Abstract
Purpose.
The herb rosemary has been reported to have antioxidant and anti-inflammatory activity. We have previously shown that carnosic acid (CA), present in rosemary extract, crosses the blood–brain barrier to exert neuroprotective effects by upregulating endogenous antioxidant enzymes via the Nrf2 transcriptional pathway. Here we investigated the antioxidant and neuroprotective activity of CA in retinal cell lines exposed to oxidative stress and in a rat model of light-induced retinal degeneration (LIRD).
Methods.
Retina-derived cell lines ARPE-19 and 661W treated with hydrogen peroxide were used as in vitro models for testing the protective activity of CA. For in vivo testing, dark-adapted rats were given intraperitoneal injections of CA prior to exposure to white light to assess protection of the photoreceptor cells. Retinal damage was assessed by measuring outer nuclear layer thickness and by electroretinogram (ERG).
Results.
In vitro, CA significantly protected retina-derived cell lines (ARPE-19 and 661W) against H2O2-induced toxicity. CA induced antioxidant phase 2 enzymes and reduced formation of hyperoxidized peroxiredoxin (Prx)2. Similarly, we found that CA protected retinas in vivo from LIRD, producing significant improvement in outer nuclear layer thickness and ERG activity.
Conclusions.
These findings suggest that CA may potentially have clinical application to diseases affecting the outer retina, including age-related macular degeneration and retinitis pigmentosa, in which oxidative stress is thought to contribute to disease progression.
Carnosic acid (CA) upregulates antioxidant enzymes via the Nrf2 transcriptional pathway. We show CA treatment protected retinal-derived cell lines against H2O2-induced toxicity, promoted survival of photoreceptor cells and preserved electroretinogram activity in rats subjected to light damage.
Introduction
Diseases of the outer retina, including retinitis pigmentosa (RP) and age-related macular degeneration (AMD), are major causes of irreversible blindness worldwide. An increase in disease incidence is expected over the next several decades due to increased life expectancy. Based on year 2000 US census data, it is estimated that in the year 2020, 0.78% of Americans older than 40 years will be blind and an additional ∼2% will have low vision due to AMD. The primary cause of AMD may be complex and involve a variety of hereditary and environmental factors. Along these lines, light-induced redox stress as well as retinaldehyde condensation with phosphatidylethanolamine (to form the toxic bis-retinoid N-retinylidene-N-retinylethanolamine [A2E]) or other toxic products of drusen-related lipids are considered major contributors to the disease.1–4 Possibly linking these two mechanisms, formation of A2E oxiranes by light stimulation has been reported in the Stargardt mouse model of AMD.5 Over the past 2 decades, several preclinical and clinical reports have suggested that light exposure, leading to oxidative and nitrosative redox stress, represents a risk factor for progression of AMD.6–8 Activation of apoptotic pathways and degeneration of both photoreceptors and retinal pigment epithelium (RPE) cells have been documented in animal models of AMD, using light-induced retinal degeneration (LIRD).9–12 Similar to LIRD, AMD is felt to progress at least at late stages because of inadequate neutralization of oxidants and free radicals, which can be accompanied by an inflammatory component.9,13,14 Therefore, protection strategies that activate endogenous antioxidant proteins in photoreceptors and RPE cells may provide at least one means for attenuating progression of AMD. Indeed, the age-related eye disease study15 showed that daily intake of vitamins E and C, beta-carotene, zinc, and copper provided ∼25% reduction in progression of late AMD in humans. Additionally, progression of genetic diseases of photoreceptors, such as RP, is thought to be affected by light-associated oxidative stress.16 These prior findings provided an impetus for our development of a novel antioxidant strategy.
Herb-derived small molecules, widely used in Asian, Greek, and folkloric European medicine, have received great attention recently for their antioxidant and anti-inflammatory activities.17–19 Two natural compounds, sulforaphane and curcumin, have been reported to protect photoreceptor and RPE cells against light-induced oxidative damage by induction of phase 2 genes via activation of the Nrf2 transcriptional pathway.20–22 A reported mechanism for this effect is reaction with a critical thiol group on the Keap1 protein, which would otherwise sequester Nrf2 in the cytoplasm and hence keep it from transcriptionally activating phase 2 enzymes in the nucleus. However, these electrophilic compounds react rather indiscriminately with other protein thiols, including reduced glutathione (GSH), thereby compromising the survival of healthy cells.23 To avoid this problem, we sought pro-electrophilic compounds that are activated by the very oxidation in redox-stressed cells that is injurious and in which GSH has already been depleted. In this manner, such pro-electrophilic drugs would avoid the serious side effect and toxicity of causing depletion of GSH in normal tissue.19,23 Along these lines, rosemary has long been known as “an herb of remembrance,” having a reputation for strengthening memory with antioxidant and anti-inflammatory properties.24 Our earlier studies in an animal model of cerebral ischemia showed that carnosic acid (CA) is a pro-electrophilic compound found in rosemary25 that readily crosses the blood–brain barrier. We found that CA subsequently undergoes oxidation at the site of redox stress, converting the compound from a pro-drug to its active electrophilic form. This electrophilic substance then upregulates a potent, endogenous antioxidant enzyme system via reaction with the Keap1 protein, resulting in activation of the Nrf2 transcriptional pathway.19 Here, we tested whether CA could protect photoreceptors and RPE cells against cell death induced by oxidation resulting from excessive photostimulation. We found CA activated phase 2 antioxidant genes and protected cells of the outer retina both in vitro and in vivo. Our findings suggest CA is a prospective therapeutic compound for attenuating retinal degeneration in disorders such as AMD and RP, in which oxidative stress plays an important role in disease progression.
Methods
Cell Culture
The human RPE ARPE-19 cell line was purchased from the American Type Culture Collection (Manassas, VA). Mouse photoreceptor-derived 661W cells26 were generously provided by Muayyad Al-Ubaidi (University of Oklahoma Health Sciences Center, Oklahoma City, OK). Cells were routinely maintained in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), 100 μg/mL streptomycin, and 100 units/mL penicillin (Invitrogen). Cells were grown in 5% CO2/balance air and 95% humidity at 37°C.
Cell Treatment and Cell Survival Assay
ARPE-19 and 661W cells were plated at a density of 7.8 × 104/cm2 for 24 hours. In order to test for possible cytoprotective effects from H2O2-induced cell death, cultures were pretreated with CA dissolved in Dimethyl sulfoxide (DMSO) at a final concentration of 10 μM (or an equivalent volume of DMSO as control) for 21 hours in serum-free medium. Oxidative stress was induced by adding H2O2 (1.5 mM for ARPE-19 and 0.5 mM for 661W cells) in a 4-hour incubation (cells were greater than 90% confluent at the time of treatment with oxidant). Cells were then stained sequentially with acridine orange and ethidium bromide for identification of live and dead cells, respectively.27 Under epifluorescence microscopy, cell survival was scored in a masked fashion and expressed as the ratio of live cells to total cells.
Immunocytochemistry
After treatment with CA or vehicle, ARPE-19 cells were fixed with 3% paraformaldehyde in PBS at room temperature for 20 minutes. Cells were then permeabilized with 0.3% Triton X-100 and incubated overnight at 4°C with an antibody against Nrf2 (no. sc-13032; Santa Cruz Biotechnology, Santa Cruz, CA). After cells were washed, they were incubated with FITC-conjugated goat-anti-rabbit IgG (no. sc-2012; Santa Cruz Biotechnology) for 1 hour at room temperature. Nuclei were counterstained with Hoechst (Sigma Aldrich, St. Louis, MO).
Reporter Gene Assay
ARPE-19 cells were seeded at 7.8 × 104/cm2 and transfected using the cell line Nucleofector kit V (Amaxa, Gaithersburg, MD) with wild-type (WT) antioxidant responsive element (ARE)-luciferase or mutant (mt)-ARE-luciferase (pGL3-promoter vector; 2.0 μg per 1 × 106 cells; Promega, Madison, WI) plus pGL3 Renilla luciferase reporter vector (0.01 μg per 1 × 106 cells). At 4 hours after transfection, cells were treated with 10 μM CA or vehicle in serum-free medium for 16 hours. Next, cells were lysed in reporter lysis buffer (Promega), and cell lysates were subjected to luciferase reporter gene assay.
Microarray Gene Expression Analysis and RT-Quantitative PCR
We used microarray analysis to examine the effect of CA on upregulation of phase II genes. ARPE-19 cells were treated with CA or vehicle. Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's protocol. After reverse transcription of total RNA, using Superscript II cDNA synthesis kit (Invitrogen), cDNA was in vitro-transcribed to biotin-labeled cRNA. Fragmented cRNA was hybridized to human genome U133 Plus 2.0 array (Affymetrix, Santa Clara, CA) at 45°C for 16 hours. Affymetrix GeneChip operating software was used for data analysis. To increase the reliability of the data, we only listed genes encountered with very high significance (P < 0.0003).
Upregulation of phase II genes induced by CA was further confirmed by quantitative RT-PCR (RT-qPCR). Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's protocol, and cDNA was synthesized using Superscript II (Invitrogen) using random hexamer primers. cDNA was used in PCR reactions with primers for HO-1, NQO1, GCLM, and xCT. Primers for β-actin, used as a housekeeping gene, were used to normalize gene expression measurements. The primer sequences were as follows: HO-1 (F-5′-GAGTTGCAGCTGCTGAG-3′ and reverse R-5′-GCATGCCTG CATTCACATG-3′), NQO1 (F-5′-CTCCATGTACTCTCTGCAAG-3′ and R-5′-GTGGTGTCTCATGAGTGTGC-3′), GCLM (F-5′-GCCATAGGTACCTCTGATC-3′ and R-5′-CTTGACAGACAACATACTGTC-3′), xCT (F-5′-GCACGATGCATACACAGGTG-3′ and R-5′-GTCCAGATGGTCAGAGACATG-3′), and β-Actin (F-5′-TGACTGACTACC TCATGAAG-3′ and R-5′-TTGCCAATGGTGATGACCTG-3′).
Western Blots
Whole-cell lysates from ARPE-19 and 661W cells were prepared for immunoblotting analysis by sonication of cell pellets in Mammalian Protein Extraction Reagent (M-PER) reagent (Pierce Biotechnology, Rockford, IL) containing a protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany). Samples were centrifuged, and supernatants were assayed for protein concentration using bicinchoninic acid (BCA) reagent (BioRad). Equal aliquots of protein samples were applied to 4% to 12% gradient SDS polyacrylamide gels (Invitrogen) and were separated by electrophoresis. Resolved proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (BioRad) and blocked by incubation in 5% nonfat dry milk in Tris-Buffered Saline-Tween 20 buffer for 1 hour at room temperature. The membrane was incubated with appropriate primary antibody for 16 hours at 4°C and then with appropriate peroxidase-conjugated secondary antibody for 1 h at room temperature. Chemiluminescence signals were detected using Super Signal West Pico chemiluminescent substrate (Pierce) and exposure of the membrane to X-ray film. Loading normalization of samples was carried out by stripping the membrane and reprobing with anti-β-actin (1:5000 dilutin). Primary antibodies against HO-1 (Assay designs, Ann Arbor, MI), NQO1 (Epitomics, Burlinggame, CA), Prx2 (Lab Frontier, Seoul, Korea), Prx-SO2/3H (Abcam, Cambridge, MA), Srxn1 (kindly provided by Sue Goo Rhee), and β-actin (Millipore, Temecula, CA) were used for Western blotting.
Light-Induced Retinal Damage
All procedures involving animals were performed according to the ARVO statement for use of Animals in Ophthalmic and Vision Research and the Sanford-Burnham Institute Guidelines for Animal Research. Sprague-Dawley rats born and raised in our animal facility under dim light (5–10 lux; 12 hours on, 12 hours off) were used in our experiments. At 5 to 6 weeks of age, rats were treated with CA or vehicle control (canola oil) for 6 days. CA was obtained from Nagase & Co. (>91% purity; Kobe, Japan), dissolved in vehicle and administered by intraperitoneal (IP) injection (25 mg/kg/day) for 6 days. Equivalent volumes of vehicle were administered to the control groups.
For the group exposed to light, after the last IP injection and 1 hour prior to light exposure, rats' pupils were dilated with 1% atropine sulfate to maximize the retinas' area affected by photostimulation. Unanesthetized rats were exposed to diffuse, cool white fluorescent light at 5000 lux for 5 hours (9 AM–2 PM) in clear plastic cages at a temperature of 22°C to 26°C. Rats were returned to their cages in the dim light environment for 7 to 9 days after the exposure to bright light. In a subgroup of rats, retinal function was evaluated by electroretinogram (ERG). Rats were subsequently euthanized, and eyes were processed for quantitative morphology analysis.
Detection and Bioavailability of CA in Retinas and Retina-Derived Cell Lines
Rats 5 to 6 weeks old were injected IP with CA blood; and retinal samples were collected at various time points after injection. Plasma was separated by centrifugation of heparinized whole blood at 4°C. Neural retinal samples from both eyes of each rat were pooled. In parallel, ARPE-19 and 661W cells were treated with 10 μM CA for 24 hours, and then cell pellets were prepared. For liquid chromatography-mass spectrometry (LC/MS/MS) analysis, plasma and retinal or cell homogenates were precipitated with acetonitrile containing an internal standard spiking solution (indomethacin, 1 μg/mL). Samples were centrifuged, and supernatants were transferred into 96-well plates for LC/MS/MS analysis. Samples were analyzed by HPLC (Prominence unit; Shimadzu, Kyoto, Japan) and an LC/MS/MS instrument (API4000 unit; Applied Biosystems, Carlsbad, CA).
Electroretinogram
Dark-adapted rats were anesthetized with ketamine (35 mg/kg) and xylazine (5 mg/kg). To dilate the pupils, we applied tropicamide (0.5%) topically to the cornea. ERGs were recorded using an Espion 2 system (Diagnosys, Lowell, MA) in a masked fashion with regard to the treatment group. Dark-adapted low and high scotopic ERG responses were elicited with 0.01 and 20 cd·s·m−2 white-flash stimuli, whereas light-adapted photopic and flicker (10 Hz) responses were recorded with a 20 cd·s·m−2 white-flash stimulus against a rod-desensitizing background light of 50 cd·m−2. An average of 5, 20, and 30 responses were collected for each flash, photopic, and flicker ERG response, respectively. Data were analyzed and exported in digital amplitude and time format using Excel software (Microsoft).
Histological Analysis
Rats were euthanized by isoflurane overdose. Eyes were gently enucleated, and the superior edge of each cornea was marked with animal tattoo ink for orientation purposes (Ketchum Manufacturing, Inc., Ontario, Canada). Eyes were fixed with 4% paraformaldehyde containing 20% isopropanol, 2% trichloroacetic acid, and 2% zinc chloride and embedded in paraffin.28 Sections (5-μm thickness) were cut along the vertical meridian from the whole eye, including the optic disc of each eyeball, and stained with hematoxylin and eosin (H&E) stain. Digitized images of the entire retina were analyzed using ImageScope software (Aperio, Vista, CA). In each hemisphere, the outer nuclear layer (ONL) thickness was measured at 500-μm intervals starting at the optic nerve head and extending toward the superior and inferior ora serrata. Values from the left and right eyes were averaged for each rat. Mean data for all rats in each group were calculated and plotted. The area under the curve (AUC) was calculated using Prism 5 software (GraphPad, San Diego, CA) and used for quantitative comparisons. All procedures and analyses were carried out by personnel masked to treatment group.
Results
CA Protects Retinal Cell Lines from Oxidative Stress-Mediated Cell Death In Vitro
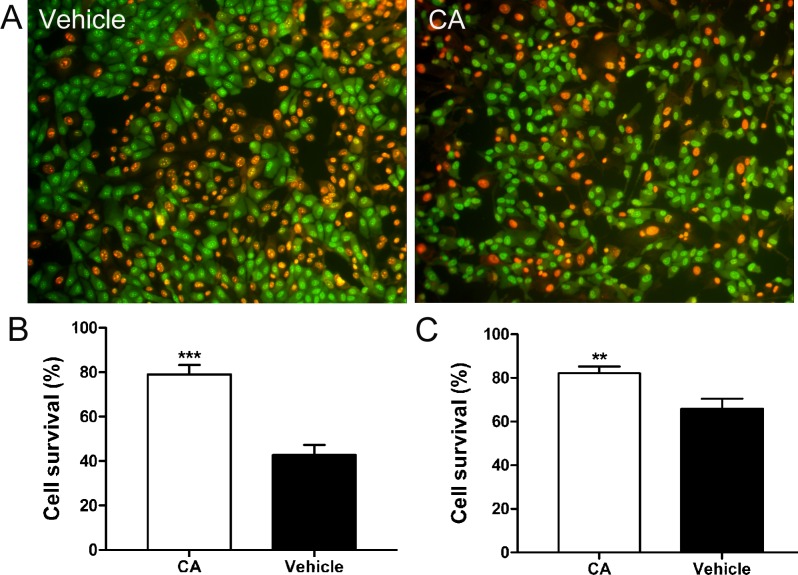
We incubated the human RPE cell line ARPE-1929 and the murine photoreceptor cell line 661W26 in a range of concentrations of CA for 21 hours prior to exposure to oxidative stress in the form of H2O2. We found that a minimum of 10 μM CA protected both cell lines against H2O2-induced oxidative damage (Fig. 1). Compared to control, CA afforded significant improvement in survival of ARPE19 cells (Fig. 1B, P < 0.0001) and 661W photoreceptors (Fig. 1C, P < 0.005).
Figure 1. .
Protective effect of CA from H2O2-induced cytotoxicity. (A) Representative images of ARPE-19 cells stained with acridine orange and ethidium bromide after exposure to H2O2 and treatment with CA or vehicle control. Live cells stained green, while dead cell nuclei stained red. (B, C) Histograms of surviving ARPE-19 cells (B) and 661W photoreceptor cells (C) treated with vehicle or 10 μM of CA in serum-free medium for 21 hours followed by a 4-hour exposure to H2O2. Values are means ± SEM. Pretreatment with CA resulted in significantly enhanced survival of both ARPE-19 cells and 661W cells (n ≥ 3 experiments, each performed in triplicate). ***P < 0.0001, **P < 0.005, Student's t-test.
CA Activates Expression of Phase 2 Genes
Nrf2 activates transcription via binding to AREs in the promoter region of phase 2 genes.23 In order to determine whether CA activates the ARE, we transfected ARPE-19 cells with a luciferase reporter vector containing wild-type or mutant ARE sequences. We found expression of the reporter gene to be significantly increased after CA treatment compared to vehicle control. Moreover, CA induced ARE reporter activity after transfection with WT-ARE vector but not with mt-ARE vector (Fig. 2A; P < 0.001).
Figure 2. .
CA induces expression of antioxidant genes and proteins in the ARPE-19 retinal cell line. (A) CA-induced activation of ARE by luciferase assay (P < 0.001 by t-test). Vaues are mean + SEM. (B) CA-induced upregulation of ARE-dependent expression of HO-1, NQO1, GCLM, and xCT genes, as shown by RT-PCR. (C) Immunoblot demonstrating upregulation of HO-1, NQO1, and SRXN1 antioxidant proteins 24 hours after 10 μM CA treatment (n ≥ 4 for each panel).
Next, we examined whether CA could induce ARE-dependent genes in ARPE-19 cells by microarray analysis and RT-PCR. By microarray analysis, we confirmed that CA induced the expression of phase 2 genes in RNA samples from ARPE-19 lysates. Treatment with CA led to significant upregulation of antioxidant genes compared to cells treated with vehicle (see Supplementary Material and Supplementary Fig. S1, http://www.iovs.org/content/53/12/7847/suppl/DC1). Additionally, using qPCR, we then validated our finding that CA induced upregulation of several ARE-related genes (heme oxygenase-1 [HO-1], NADPH quinoneoxido-reductase 1 [NQO1], glutamyl cysteine ligase modifier subunit [GCLM], and Na+-independent-cystine/glutamate exchanger [xCT]) (Fig. 2B). Moreover, we examined the effect of CA on protein expression by immunoblotting and found that treatment of ARPE-19 cells with CA also resulted in upregulation of several phase 2 proteins, including HO-1, NQO1, and sulfiredoxin (SRXN1) (Fig. 2C).
Nuclear Translocation of Nrf2
In the absence of stress, the transcription factor Nrf2 is retained in the cytoplasm through its binding to the Keap1 protein. Oxidants such as electrophiles can react with a critical cysteine residue on Keap1 to induce conformational changes that release Nrf2. Nrf2 thus liberated translocates into the cell nucleus where it binds to the ARE to induce transcription of targets including phase 2 genes.30–32 Using an immunocytochemical approach, we initially examined whether CA can induce nuclear translocation of Nrf2 in ARPE-19 cells. Nrf2 remained in the cytoplasm in vehicle-treated cells but translocated predominantly to nuclei of CA-treated cells (see Supplementary Material and Supplementary Figs. S2A, S2B, http://www.iovs.org/content/53/12/7847/suppl/DC1). This observation is consistent with our reporter gene assays, microarray data, and qRT-PCR results showing that CA activates the ARE in ARPE-19 cells (Fig. 2 and Supplementary Fig. S1, http://www.iovs.org/content/53/12/7847/suppl/DC1).
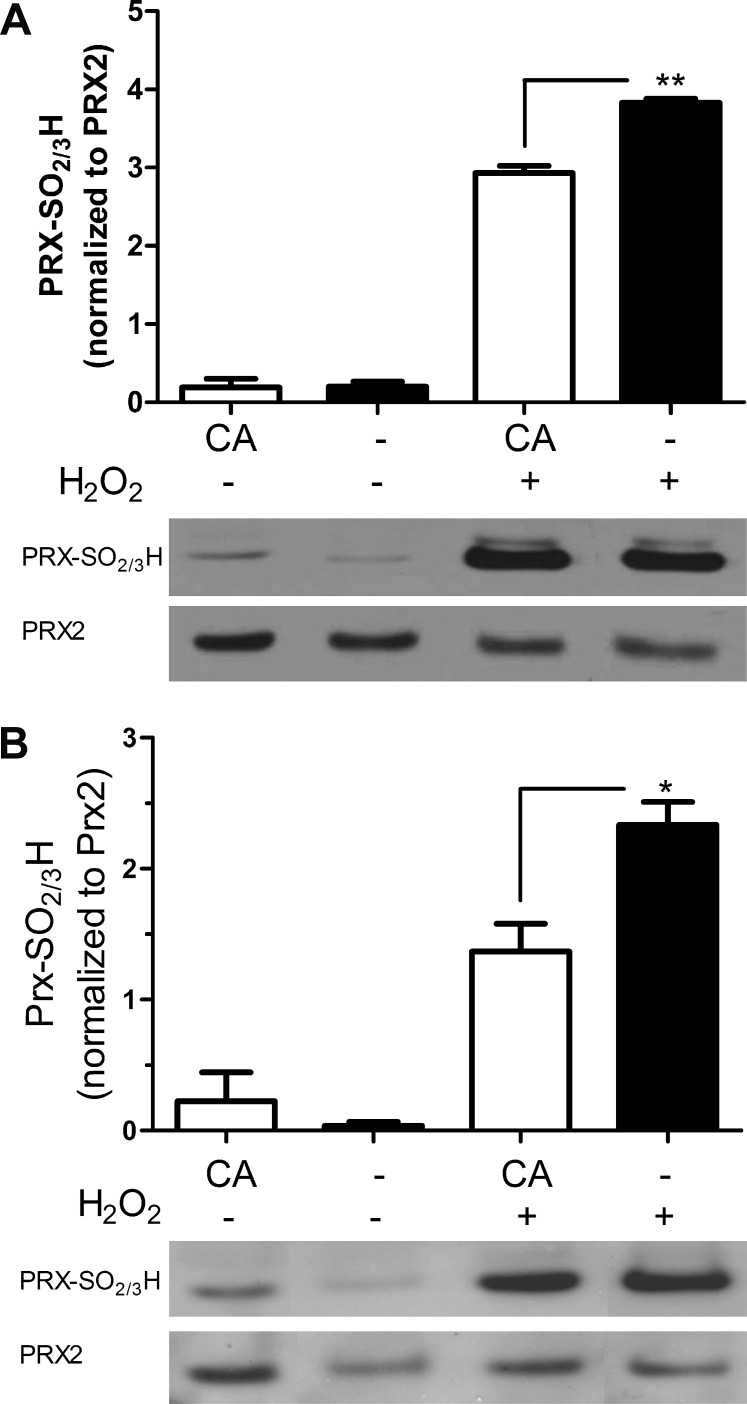
CA Inhibits Hyperoxidation of Peroxiredoxin 2
Peroxiredoxins (Prxs) are members of a superfamily of thiol peroxidases that catalyze reduction of reactive oxygen species (ROS) and thus play an important role in antioxidant defense and redox signaling. Prx2 is particularly important for protection in the nervous system.33 We and others have shown that the cysteine in the active site of Prx2 is hyperoxidized after intense ROS exposure to a cysteine sulfinic or sulfonic acid derivative (Cys-SO2/3) with resulting loss of protective peroxidase activity.33–35 To combat this effect, the enzyme Srxn1 can reduce and thus reactivate hyperoxidized Prx2-SO2H. However, basal levels of Srxn1 are generally low in cells, so the enzyme must be transcriptionally induced.36,37 A major pathway for Srxn1 induction involves Nrf2-mediated transcriptional activation via ARE sites in the promoter region of the Srxn1 gene.38–40 Accordingly, in the present study, we found that CA increased expression of the SRXN1 protein (Fig. 2C). As H2O2-induced hyperoxidation of Prx2 in ARPE-19 and photoreceptor cell lines, we next determined whether treatment with CA could ameliorate this effect. We found that preincubation with CA significantly inhibited H2O2-induced Prx2 hyperoxidation in both ARPE-19 (Fig. 3A) and 661W (Fig. 3B) cells. Taken together, these findings are consistent with the notion that CA-mediated cytoprotection may be afforded in part by induction of Srxn1 and consequent reduction of hyperoxidized Prx2.33,36
Figure 3. .
CA reduces hyperoxidation of Prx2. CA treatment of ARPE-19 retinal cells (A) and 661W photoreceptor cells (B) significantly protected against H2O2-induced hyperoxidation of Prx2 (n = 4; *P < 0.05; **P < 0.006 by t-test). Values are means ± SEM.
Therapeutic Levels of CA in Retina-Derived Cell Lines In Vitro and Rat Retinas In Vivo
From our previous work, we knew that CA readily crosses the blood–brain barrier (and therefore presumably the blood–retina barrier).19 Based on additional published data,19,41 we estimated that a dose of ∼20 mg/kg CA administered parenterally would be needed to achieve a concentration of ∼10 μM CA in the retina. Thus, we bracketed that dose, administering CA IP as a depot in an oil excipient at 1, 5, 10, 25, and 50 mg/kg. LC/MS/MS analysis showed that the concentration of CA after IP injection remained approximately stable in the plasma if monitored 24 or 48 hours after a single administration. We found that daily dosing at 25 mg/kg roughly doubled the plasma concentration by 2 days after the initial injection compared to the level measured after 1 day, yielding a concentration of ∼18 μM in the plasma and ∼12 μM in the retina (see Supplementary Material and Supplementary Fig. S3, http://www.iovs.org/content/53/12/7847/suppl/DC1). A dose of 50 mg/kg CA compared to 25 mg/kg only increased the plasma level by 1.2-fold. As the 25 mg/kg dose produced retinal levels of CA approximating those that were found to be protective in vitro, we chose that dose for further analysis.
In Vivo Protection from LIRD by CA
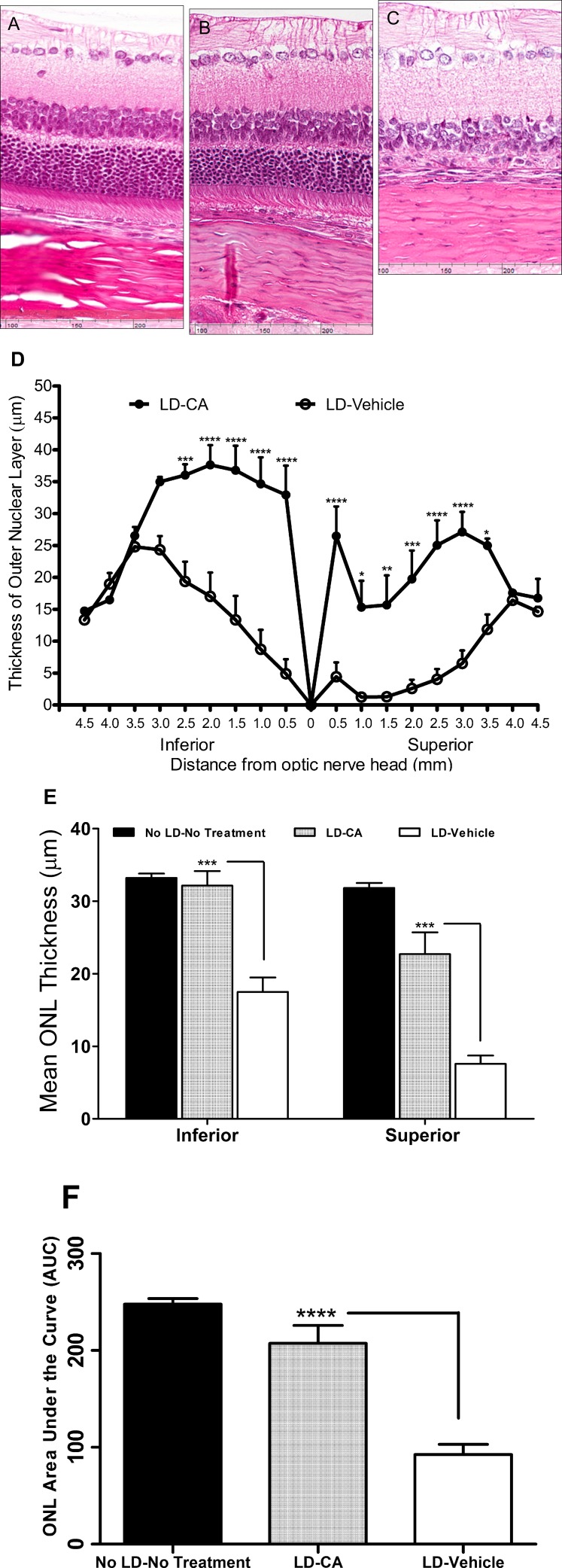
For the light-induced damage model, Sprague-Dawley rats were reared from birth under dim light conditions (5–10 lux), and our protocol for subsequent bright light exposure was modified from that of Anderson and colleagues.42 In control animals not exposed to light, treatment with CA did not manifest any effect on retinal histology compared to vehicle (see Supplementary Material and Supplementary Fig. S4, http://www.iovs.org/content/53/12/7847/suppl/DC1). Quantitative histology of rat eyes 7 to 10 days after bright light exposure (5000 lux) revealed a significant reduction in ONL thickness that was more severe in the superior than the inferior retina (Figs. 4A–D). While the exact cause for this regional variation in light susceptibility is unknown,43 it may in part reflect the positioning of the lights in the visual field relative to the retina. In rats treated with vehicle alone, the mean ONL thickness was reduced ∼50% in the inferior retina and ∼70% in the superior retina compared to those treated with a dose of 25 mg/kg CA (P < 0.001) (Fig. 4E). Looked at another way, we observed only 3% photoreceptor loss in light-exposed/CA-treated rats compared to normal (unexposed/nontreated) rats in the inferior retina and 29% loss in the superior retina. In contrast, in light-exposed/vehicle-treated rats compared to normal rats, we observed 47% loss in the inferior retina (P < 0.001) and 76% in the superior retina (P < 0.001) (Fig. 4E). Moreover, for rats treated with CA, the decrease in AUC analysis was not significantly different from that in normal (unexposed/nontreated) rats. However, for light-exposed/vehicle-treated rats, the AUC was significantly reduced compared to both normal and light-exposed/CA-treated animals (P < 0.0001) (Fig. 4F). The neuroprotective effect of CA was further confirmed by TUNEL staining of retinal sections. After exposure to damaging light, the number of TUNEL-positive apoptotic photoreceptor cells in rats treated with CA was significantly reduced compared to vehicle (see Supplementary Material and Supplementary Fig. S5, http://www.iovs.org/content/53/12/7847/suppl/DC1). These findings suggest that pretreatment with CA prior to light exposure offers significant protection of photoreceptors in this animal model of AMD, at least at the histological level.
Figure 4. .
CA protects photoreceptors from light damage in the intact retina. (A–C) Eye sections from normal rats stained with H&E stain in the superior retinal area, 500 to 1000 μm from the ONH (A), after light insult and treatment with CA (25 mg/kg) (B), and after light insult with vehicle treatment (C). (D) Graph shows retinal ONL thickness in rats treated with CA or vehicle and exposed to damaging light. Values are means ± SEM. (E) ONL thickness in the inferior and the superior hemispheres of control retinas or after light damage (LD) with and without CA treatment. (F) AUC analysis for the three different treatment groups. Significantly thicker ONLs were observed in rats treated with CA than vehicle controls (n = 7 for No LD, 15 for LD + vehicle, 14 for LD + CA; *P < 0.05, **P < 0.01; ***P < 0.001; ****P < 0.0001 by ANOVA). Values are means ± SEM.
ERG Evidence for CA-Mediated Improvement in Retinal Function after Light Damage
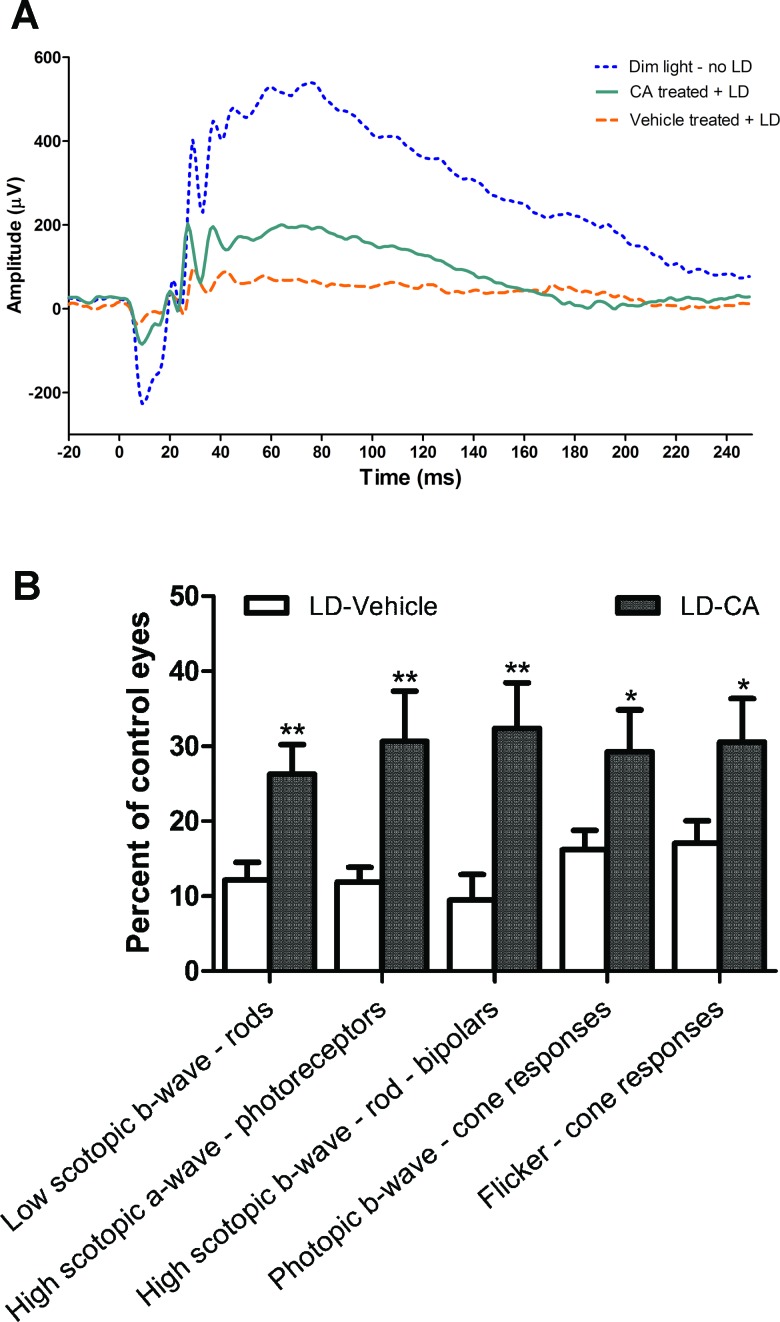
Next, we sought to characterize the ability of CA to afford functional protection from light-induced damage by using an electrophysiological analysis. In general, in an ERG, the rising phase of the a-wave reflects photoreceptor function, while the b-wave reflects activity of cells distal to the photoreceptors, including bipolar cells. Moreover, intact synaptic transmission emanating from photoreceptors would be necessary to generate a normal b-wave. Additionally, by varying the level of dark adaptation and light stimulation parameters, ERG measurements can be obtained that are relatively selective for cone or rod photoreceptors. Our ERG results showed that treatment with CA attenuated the loss in amplitude of both the a-wave and the b-wave when measured 7 days after LIRD (Figs. 5A, 5B). Scotopic and photopic protocols afforded evidence that both cone and rod functions were relatively preserved under both conditions when animals were pretreated with CA. In summary, our findings suggest that treatment with CA prevented loss of photoreceptor function to a significant degree in the face of light-damaging insults.
Figure 5. .
Beneficial effect of CA treatment on retinal electrophysiology by ERG analysis. (A) Representative ERG traces from rat retinas of dim light-exposed control, CA-treated after light damage (LD), and vehicle-treated after LD. LD resulted in significant loss of ERG responses in vehicle-treated animals. Administration of CA enhanced ERG responses compared to vehicle (n = 5 for dim light/no LD, n = 7 for CA-treated/LD, n = 8 for vehicle-treated/LD groups). The initial downward deflection in the voltage response represents the a-wave, while the subsequent positive voltage response is the b-wave, as described in text (see Results section). (B) In rats treated with CA compared to vehicle, there was significant protection from light damage on both the ERG a-wave and b-wave (n = 8 for LD-vehicle, 7 for LD-CA; values normalized to the “no treatment” group, where n = 5; *P < 0.05; **P < 0.005). Values are means ± SEM.
Discussion
Oxidative stress has been shown to be a factor in the progression of a number of retinal diseases, including those affecting photoreceptors and RPE cells, such as RP and AMD. Due to rapid growth in the aging population, the prevalence of visual disabilities is expected to drastically increase during the next 20 years.44 For example, AMD is the leading cause of visual impairment in the elderly and accounts for over 54% of all irreversible vision loss in Caucasians.45 Progression of AMD and RP may occur at least in part because of light exposure, although other factors are clearly also critically important. The fact that excessive light evokes the generation of ROS coupled with the susceptibility of RPE and photoreceptor cells to oxidative stress suggests involvement of this process as one pathway to cell injury in the outer retina.1,2,46 The RPE cell layer plays a crucial role in the maintenance and survival of adjacent photoreceptors via phagocytosis of the photoreceptor outer segments.47 These cells also provide trophic support to the neural retina by supplying neurotrophic factors.48,49 Thus, rescue of both photoreceptor and RPE cells will be critical for preventing the onset and progression of AMD. Here, we present evidence that treatment with CA prior to exposure to oxidative stress or damaging light protects both form and function in the rat retina.
Our group previously reported that CA protects cerebrocortical neurons from oxidative stress, excitotoxicity, and ischemic injury via Nrf2-mediated induction of phase 2 antioxidant enzymes.19 Importantly, GSH, the major cell reductant, was increased in the brain following CA treatment, rather than decreased as observed with previously studied electrophilic compounds. We showed that CA was different from these other compounds by virtue of being a pro-electrophile that becomes activated to an electrophile by oxidative stress, the very injury that it then protects from by inducing various antioxidant genes. These findings and the strong association of progression of retinal damage with oxidative stress in the dry form of macular degeneration and in RP suggested that CA-induced antioxidant pathways could prove beneficial in these diseases. Based on our previous results, we hypothesized that CA would be activated by the oxidative stress associated with light-induced damage in the retina and subsequently boost the expression of endogenous phase 2 genes without depleting the endogenous reducing capacity of normal cells. This approach of boosting endogenous phase 2 gene expression, which results in synthesis of proteins with a broad spectrum of antioxidant, detoxification, and reductive activities, is attractive due to the well-known and long-lasting cytoprotective effects of these gene products. In contrast, an antioxidant-based therapy would have to be continuously supplied to the injured cells in order to remain effective, as the agents are continuously consumed in the neutralization process.
In this study, we found that the pro-electrophilic drug CA promoted survival of retina-derived cell lines, 661W photoreceptors and ARPE-19 RPEs, against H2O2-induced cell death in vitro. Accounting for this effect, CA induced nuclear translocation of Nrf2 and then, as shown in our reporter gene assays, activated transcription of phase 2 genes. We confirmed these results by microarray and qPCR analyses. Transcriptional activation resulted in upregulation of antioxidant/detoxifying proteins, including HO-1, NQO1, GCLM, xCT, and SRXN1.
Several electrophilic compounds have been found to activate the Nrf2 pathway in RPE cells, which have the potential of protecting photoreceptors against oxidative stress.20–22 Unlike these compounds, however, an advantage of CA-induced activation of the Nrf2 pathway is that it preserves the endogenous reducing capacity of healthy cells by avoiding reaction with glutathione; this is accomplished by the targeted activation of CA in the oxidatively stressed tissue.
One of the phase 2 proteins that we found to be upregulated by CA was SRXN1, which is responsible for enzymatically reversing hyperoxidation of the active site cysteine of Prxs from the sulfinic acid derivative to the free thiol form (from -SO2H to -SOH). CA should thus afford cytoprotection in part by allowing Prxs to detoxify additional ROS.50 Indeed, our data demonstrate that CA protects the retina-derived cell lines ARPE-19 and 661W from H2O2-induced hyperoxidation of Prx2.
Importantly, our data also indicate that CA crosses the blood–retina barrier when administered by IP injection and further accumulates in the retina after multiple doses. After CA treatment, compared to control, we found histological evidence that a significantly greater number of photoreceptors survived in rats after exposure to damaging light. Additionally, we observed significantly increased amplitudes of the ERG a-wave and b-wave after CA treatment in the face of a light-damaging insult, indicating functional preservation of photoreceptors and more distal retinal elements, predominantly bipolar and Müller cells, respectively. Hence, our ERG findings are also consistent with the notion that CA attenuated light-induced retinal dysfunction in photoreceptors and the inner retina.
In summary, our results suggest that the pro-electrophilic drug CA, after conversion to its active electrophilic form, can attenuate oxidative damage to the retina in the face of light-induced insult. CA acts, at least in part, via activation of the Nrf2 transcriptional pathway to activate endogenous antioxidant phase 2 genes. Hence, such pro-electrophilic drug therapy may prove to be a useful strategy to ameliorate retinal oxidative stress that occurs as a result of exposure to light or other factors and, hence, to prevent progression of AMD and RP.
Supplementary Material
Acknowledgments
We thank Sue Goo Rhee of Ewha Woman's University, Seoul, South Korea, for the Srxn1 antibody and Carmen Sunico for performing early immunoblot experiments with the antibody. The 661W cells were generously provided by Muayyad Al-Ubaidi (University of Oklahoma Health Sciences Center, Oklahoma City, OK). We are also indebted to Biju B. Thomas of the University of Southern California, Los Angeles, and Magdalene J. Seiler of the University of California, Irvine, for providing the light box to induce retinal degeneration.
Footnotes
Supported in part by National Institutes of Health Grants R01EY05477, P01 HD29587, P01 ES016738, and P30 NS076411, and by Allergan, Inc., Irvine, California.
Disclosure: T. Rezaie, None; S.R. McKercher, None; K. Kosaka, None; M. Seki, None; L. Wheeler, None; V. Viswanath, None; T. Chun, None; R. Joshi, None; M. Valencia, None; S. Sasaki, None; T. Tozawa, None; T. Satoh, P; S.A. Lipton, P
References
- 1.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134 [DOI] [PubMed] [Google Scholar]
- 2.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 3.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293 [DOI] [PubMed] [Google Scholar]
- 4.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97:7154–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radu RA, Mata NL, Bagla A, Travis GH. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt's macular degeneration. Proc Natl Acad Sci U S A. 2004;101(16):5928–5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruickshanks KJ, Klein R, Klein BE. Sunlight and age-related macular degeneration. The Beaver Dam Eye Study. Arch Ophthalmol. 1993;111:514–518 [DOI] [PubMed] [Google Scholar]
- 7.Marc RE, Jones BW, Watt CB, et al. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008;14:782–806 [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor HR, West S, Munoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110:99–104 [DOI] [PubMed] [Google Scholar]
- 9.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306 [DOI] [PubMed] [Google Scholar]
- 10.Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–473 [PubMed] [Google Scholar]
- 11.Hafezi F, Marti A, Munz K, Reme CE. Light-induced apoptosis: differential timing in the retina and pigment epithelium. Exp Eye Res. 1997;64:963–970 [DOI] [PubMed] [Google Scholar]
- 12.Hafezi F, Steinbach JP, Marti A, et al. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med. 1997;3:346–349 [DOI] [PubMed] [Google Scholar]
- 13.Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442 [DOI] [PubMed] [Google Scholar]
- 14.Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no 9. Arch Ophthalmol. 2001;119:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815 [DOI] [PubMed] [Google Scholar]
- 17.Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006;40:223–231 [DOI] [PubMed] [Google Scholar]
- 18.Satoh T, Izumi M, Inukai Y, et al. Carnosic acid protects neuronal HT22 Cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008;434:260–265 [DOI] [PubMed] [Google Scholar]
- 19.Satoh T, Kosaka K, Itoh K, et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanito M, Masutani H, Kim YC, et al. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Ophthalmol Vis Sci. 2005;46:979–987 [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci U S A. 2004;101:10446–10451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandal MN, Patlolla JM, Zheng L, et al. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46:672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh T, Lipton SA. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007;30:37–45 [DOI] [PubMed] [Google Scholar]
- 24.al-Sereiti MR, Abu-Amer KM, Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol. 1999;37:124–130 [PubMed] [Google Scholar]
- 25.Chipault JR, Mizumo GR, Hawkins JM. The antioxidant properties of natural spices. Food Res. 1952;17:46–55 [Google Scholar]
- 26.Tan E, Ding XQ, Saadi A, et al. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:764–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mironova EV, Evstratova AA, Antonov SM. A fluorescence vital assay for the recognition and quantification of excitotoxic cell death by necrosis and apoptosis using confocal microscopy on neurons in culture. J Neurosci Methods. 2007;163:1–8 [DOI] [PubMed] [Google Scholar]
- 28.Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42:1838–1850 [DOI] [PubMed] [Google Scholar]
- 29.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169 [DOI] [PubMed] [Google Scholar]
- 30.Dinkova-Kostova AT, Liby KT, Stephenson KK, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102:4584–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394 [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140 [DOI] [PubMed] [Google Scholar]
- 33.Fang J, Nakamura T, Cho DH, Gu Z, Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18742–18747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552 [DOI] [PubMed] [Google Scholar]
- 35.Rhee SG, Jeong W, Chang TS, Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int. 2007;(suppl):S3–S8 [DOI] [PubMed] [Google Scholar]
- 36.Papadia S, Soriano FX, Léveillé F, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo HA, Chae HZ, Hwang SC, et al. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656 [DOI] [PubMed] [Google Scholar]
- 38.Singh A, Ling G, Suhasini AN, et al. Nrf2-dependent sulfiredoxin-1 expression protects against cigarette smoke-induced oxidative stress in lungs. Free Radic Biol Med. 2009;46:376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soriano FX, Baxter P, Murray LM, et al. Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin. Mol Cells. 2009;27:279–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soriano FX, Leveille F, Papadia S, et al. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107:533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan H, Wang L, Li X, et al. High-performance liquid chromatography method for determination of carnosic acid in rat plasma and its application to pharmacokinetic study. Biomed Chromatogr. 2009;23:776–781 [DOI] [PubMed] [Google Scholar]
- 42.Tanito M, Kaidzu S, Anderson RE. Delayed loss of cone and remaining rod photoreceptor cells due to impairment of choroidal circulation after acute light exposure in rats. Invest Ophthalmol Vis Sci. 2007;48:1864–1872 [DOI] [PubMed] [Google Scholar]
- 43.Tanito M, Kaidzu S, Ohira A, Anderson RE. Topography of retinal damage in light-exposed albino rats. Exp Eye Res. 2008;87:292–295 [DOI] [PubMed] [Google Scholar]
- 44.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572 [DOI] [PubMed] [Google Scholar]
- 45.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485 [DOI] [PubMed] [Google Scholar]
- 46.Bok D. New insights and new approaches toward the study of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14619–14621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700 [DOI] [PubMed] [Google Scholar]
- 48.Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91–101 [DOI] [PubMed] [Google Scholar]
- 49.Ishida K, Yoshimura N, Yoshida M, et al. Expression of neurotrophic factors in cultured human retinal pigment epithelial cells. Curr Eye Res. 1997;16:96–101 [DOI] [PubMed] [Google Scholar]
- 50.Chang TS, Jeong W, Woo HA, et al. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.