Abstract
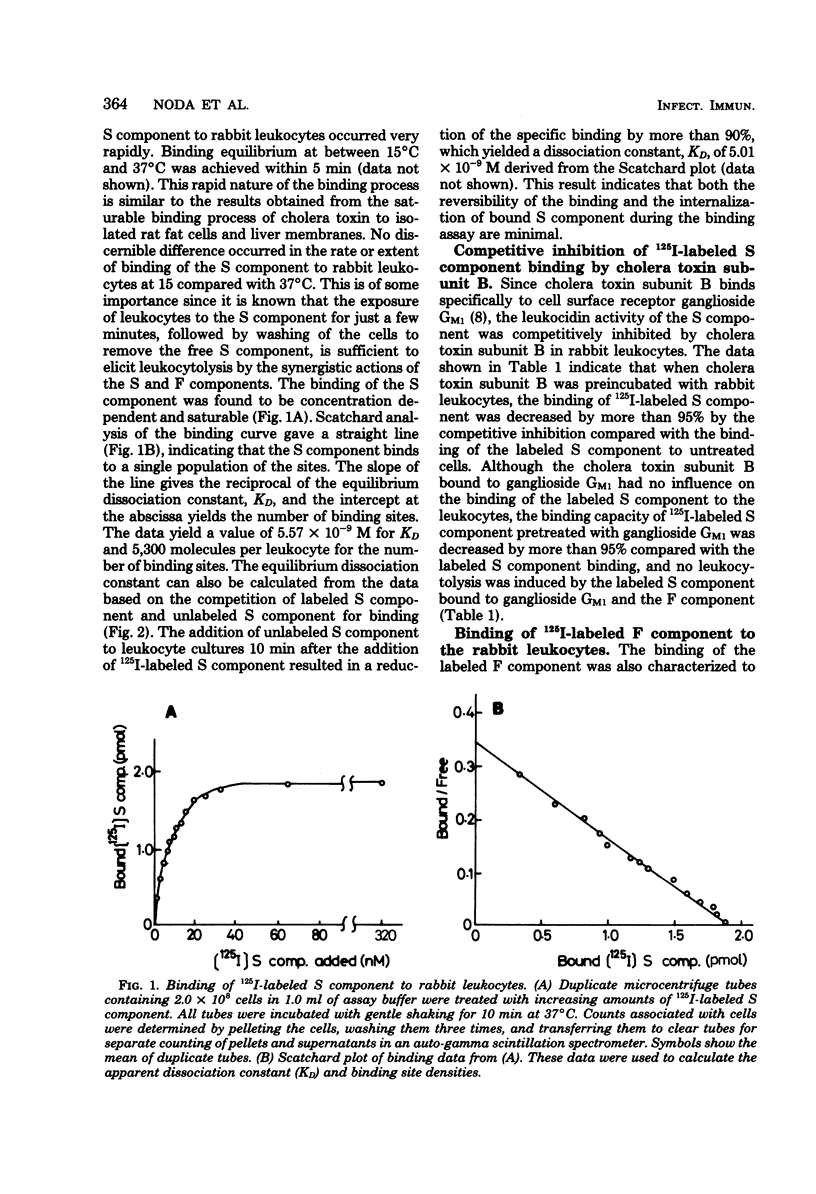
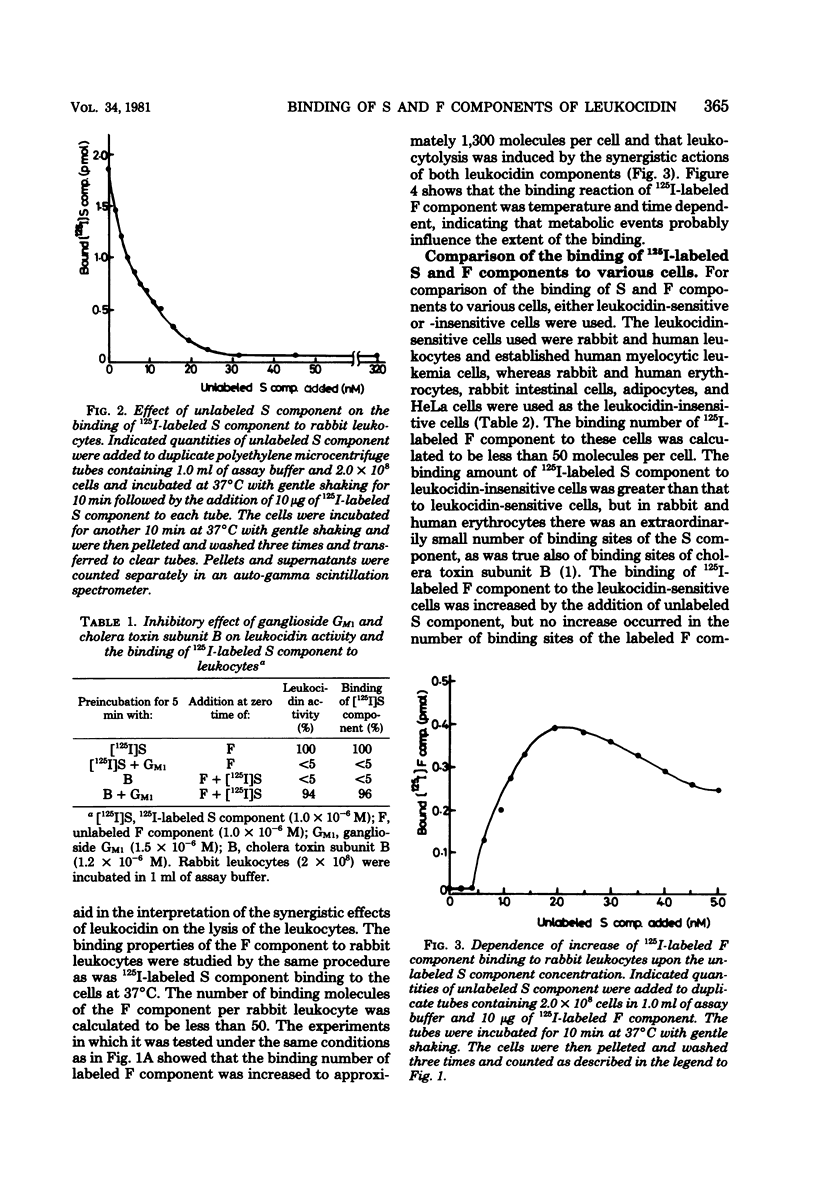
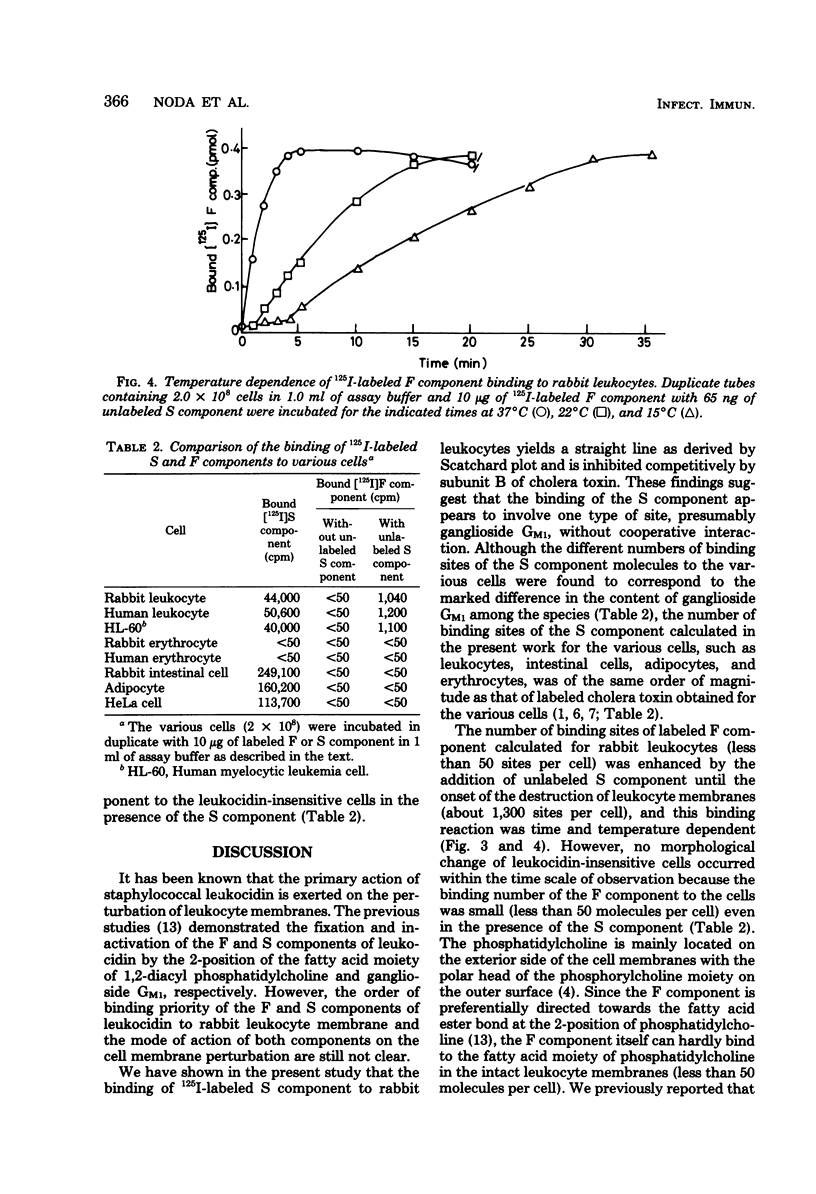
The binding of 125I-labeled S component to rabbit polymorphonuclear leukocytes was found to be concentration dependent and saturable at 37°C. Scatchard analysis of the binding curve gave a straight line, indicating that S component binds to a single population of sites. The dissociation constant, KD, derived from the Scatchard plot was 5.57 × 10−9 M, and the number of binding sites per leukocyte was calculated to be approximately 5,300. Unlabeled S component (10−8 M) or subunit B of cholera toxin (10−7 M) readily competed with 125I-labeled S component binding, and the labeled S component, preincubated with ganglioside GM1 at equimolar proportions for 5 min, lost the binding capacity to the leukocyte membranes. The binding number of 125I-labeled F component to leukocidin-sensitive cells, such as rabbit polymorphonuclear leukocytes and the established human myelocytic leukemia cells, in the absence and in the presence of the unlabeled S component (2.1 nM), was calculated to be 50 and 1,300 molecules per cell, respectively. This increased binding of the labeled F component was time and temperature dependent. The binding number of labeled F component to other cell types comparatively insensitive to leukocidin, such as erythrocytes, adipocytes, intestinal cells, and HeLa cells, was calculated to be less than 50 molecules per cell in spite of the sufficient amount of unlabeled S component bound to their cells. These observations are consistent with the view that in rabbit leukocyte the S component, preferentially bound to the cell surface at 5,300 molecules per cell, contributes to enhance the F component binding up to about 1,300 molecules per cell and may thus play a role of synergistic action of both leukocidin components on the cell membranes in the leukocytolysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., VAN HEYNINGEN W. E. Staphylococcal leucocidins. Br J Exp Pathol. 1957 Apr;38(2):123–137. [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic synthesis and rapid translocation of phosphatidylcholine by two methyltransferases in erythrocyte membranes. Proc Natl Acad Sci U S A. 1978 May;75(5):2348–2352. doi: 10.1073/pnas.75.5.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lindholm L., Lönnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. I. Binding properties and interference with lectin-induced cellular stimulation. J Exp Med. 1974 Apr 1;139(4):801–819. doi: 10.1084/jem.139.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Månsson J., Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO I., PAPPENHEIMER A. M., Jr An early effect of diphtheria toxin on the metabolism of mammalian cells growing in culture. J Exp Med. 1960 Aug 1;112:329–349. doi: 10.1084/jem.112.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Watanuki A. Effect of diphtheria toxin on lysosome activity in leukocytes and Ehrlich ascites tumor cells. Jpn J Exp Med. 1970 Apr;40(2):87–100. [PubMed] [Google Scholar]
- Levine P. H., Weintraub L. R. Preparation of suspensions of small bowel mucosal epithelial cells. J Lab Clin Med. 1970 Jun;75(6):1026–1029. [PubMed] [Google Scholar]
- Noda M., Hirayama T., Kato I., Matsuda F. Crystallization and properties of staphylococcal leukocidin. Biochim Biophys Acta. 1980 Nov 17;633(1):33–44. doi: 10.1016/0304-4165(80)90035-5. [DOI] [PubMed] [Google Scholar]
- Noda M., Kato I., Hirayama T., Matsuda F. Fixation and inactivation of staphylococcal leukocidin by phosphatidylcholine and ganglioside GM1 in rabbit polymorphonuclear leukocytes. Infect Immun. 1980 Aug;29(2):678–684. doi: 10.1128/iai.29.2.678-684.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Luberoff D. E. A new method for separating lymphocytes and granulocytes from human peripheral blood using programmed gradient sedimentation in an isokinetic gradient. Immunology. 1973 Jan;24(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Soboll H., Ito A., Schaeg W., Blobel H. Leukozidin von Staphylokokken verschiedener Herkunft. Zentralbl Bakteriol Orig A. 1973 Jul;224(2):184–193. [PubMed] [Google Scholar]
- WOODIN A. M. Fractionation of a leucocidin from Staphylococcus aureus. Biochem J. 1959 Oct;73:225–237. doi: 10.1042/bj0730225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODIN A. M. Purification of the two components of leucocidin from Staphylococcus aureus. Biochem J. 1960 Apr;75:158–165. doi: 10.1042/bj0750158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Kato I. Purification and some properties of staphylococcal alpha-toxin. Jpn J Exp Med. 1974 Apr;44(2):165–178. [PubMed] [Google Scholar]
- Wenk K., Blobel H. Untersuchungen an "Leukozidinen" von Staphylokokken verschiedener Herkunft. Zentralbl Bakteriol Orig. 1970 May;213(4):479–487. [PubMed] [Google Scholar]


