Abstract
Background and methods
We compared surgical procedures and outcomes, including duration of recovery period, in eyes with proliferative diabetic retinopathy that underwent 25-gauge microincision vitrectomy surgery with those that underwent 20-gauge vitrectomy. Seventy-two eyes from 53 patients that underwent 20-gauge vitrectomy in 2006 and 87 eyes from 55 patients that underwent 25-gauge vitrectomy in 2010 were studied. The surgical procedures, ie, number of vitreous procedures, operating time, and ratio of type of intraocular tamponade were compared between the two groups. In addition, the outcomes, ie, postoperative complications, anatomical success, postoperative best-corrected visual acuity (BCVA), and duration of hospitalization as an indicator of the postoperative recovery period were also compared.
Results
There were no significant differences in surgical procedures or ratio of cases with postoperative complications between cases with 20-gauge and 25-gauge vitrectomy. The final anatomical success rate was 100% in the two groups. BCVA at 6 months after the final vitrectomy was significantly better than the preoperative BCVA for both types of vitrectomy (P < 0.05), and was not significantly different between the two groups. The average duration of hospitalization was 19.5 days after 20-gauge vitrectomy, which was significantly longer than the 11.0 days after 25-gauge vitrectomy (P < 0.001).
Conclusion
These results indicate that the anatomical and functional improvements after 25-gauge microincision vitrectomy surgery are not significantly different from those after 20-gauge vitrectomy in eyes with proliferative diabetic retinopathy. However, the significantly shorter recovery period after 25-gauge microincision vitrectomy surgery suggests that it is less traumatic than 20-gauge vitrectomy.
Keywords: vitrectomy, 25-gauge vitrectomy, microincision vitrectomy surgery, diabetic retinopathy, postoperative recovery
Introduction
Proliferative diabetic retinopathy (PDR) is an ocular disorder characterized by retinal ischemia and recurrent retinal neovascularization. Panretinal laser photocoagulation is the primary treatment for PDR,1 and vitrectomy is performed for more complicated cases, eg, those with vitreous hemorrhage and tractional retinal detachment.2
Vitrectomy was performed exclusively with 20-gauge (Φ 0.9 mm) instruments from the 1970’s, but vitrectomy with 25-gauge instruments (Φ 0.5 mm), ie, microincision vitrectomy surgery (MIVS), has been done since its introduction by Fujii et al in 2002.3 Although the indications for 25-gauge MIVS were initially limited to less surgically complex vitreoretinal pathology, eg, macular hole, preretinal membrane, and simple vitreous hemorrhage,4,5 recent improvements in technology and instrumentation have expanded the use of 25-gauge vitrectomy to include more complex disorders, such as rhegmatogenous retinal detachments6–9 and PDR.10–12
Twenty-five gauge MIVS has several advantages over conventional 20-gauge vitrectomy.13 For example, 25-gauge MIVS is performed with smaller incisions and without the need for peritomy. Thus, surgery is less traumatic, with less postoperative inflammation,14 lower induced astigmatism,5 and reduced patient discomfort. These advantages of 25-gauge vitrectomy appear to facilitate faster recovery in PDR patients. However, data are not available on duration of the postoperative recovery period in PDR cases undergoing 25-gauge MIVS.
The purpose of this study was to compare the effect of 25-gauge vitrectomy with that of 20-gauge vitrectomy on surgical procedures undertaken and their outcomes, including duration of the postoperative recovery period in PDR cases.
Materials and methods
The procedures used in this study conformed to the tenets of the Declaration of Helsinki and were approved by the institutional review board of Osaka Rosai Hospital. All patients received an explanation of the procedures to be used and possible consequences, and all signed an informed consent form.
Patients
Twenty-five gauge MIVS was first used at the Osaka Rosai Hospital, Osaka, Japan, in 2007 for eyes with PDR, and 20-gauge PDR vitrectomy has been completely replaced by 25-gauge MIVS since 2009. PDR cases that underwent primary vitrectomy in 2006 were reviewed for the surgical procedures used and outcomes of 20-gauge vitrectomy. To minimize the effect of a learning curve for 25-gauge MIVS on the surgical results, PDR cases that had primary vitrectomy in 2010, one year after the complete replacement of 20-gauge by 25-gauge vitrectomy, were selected for this study. All cases underwent vitrectomy by two experienced surgeons (KE, TI) to minimize any potential differences in surgical results between surgeons.
The indications for vitrectomy were PDR with nonclearing vitreous hemorrhage, active fibrovascular proliferation, and tractional retinal detachment involving or threatening the macula. Eyes that had undergone intraocular surgery and intravitreal bevacizumab within 6 months of the vitrectomy, and eyes that had less than 6 months of follow-up were excluded.
Surgical procedures, ie, number of vitreous procedures, operating time, and type of intraocular tamponade, were compared between the cases that underwent 20-gauge and 25-gauge vitrectomy. In addition, the surgical outcomes, ie, anatomical success, postoperative complications, postoperative best-corrected visual acuity (BCVA), and length of hospitalization, were also compared. The operating time was the sum of the durations of all surgical procedures in cases with multiple vitrectomies. Eyes with vitreous cavities that were still filled with silicone oil at the last examination were considered to be anatomical failures.
The length of hospitalization after vitrectomy was selected as an indicator of the postoperative recovery period. At our hospital, criteria for hospital discharge were no signs of infection for 5 consecutive days after the vitrectomy,13,16 no postoperative complications such as retinal detachment and nonclearing vitreous hemorrhage, and no patient discomfort. After discharge from hospital, the patients were allowed to return to normal life but were reminded to use topical medications and to take care not to acquire infections.
Vitrectomy
Standard three-port vitrectomy was performed by the two surgeons (KE, TI) using the 20-gauge or 25-gauge vitrectomy system under local anesthesia. The Accurus® (Alcon Laboratories Inc, Fort Worth, TX) surgical vitrectomy system was used. Phacoemulsification and intraocular lens implantation was performed on all phakic eyes through a 2.5 mm sclerocorneal incision in cases with 20-gauge vitrectomy or through a 2.5 mm clear corneal incision in 25-gauge vitrectomy cases.
For 20-gauge vitrectomy, sclerotomies were performed vertically using a vitreoretinal blade after conjunctival peritomy, and the surgical instruments were inserted into the vitreous cavity through the sclerotomy sites. For 25-gauge MIVS, a trocar was inserted obliquely17 using a one-step entry method through the conjunctiva and sclera, and the transscleral cannula was left in place. Surgical instruments were inserted into the vitreous through the cannula.
Core vitrectomy was performed with a high-speed (2500 cycles per minute) vitreous cutter. In cases with 20-gauge vitrectomy, fibrovascular membranes were usually removed using a bimanual technique with membrane scissors and forceps. In the 25-gauge vitrectomy cases, the membranes were initially cut and removed using only the cutter, and if needed, a bimanual technique was performed using membrane forceps and a cutter with a chandelier illumination fiber. Intraoperative bleeding was controlled by temporarily raising the intraocular pressure or by endocautery, or both. Peripheral vitrectomy and vitreous base shaving were performed with scleral indentation using a halogen light source during 20-gauge vitrectomy or a xenon chandelier illumination fiber during 25-gauge vitrectomy. Panretinal laser photocoagulation was performed up to the peripheral retina. The intraocular pressure was decreased to identify and treat any bleeding. All retinal breaks were photocoagulated. Fluid-gas exchange was performed for eyes with retinal breaks and rhegmatogenous retinal detachment, and vitreous tamponade was performed with gas or silicone oil. When we judged that the patient would have difficulty in maintaining a prone position or the eye would require longer-lasting tamponade agents, silicone oil was injected into the vitreous cavity.
On completion of surgery, the conjunctiva and sclerotomy sites were sutured after 20-gauge vitrectomy. After 25-gauge vitrectomy, no transconjunctival sutures were used, except when wound leakage from the sclerotomy was present.
During hospitalization, the eye was patched and received topical levofloxacin (Cravit®, Santen Pharmaceutical Co, Ltd, Osaka, Japan), anti-inflammatory diclofenac sodium (Diclod®, Wakamoto Co, Ltd, Tokyo, Japan), and betamethasone sodium phosphate (Rinderon®, Shionogi Co, Ltd, Osaka, Japan) three times daily.
Statistical analyses
Statistical analyses were performed using the SPSS program (Sigma Plot 12, Systat Software Inc, San Jose, CA). Data are presented as the mean and standard deviation. The BCVA was measured in decimal units and converted to the logarithm of the minimum angle resolution (logMAR) units for the statistical analyses. From earlier reports,18,19 counting fingers, hand motion, light perception, and no light perception vision were set to 1.85, 2.30, 2.80, and 2.90 logMAR units, respectively. Because the data were not normally and equally distributed, significant differences between the cases with 20-gauge and 25-gauge vitrectomy were tested using the Mann–Whitney rank sum test. Friedman repeated-measures analysis of variance on ranks was performed to compare perioperative BCVA within subjects, followed by Dunn’s method to detect significant differences between each postoperative time point and previtrectomy BCVA. Significance differences in ratio between the cases with 20-gauge and 25-gauge vitrectomy were determined by Chi-square tests. A P value <0.05 was considered to be statistically significant.
Results
The percentage of PDR eyes that underwent 25-gauge MIVS by the two surgeons was 0% (0 of 80 eyes) in 2006, 66% (97 of 146 eyes) in 2007, 96% (128 of 134 eyes) in 2008, 100% (111 of 111 eyes) in 2009, and 100% (96 of 96 eyes) in 2010. According to the study criteria, 72 eyes from 53 patients who underwent 20-gauge vitrectomy in 2006 and 87 eyes from 55 patients who underwent 25-gauge MIVS in 2010 were analyzed.
Patient demographics and preoperative ocular status are shown in Table 1. The gender distribution, age, and major systemic data between the cases with 20-gauge and 25-gauge vitrectomy were not significantly different. Preoperative BCVA, intraocular pressure, ratio of phakic/pseudophakic eyes, ratio of eyes with preoperative photocoagulation, ratio of eyes with preoperative neovascular glaucoma, and indications for vitrectomy were not significantly different between the two groups.
Table 1.
Patients’ demographics and preoperative ocular status
| 20-gauge | 25-gauge | P value | |
|---|---|---|---|
| Eyes/patients (n) | 72/53 | 87/55 | |
| male:female | 34:19 | 36:19 | 0.952* |
| Age (yrs) | |||
| Mean ± SDs | 57.6 ± 11.8 | 58.7 ± 11.0 | 0.432** |
| Range | 26–82 | 33–78 | |
| Preoperative BCVA | |||
| LogMAR ± SDs | 0.99 ± 0.70 | 0.99 ± 0.66 | 0.781** |
| Range | LP-0.9 | LP-1.2 | |
| Preoperative intraocular pressure (mmHg) | |||
| Mean ± SDs | 15.4 ± 3.4 | 14.3 ± 3.4 | 0.076** |
| Range | 8–25 | 8–28 | |
| Phakic:pseudophakic eyes | 59:13 | 69:18 | 0.829* |
| Preoperative photocoagulation (n [%]) | 61 [84.7] | 77 [88.5] | 0.641* |
| Preoperative neovascular glaucoma (n [%]) | 6 [8.3] | 2 [2.3] | 0.171* |
| Indications for vitrectomy | 0.108* | ||
| Non-clearing vitreous hemorrhage (n [%]) | 37 [51.4] | 59 [67.8] | |
| Active fibrovascular proliferation (n [%]) | 20 [27.8] | 16 [18.4] | |
| Tractional retinal detachment involving or threatening the macula (n [%]) | 15 [20.8] | 12 [13.8] | |
| Systemic data | |||
| Mean hemoglobin A1c (%) ± SDs | 7.4 ± 1.4 | 7.3 ± 1.6 | 0.269** |
| Mean blood urea nitrogen (mg/dL) ± SDs | 21.8 ± 13.6 | 17.5 ± 7.4 | 0.131** |
| Mean creatine (mg/dL) ± SDs | 1.6 ± 2.5 | 1.1 ± 0.9 | 0.342** |
Notes:*Chi-square or **Mann–Whitney rank sum test was performed to compare between the groups.
Abbreviations: BCVA best-corrected visual acuity; logMAR, logarithm of minimal angle of resolution; SD, standard deviation; LP, light perception.
The surgical procedures and outcomes are summarized in Table 2. Differences in the number of vitreous procedures, operating time, percentage of eyes with different types of intraocular tamponade, and percentage of cases with postoperative complications between 20-gauge and 25-gauge vitrectomy were not statistically significant. Endophthalmitis was not observed in either group. Four of 72 eyes (5.6%) that had 20-gauge vitrectomy and three of 87 eyes (3.5%) that had 25-gauge MIVS required additional vitrectomy for postoperative retinal detachment or nonclearing vitreous hemorrhage. The final anatomical success rate was 100% in both groups.
Table 2.
Surgical procedures and outcomes
| 20-gauge (n = 72) | 25-gauge (n = 87) | P value | |
|---|---|---|---|
| Number of vitreous procedures | |||
| Mean ± SDs | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.524** |
| Range | 1–2 | 1–2 | |
| Operating time† (minutes) | |||
| Mean ± SDs | 55.5 ± 46.8 | 44.9 ± 21.2 | 0.089** |
| Range | 25–390 | 16–96 | |
| Intraocular tamponade | 0.516* | ||
| None (n [%]) | 64 [88.9] | 77 [88.5] | |
| Gas (n [%]) | 7 [9.7] | 10 [11.5] | |
| Silicone oil (n [%]) | 1 [1.4] | 0 [0.0] | |
| Postoperative complications | |||
| Tractional or rhegmatogenous retinal detachment (n [%]) | 2 [2.8] | 1 [1.1] | 0.868* |
| Nonclearing vitreous hemorrhage (n [%]) | 2 [2.8] | 2 [2.3] | 0.751* |
| Neovascular glaucoma (n [%]) | 1 [1.4] | 1 [1.1] | 0.562* |
| Endophthalmitis (n [%]) | 0 [0.0] | 0 [0.0] | |
| Final anatomical success (n [%]) | 72 [100] | 87 [100] | |
| BCVA at time of hospital discharge | |||
| LogMAR ± SDs | 0.68 ± 0.57 | 0.74 ± 0.60 | 0.583** |
| Range | NLP-1.0 | CF-1.0 | |
| BCVA 6 months after final surgery | |||
| LogMAR ± SDs | 0.54 ± 0.63 | 0.51 ± 0.62 | 0.496** |
| Range | NLP-1.2 | NLP-1.2 | |
| Length of hospitalization (days) | 19.5 ± 12.6 | 11.0 ± 4.1 | <0.001** |
Notes:*Chi-square or **Mann–Whitney rank sum test was performed to compare between the groups;
operating time is the sum of all of surgical procedures in cases with multiple vitrectomies.
Abbreviations: BCVA, best-corrected visual acuity; logMAR, logarithm of minimal angle of resolution; SD, standard deviation; NLP, no light perception; CF, counting finger.
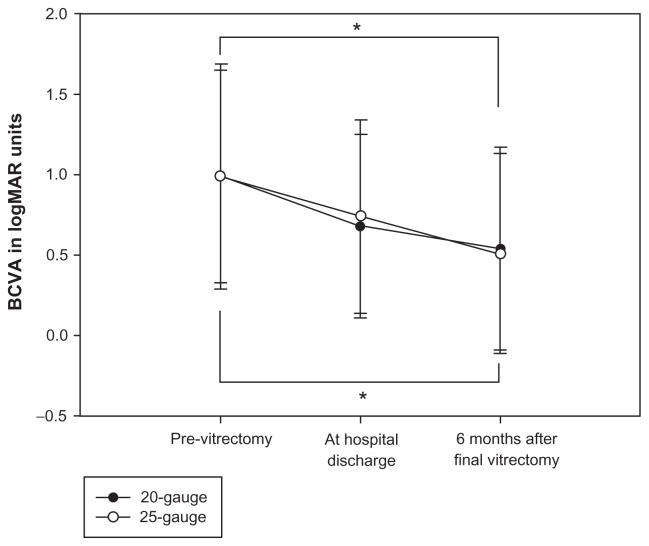
BCVA at 6 months after final vitrectomy was significantly better than preoperative BCVA in both groups (P < 0.05, Figure 1). BCVA at the time of hospital discharge and 6 months after final vitrectomy was not significantly different between the two groups (Table 2). The average length of hospitalization was 19.5 days after 20-gauge vitrectomy and 11.0 days after 25-gauge vitrectomy. The duration of hospital stay was significantly longer after 20-gauge vitrectomy (P < 0.001).
Figure 1.
Perioperative visual acuity.
Notes: The abscissa represents time and the ordinate represents best-corrected visual acuity (BCVA) in logarithm of minimum angle resolution (logMAR) units. Statistical analyses were performed by Friedman repeated-measures analysis of variance on ranks, followed by Dunn’s method to detect significant differences between each time point and previtrectomy (*P < 0.05).
Discussion
Our results show that BCVA at 6 months after final vitrectomy was significantly better than preoperative BCVA after both 20-gauge and 25-gauge vitrectomy. In addition, BCVA at the time of hospital discharge and at 6 months after final vitrectomy was not significantly different between the two groups. However, duration of hospitalization was significantly shorter in cases with 25-gauge vitrectomy than in those with 20-gauge vitrectomy. The surgical procedures, ie, number of vitreous procedures, operating time, and percentages of type of intraocular tamponade were not significantly different between the cases with 20-gauge and 25-gauge vitrectomy.
Twenty-five gauge MIVS differed from 20-gauge vitrectomy in that both conjunctiva peritomy at the beginning and peritomy closure on completion of surgery were not necessary with the 25-gauge MIVS. Another difference was that the surgical instruments used, especially the vitreous cutter, were smaller for 25-gauge vitrectomy than for 20-gauge vitrectomy. In PDR cases with fibrovascular proliferation, the fibrovascular membranes were initially cut and removed bimanually with cutters, scissors, and forceps in both 20-gauge and 25-gauge vitrectomy. The smaller size of the 25-gauge instruments permitted the vitreous cutter to be inserted between the fibrovascular membranes and retina in most cases. This allowed membrane segmentation, dissection, and removal using only the vitreous cutter. Eliminating the need for conjunctival peritomy and exchange of vitreous instruments are considered to shorten the operating time for 25-gauge MIVS, and an earlier study reported that the operating time in cases with 25-gauge vitrectomy was significantly shorter than that of 20-gauge vitrectomy.10 On the other hand, the smaller instruments used in 25-gauge MIVS have lower aspiration volumes20 which could lead to a longer operating time. In a prospective, randomized, clinical trial, Kellner et al21 reported that in cases requiring more intraocular manipulations, the time reduction at surgical opening and closing with 25-gauge MIVS was offset by a longer vitrectomy time and retinal manipulation time. This agrees with our findings.
Differences in the number of postoperative complications and number of vitreous procedures between 20-gauge and 25-gauge vitrectomy were not significant. Bacterial endophthalmitis did not occur in either group.
Twenty-five gauge MIVS was initially introduced as a system for sutureless transconjunctival vitrectomy surgery.3 However, accumulating experience has shown that an increased risk of postoperative endophthalmitis is of considerable concern with 25-gauge MIVS, especially because of postoperative hypotony.13,16 Thus, in our PDR cases that underwent 25-gauge MIVS in 2010, an oblique incision was used to create the transconjunctival sclerotomy,17 and transconjunctival sutures were used when a leaky wound was found.
The final anatomical success rate was 100% in both groups, and BCVA 6 months after final vitrectomy was not significantly different between the two groups. These results suggest that 25-gauge MIVS has an anatomical and functional success rate that is comparable with that achieved by 20-gauge vitrectomy in eyes with PDR.
Length of hospitalization was significantly shorter after 25-gauge vitrectomy than after 20-gauge vitrectomy. BCVA at the time of hospital discharge was not significantly different between cases with 20-gauge and 25-gauge vitrectomy. As already described, 25-gauge MIVS has some advantages for early postoperative recovery over 20-gauge vitrectomy. Because conjunctival peritomy was not necessary, there was less postoperative discomfort reported by the patients. The smaller 25-gauge instruments had lower aspiration volumes, leading to less use of irrigation solution, which should theoretically reduce postoperative inflammation.14 The smaller size of the 25-gauge instruments has also been demonstrated to induce less postoperative astigmatism.15 These characteristics of 25-gauge MIVS were probably why earlier postoperative recovery was documented after 25-gauge vitrectomy than after 20-gauge vitrectomy. In fact, the length of hospitalization in PDR cases undergoing 25-gauge vitrectomy in 2010 was about 8 days shorter than that in PDR cases undergoing 20-gauge vitrectomy in 2006.
There are some limitations to this retrospective study. The patients were not randomized preoperatively, and the study periods were different for cases with 20-gauge and 25-gauge vitrectomy. However, to minimize patient selection bias, PDR cases that underwent 20-gauge vitrectomy in 2006 were compared with cases that underwent 25-gauge vitrectomy in 2010 because all of the vitrectomies in PDR cases were performed using 20-gauge and 25-gauge systems in 2006 and 2010. There were no significant differences in patient demographics or preoperative ocular status between the two groups. In addition, to minimize the effect of a learning curve for 25-gauge MIVS on the surgical results, PDR cases that underwent 25-gauge vitrectomy in 2010 were studied because 2009 was the first year in which the conventional 20-gauge vitrectomy was fully substituted by 25-gauge MIVS.
In conclusion, we compared the surgical procedures used and outcomes of 25-gauge MIVS with those of 20-gauge vitrectomy in eyes with PDR. The results show that the anatomical and functional success after 25-gauge vitrectomy was not significantly different from that after 20-gauge vitrectomy. However, recovery time was significantly shorter after 25-gauge vitrectomy when the length of hospitalization after the vitrectomy was used as an indication of postoperative recovery. This would suggest that 25-gauge MIVS is less traumatic than 20-gauge vitrectomy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383–396. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- 2.Ho T, Smiddy WE, Flynn HW., Jr Vitrectomy in the management of diabetic eye disease. Surv Ophthalmol. 1992;37:190–202. doi: 10.1016/0039-6257(92)90137-i. [DOI] [PubMed] [Google Scholar]
- 3.Fujii GY, De Juan E, Jr, Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109:1807–1812. doi: 10.1016/s0161-6420(02)01179-x. [DOI] [PubMed] [Google Scholar]
- 4.Lakhanpal RR, Humayun MS, de Juan E, Jr, et al. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology. 2005;112:817–824. doi: 10.1016/j.ophtha.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 5.Ibarra MS, Hermel M, Prenner JL, Hassan TS. Longer-term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am J Ophthalmol. 2005;139:831–836. doi: 10.1016/j.ajo.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Lai MM, Ruby AJ, Sarrafizadeh R, et al. Repair of primary rhegmatogenous retinal detachment using 25-gauge transconjunctival sutureless vitrectomy. Retina. 2008;28:729–734. doi: 10.1097/IAE.0b013e318162b01c. [DOI] [PubMed] [Google Scholar]
- 7.Von Fricken MA, Kunjukunju N, Weber C, Ko G. 25-Gauge sutureless vitrectomy versus 20-gauge vitrectomy for the repair of primary rhegmatogenous retinal detachment. Retina. 2009;29:444–450. doi: 10.1097/IAE.0b013e318196b19c. [DOI] [PubMed] [Google Scholar]
- 8.Mura M, Tan SH, De Smet MD. Use of 25-gauge vitrectomy in the management of primary rhegmatogenous retinal detachment. Retina. 2009;29:1299–1304. doi: 10.1097/IAE.0b013e3181aa0f5f. [DOI] [PubMed] [Google Scholar]
- 9.Bourla DH, Bor E, Axer-Siegel R, Mimouni K, Weinberger D. Outcomes and complications of rhegmatogenous retinal detachment repair with selective sutureless 25-gauge pars plana vitrectomy. Am J Ophthalmol. 2010;149:630–634. doi: 10.1016/j.ajo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Yang SJ, Yoon SY, Kim JG, Yoon YH. Transconjunctival sutureless vitrectomy for the treatment of vitreoretinal complications in patients with diabetes mellitus. Ophthalmic Surg Lasers Imaging. 2009;40:461–466. doi: 10.3928/15428877-20090901-04. [DOI] [PubMed] [Google Scholar]
- 11.Farouk MM, Naito T, Sayed KM, et al. Outcomes of 25-gauge vitrectomy for proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:369–376. doi: 10.1007/s00417-010-1506-7. [DOI] [PubMed] [Google Scholar]
- 12.Ozone D, Hirano Y, Ueda J, Yasukawa T, Yoshida M, Ogura Y. Outcomes and complications of 25-gauge transconjunctival sutureless vitrectomy for proliferative diabetic retinopathy. Ophthalmologica. 2011;226:76–80. doi: 10.1159/000328407. [DOI] [PubMed] [Google Scholar]
- 13.Recchia FM, Scott IU, Brown GC, Brown MM, Ho AC, Ip MS. Small-gauge pars plana vitrectomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2010;117:1851–1857. doi: 10.1016/j.ophtha.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Inoue Y, Kadonosono K, Yamakawa T, et al. Surgically-induced inflammation with 20-, 23-, and 25-gauge vitrectomy systems: an experimental study. Retina. 2009;29:477–480. doi: 10.1097/IAE.0b013e31819a6004. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto F, Okamoto C, Sakata N, et al. Changes in corneal topography after 25-gauge transconjunctival sutureless vitrectomy versus after 20-gauge standard vitrectomy. Ophthalmology. 2007;114:2138–2141. doi: 10.1016/j.ophtha.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Oshima Y, Kadonosono K, Yamaji H, et al. Multicenter survey with a systemic overview of acute-onset endophthalmitis after transconjunctival microincision vitrectomy surgery. Am J Ophthalmol. 2010;150:716–725. doi: 10.1016/j.ajo.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Guajardo L, Pareja-Esteban J, Teus-Guezala MA. Oblique sclerotomy technique for prevention of incompetent wound closure in transconjunctival 25-gauge vitrectomy. Am J Ophthalmol. 2006;141:1154–1156. doi: 10.1016/j.ajo.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–1240. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 19.Grover S, Fishman GA, Anderson RJ, et al. Visual acuity impairment in patients with retinitis pigmentosa at ages 45 years or older. Ophthalmology. 1999;106:1780–1785. doi: 10.1016/S0161-6420(99)90342-1. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Kusaka S, Oshima Y, Fujikado T. Analyses of cutting and aspirating properties of vitreous cutters with high-speed camera. Retina. 2008;28:749–754. doi: 10.1097/IAE.0b013e3181631907. [DOI] [PubMed] [Google Scholar]
- 21.Kellner L, Wimpissinger B, Stolba U, Brannath W, Binder S. 25-gauge vs 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br J Ophthalmol. 2007;91:945–948. doi: 10.1136/bjo.2006.106799. [DOI] [PMC free article] [PubMed] [Google Scholar]