral test. For instance FVB, known to be hyperactive in a novel open field compared to other strains (Millstein & Holmes 2007) showed lesser activity in the PhenoTyper. Locomotor activity is known to be influenced by environmental and emotional states. This relation is not linear as shown in different outcomes of corticotropin-releasing factor on activity depending on the environment (hypoactivity during novelty exposure, but hyperactivity in familiar environment (Britton & Indyk 1990)).
Knocking out the major splice variants of the functionally not annotated gene specc1/cytospinB produced a delay in avoidance learning, whereas many general activity parameters, circadian rhythms and sensory-motor abilities were unaffected. This delay can be caused by a specific impairment in learning the association between the entrance and the light stimulus or could be the result of a difference in the perceived aversiveness of the light stimulus. In either case, this example illustrates the sensitivity of this paradigm to pick up avoidance learning phenotypes using subtle aversive learning stimuli in a home cage environment. The encoded protein contains prominent protein-protein interaction domains, a coiled-coil domain and a calponin-homology domain. Its expression level is relatively low in adult brain but is upregulated in several behavioral paradigms and disease models (GeneNetwork 2012; European Bioinformatics Institute 2012). Calponin-homology domains are also found in spectrin, λ-actinin, many microtubule-associated proteins and several rho/ras GTPase-activating and GDP exchange factors, which implies that Specc1/CytospinB might, like these other proteins, be involved in cytoskeleton remodeling. Interestingly, cytoskeleton remodeling small GTPases have a strong link to cognitive abilities in different mammals including humans (reviewed in (van Galen & Ramakers 2005)). Moreover, of the 13 top SNPs reported by Verweij et al. (2010) for harm avoidance in humans, rs971718 on chr 17 (P-value 3.8×10−5, effect size −.23) is located 6 Mb upstream from SPECC1 (Verweij et al. 2010).
Together, the results described in this manuscript show that subtle learning stimuli in a home cage environment provide sufficient salience to drive avoidance learning. Due to the efficiency achieved with automated home cage screening, we anticipate that learning protocols in home cage settings will gain popularity in large scalemouse phenotyping efforts. Such efforts, especially when combined with drug screening or lesion studies, will further validate the avoidance learning paradigm as a novel cognition test in mice.
Supplementary Material
Acknowledgments
We thank Rolinka van der Loo for operating the PhenoTyper systems and Ruud Wijnands for assistance, Joost Hoetjes and Joke Wortel for genotyping and generating transposon mice, Chris van der Meer, Bert Tersteeg, Marieke Boin and Lieselotte Thoolen for mouse breeding and animal care, Jurjen Broeke and Arthur de Jong for help with MatLab programming and Dr. Douglas Armstrong for excellent suggestions. We also thank Noldus Information Technology for supplying software free of charge and hardware at cost price and Ben Loke, Cecilia Herrera, Willem van der Veer and Raymond de Heer for development of hardware, software and test scripts. This work was supported by Agentschap NL (NeuroBSIK Mouse Phenomics Consortium, BSIK03053), the Netherlands Organization for Scientific Research (Pionier/VICI900-01-001 and ZonMW 903-42-095 to M.V.; VENI-451-08-025 and VIDI-016-065-318 to S.v.d.S), and the European Union Seventh Framework Program under grant agreement no. HEALTH-F2-2009-241498 (EUROSPIN project, to M.V.) and HEALTH-F2-2009-242167 (SynSys project to A.B.S. and M.V.).
Footnotes
The authors declare no conflict of interest. M.L. and B.K. are full time employees of Sylics (Synaptologics BV), a private, VU University spin-off company that offers mouse phenotyping services using AHCODA. A.B.S. and M.V. participate in a holding that owns Sylics shares and have received consulting fees from Sylics.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- Baarendse PJJ, van Grootheest G, Jansen RF, Pieneman AW, Ogren SO, Verhage M, Stiedl O. Differential involvement of the dorsal hippocampus in passive avoidance in C57bl/6J and DBA/2J mice. Hippocampus. 2008;18:11–19. doi: 10.1002/hipo.20356. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Pooler O, Flaherty L. Inbred strain variation in contextual and cued fear conditioning behavior. Mamm Genome. 2001;12:651–656. doi: 10.1007/s003350020039. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 2004;3:149–157. doi: 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- Britton DR, Indyk E. Central effects of corticotropin releasing factor (CRF): evidence for similar interactions with environmental novelty and with caffeine. Psychopharmacology. 1990;101:366–370. doi: 10.1007/BF02244055. [DOI] [PubMed] [Google Scholar]
- Brunner D, Nestler E, Leahy E. In need of high-throughput behavioral systems. Drug Discov Today. 2002;7(Suppl 18):S107–S112. doi: 10.1016/s1359-6446(02)02423-6. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dziegielewski SF. DSM-IV-TR in Action. John Wiley and Sons; Hoboken, New Jersey, USA: 2010. [Google Scholar]
- European Bioinformatics Institute. European Bioinformatics Institute Homepage, EBI. [Date of last access March 3, 2011];EMBL - EBI. 2012 URL http://www.ebi.ac.uk/
- Finkel D, McGue M. Sex differences and nonadditivity in heritability of the Multidimensional Personality Questionnaire Scales. J Pers Soc Psychol. 1997;72:929–938. doi: 10.1037//0022-3514.72.4.929. [DOI] [PubMed] [Google Scholar]
- Gagne JR, Saudino KJ. Wait for it! A twin study of inhibitory control in early childhood. Behav Genet. 2010;40:327–337. doi: 10.1007/s10519-009-9316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Galen EJM, Ramakers GJA. Rho proteins, mental retardation and the neurobiological basis of intelligence. Prog Brain Res. 2005;147:295–317. doi: 10.1016/S0079-6123(04)47022-8. [DOI] [PubMed] [Google Scholar]
- GeneNetwork. [Date of last access March 3, 2011];GeneNetwork Search. 2012 URL http://www.genenetwork.org/
- Geurts AM, Wilber A, Carlson CM, Lobitz PD, Clark KJ, Hackett PB, McIvor RS, Largaespada DA. Conditional gene expression in the mouse using a Sleeping Beauty gene-trap transposon. BMC Biotechnol. 2006;6:30. doi: 10.1186/1472-6750-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH. A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci USA. 2008;105:20575–20582. doi: 10.1073/pnas.0809053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot AS, de Sonneville LM, Stins JF, Boomsma DI. Familial influences on sustained attention and inhibition in preschoolers. J Child Psychol Psychiatry. 2004;45:306–314. doi: 10.1111/j.1469-7610.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- de Heer RC, Schenke M, Kuurman WW, Spruijt BM. Learning (in) the PhenoTyper: an integrative approach to conducting cognitive behavioural challenges in a home cage environment. Conference abstract; Proceedings of Measuring Behavior; 26–29 August; Maastricht, The Netherlands. 2008. p. 57. [Google Scholar]
- Hurst JL, West RS. Taming anxiety in laboratory mice. Nat Methods. 2010;7:825–826. doi: 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- Jhuang H, Garrote E, Mutch J, Yu X, Khilnani V, Poggio T, Steele AD, Serre T. Automated home-cage behavioural phenotyping of mice. Nat Commun. 2010;1 doi: 10.1038/ncomms1064. [DOI] [PubMed] [Google Scholar]
- Jones N, King SM. Influence of circadian phase and test illumination on pre-clinical models of anxiety. Physiol Behav. 2001;72(1–2):99–106. doi: 10.1016/s0031-9384(00)00388-7. [DOI] [PubMed] [Google Scholar]
- Kas MJ, de Mooij-van Malsen AJ, Olivier B, Spruijt BM, van Ree JM. Differential genetic regulation of motor activity and anxiety-related behaviors in mice using an automated home cage task. Behav Neurosci. 2008;122:769–776. doi: 10.1037/0735-7044.122.4.769. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lad HV, Liu L, Paya-Cano JL, Parsons MJ, Kember R, Fernandes C, Schalkwyk LC. Behavioural battery testing: evaluation and behavioural outcomes in 8 inbred mouse strains. Physiol Behav. 2010;99:301–316. doi: 10.1016/j.physbeh.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- McGaugh JL. Time-dependent processes inmemory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- de Mooij-van Malsen JG, Yu KL, Veldman H, Oppelaar H, van den Berg LH, Olivier B, Kas MJ. Variations in ventral root axonmorphology and locomotor behavior components across different inbred strains of mice. Neuroscience. 2009;164:1477–1483. doi: 10.1016/j.neuroscience.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, Järvelin MR, Taanila A, Flint J. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noldus LP, Spink AJ, Tegelenbosch RA. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods. 2001;33:398–414. doi: 10.3758/bf03195394. [DOI] [PubMed] [Google Scholar]
- O’Leary TP, Savoie V, Brown RE. Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.08.030. URL http://www.ncbi.nlm.nih.gov/pubmed/20801160. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, de Geus EJ, Hoekstra RA, Bartels M, van Leeuwen M, Verhulst FC, Posthuma D, Boomsma DI. Attention problems, inhibitory control, and intelligence index overlapping genetic factors: a study in 9-, 12-, and 18-year-old twins. Neuropsychology. 2009;23:381–391. doi: 10.1037/a0014915. [DOI] [PubMed] [Google Scholar]
- Roberts A, Pardo-Manuel de Villena F, Wang W, McMillan L, Threadgill DW. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: implications for QTL discovery and systems genetics. Mamm Genome. 2007;18(6–7):473–481. doi: 10.1007/s00335-007-9045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA. Measurement scale does moderate the association between the serotonin transporter gene and trait anxiety: comments on Munafo et al. Mol Psychiatry. 2005;10:892–893. doi: 10.1038/sj.mp.4001708. author reply 895-897. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Radulovic J, Lohmann R, Birkenfeld K, Palve M, Kammermeier J, Sananbenesi F, Spiess J. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav Brain Res. 1999;104(1–2):1–12. doi: 10.1016/s0166-4328(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Stins JF, van Baal GC, Polderman TJ, Verhulst FC, Boomsma DI. Heritability of Stroop and flanker performance in 12-year old children. BMC Neurosci. 2004;5:49. doi: 10.1186/1471-2202-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöke A, Schürhoff F, Mathieu F, Meary A, Ionescu S, Leboyer M. Tests of executive functions in first-degree relatives of schizophrenic patients: a meta-analysis. Psychol Med. 2005;35:771–782. doi: 10.1017/s0033291704003460. [DOI] [PubMed] [Google Scholar]
- Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. Oikos. 2005;108:643–647. [Google Scholar]
- Verweij KJ, Zietsch BP, Medland SE, Gordon SD, Benyamin B, Nyholt DR, McEvoy BP, Sullivan PF, Heath AC, Madden PA, Henders AK, Montgomery GW, Martin NG, Wray NR. A genome-wide association study of Cloninger’s temperament scales: implications for the evolutionary genetics of personality. Biol Psychol. 2010;85:306–317. doi: 10.1016/j.biopsycho.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser L, van den Bos R, Spruijt BM. Automated home cage observations as a tool to measure the effects of wheel running on cage floor locomotion. Behav Brain Res. 2005;160:382–388. doi: 10.1016/j.bbr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- de Visser L, van den Bos R, Kuurman WW, Kas MJ, Spruijt BM. Novel approach to the behavioural characterization of inbred mice: automated home cage observations. Genes Brain Behav. 2006;5:458–466. doi: 10.1111/j.1601-183X.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Voikar V, Colacicco G, Gruber O, Vannoni E, Lipp HP, Wolfer DP. Conditioned response suppression in the IntelliCage: assessment of mouse strain differences and effects of hippocampal and striatal lesions on acquisition and retention of memory. Behav Brain Res. 2010;213:304–312. doi: 10.1016/j.bbr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Cooper SF, Crabbe JC. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J Subst Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- Wray NR, James MR, Gordon SD, Dumenil T, Ryan L, Coventry WL, Statham DJ, Pergadia ML, Madden PA, Heath AC, Montgomery GW, Martin NG. Accurate, large-scale genotyping of 5HTTLPR and flanking single nucleotide polymorphisms in an association study of depression, anxiety, and personality measures. Biol Psychiatry. 2009;66:468–476. doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata S, Takahashi Y, Kijima N, Maekawa H, Ono Y, Ando J. Genetic and environmental etiology of effortful control. Twin Res Hum Genets. 2005;8:300–306. doi: 10.1375/1832427054936790. [DOI] [PubMed] [Google Scholar]
- Youn J, Ellenbroek BA, van Eck I, Roubos S, Verhage M, Stiedl O. Finding the right motivation: genotype-dependent differences in effective reinforcements for spatial learning. Behav Brain Res. 2012;226:397–403. doi: 10.1016/j.bbr.2011.09.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.