Synopsis
The purposes of this article are to discuss key factors for assessing joint function, to present some recent findings and to address the future directions for evaluating the function of the ACL-injured/reconstructed knees. Well-designed studies, using state-of-the art tools to assess knee kinematics under in vivo, dynamic, high-loading conditions, are necessary to evaluate the relative performance of different procedures for restoring normal joint motion.
Keywords: Anterior cruciate ligament, In-vivo knee kinematics, Dynamic stereo X-ray system, Knee, Anatomic, Double-bundle, Single-bundle
Introduction
The goals of anterior cruciate ligament (ACL) reconstruction are to restore stability and enable return to unrestricted function over the short term, and ideally to protect joint health over the long term. Current treatment approaches are moderately successful for restoring function, with most individuals able to return to their pre-injury level of sports activity [1,2]. However, surgical ligament reconstruction does not appear to prevent the development of osteoarthritis after ACL injury [3,4,5]. While there are many factors that may contribute to joint degeneration, persistent abnormal knee mechanics are often implicated in the initiation and progression of osteoarthritis (OA) in the ACL-injured/reconstructed knee [6,7]. Concerns about the high rates of OA are largely responsible for the increased interest over the last several years on more anatomical approaches to ACL repair and reconstruction. Though no current techniques can restore the true insertion site anatomy or physiology of the native ACL, procedures that attempt to improve tunnel placement and graft geometry to more closely resemble the original ligament are gaining in popularity. The premise for these newer procedures is that better restoration of native anatomy will lead to more normalized joint mechanics and improved long-term joint health.
Ultimately, determining the efficacy of these procedures for reducing OA risk after ACL injury will require large, well-designed clinical studies with long-term follow-up to directly evaluate joint degeneration. However, considerable insights about the relative merits of different ACL reconstruction techniques can be obtained over the short term by investigating their effectiveness for restoring normal joint function. The goals of this narrative review are to discuss key factors for assessing joint function, present some recent findings and propose future directions for evaluating the function of the ACL-injured/reconstructed knee.
Methodological considerations for assessing knee function
The case for in vivo, human studies
Definitive conclusions on the relative efficacy of different ACL reconstruction techniques can only be drawn from in vivo, human studies. Cadaveric studies have contributed a wealth of information on the basic biomechanics and passive structural properties of the knee, and can be beneficial for development and initial evaluation of surgical techniques. However, cadaver studies cannot reproduce the complex combination of gravitational, inertial and active muscular forces that influence knee mechanics during functional activities. Cadaver studies also represent only “time zero” conditions, and cannot account for biological responses (such as healing, remodeling, tunnel enlargement, etc.) that can have a significant influence on knee and ligament function. Conversely, animal models are well suited for studying biological tissue response, but they differ too extensively in joint morphology and function for direct transfer of kinematic findings to humans (with the possible exception of large primates, which are rarely used for orthopaedic studies due to cost and ethical considerations).
Laxity vs. stability
In vivo knee function can be evaluated under a wide range of conditions. Which measures are most relevant to outcomes after ACL reconstruction? With the progression from laxity testing to static weight-bearing to dynamic, functional activities, assessment becomes more technically demanding but also potentially more relevant to joint health. Central to most theories relating joint mechanics to OA development is the idea that altered joint contact patterns and joint loads, encountered during routine activities, can be detrimental to long-term cartilage health [6,8]. These theories would suggest that the most measures of joint function are those that reflect the behavior of the knee during common, functional activities. It is especially important to distinguish between evaluations of laxity vs. assessments of dynamic knee function and functional stability. In a recent review, Musahl et al. stated, “In biomechanical terms, laxity is the passive response of a joint to an externally applied force or torque. Stability, on the other hand, is a functional measure; that is, a knee, regardless of laxity, is only unstable if it “gives way” during functional activities.”[9]. Laxity tests are typically performed without the compressive joint forces required to properly engage the conforming condylar surfaces, which play an important role in joint stabilization. Thus, while laxity tests may be effective for identifying structural deficits, the results cannot predict joint behavior during dynamic, functional activities. In fact, many studies relating static laxity and clinical/functional outcomes have reported at best weak correlations [10,11,12,13,14].
Knee function is task-dependent
In vivo studies incorporating body-weight loading and active muscular control provide a much more comprehensive and realistic picture of the natural function of the knee joint as a complex neuromusculoskeletal system. However, the knee has a wide envelope of possible motions, and joint function is highly activity-dependent. Patterns of joint motion and articular contact vary considerably with loading and activity, even during similar ranges of knee flexion[15,16]. Knee tissues are highly viscoelastic and respond nonlinearly to load magnitude and loading rate [17,18], so the behavior of the knee under low-demand conditions cannot be simply “scaled up” to predict behavior during functional activities. Thus, studies incorporating body-weight loading during quasi-static activities (e.g. sequential fixed knee angles [19]) or low-effort movements (e.g. half-speed gait [20]) may not predict knee behavior during more complex, demanding tasks. This may be especially important for ACL-injured athletes, who will routinely expose their joints to high-magnitude, rapidly changing loads after returning to sports.
Measurement options for dynamic, in vivo studies
Meaningful characterization of dynamic joint function during common activities poses unique challenges, especially for measurements directly relevant to soft tissue behaviour. Peak ACL strains during activities of daily living are in the order of 4% or less [21]; for a typical ACL size this represents a length change of only 1.2 mm. Assessing articular contact kinematics (arthrokinematics) requires a measurement error substantially smaller than the thickness of the cartilage layer (typically 2-4 mm thick for the tibio-femoral joint). The most widely utilized technology for studying knee function after ACL reconstruction is video-motion analysis, which tracks motion of multiple skin-mounted markers placed on the thigh and shank to determine limb movement. This technology is non-invasive, widely available and reliable, and has been effective for identifying differences in knee kinematics between ACL-intact, ACL-deficient and ACL-reconstructed joints (as described below). But, conventional motion analysis cannot achieve the sub-millimeter accuracy required for tissue-relevant measurements, because of the displacement of skin-mounted markers relative to underlying bone [22,23,24]. Magnetic resonance imaging (MRI) can achieve sub-millimeter accuracy and enables direct visualization of soft tissue, but sample rates are too slow and the imaging environment is too restrictive for most functional movement tasks.
Dynamic radiographic imaging enables direct visualization and 3D tracking of bone motion, and has been gaining in popularity over the last decade. While some measurements have been performed using a single imaging plane, dual or biplane imaging systems are generally required to obtain sub-mm resolution in all three movement planes. Many systems are now in use across the United States, with capabilities that vary based on the specific equipment and analysis techniques employed. Conventional “C-arm” fluoroscopy systems are limited by low frame rates (30Hz or less) and long exposure times (8 ms or longer), but are adequate for quasi-static and low-speed activities [25,26]. Custom-built systems can achieve much higher sample rates and have validated sub-mm accuracy for more physically demanding tasks, such as running [27,28,29].
Dynamic knee function: Traditional ACL Reconstruction
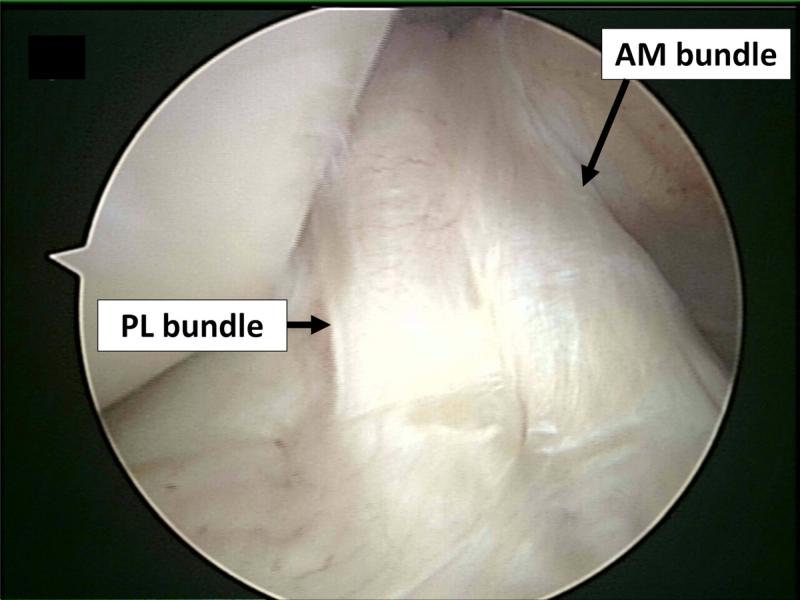
The ACL is often described as consisting of two functional bundles, the anteromedial (AM) and the posterolateral (PL) bundles, named in relation to their typical orientation and insertion on the tibia and femur (Figure 1) [30,31]. The AM and PL bundles function synergistically to provide both anterior and rotational stability of the knee. Cadaver studies suggest that the AM bundle is taut throughout the range of motion of the knee, reaching a maximum tension between 45° and 60°, whereas the PL bundle is tight primarily in extension [32,33,34,35].
Figure 1.
Arthroscopic view of the intact ACL, in 90 degrees of knee flexion. The intact native ACL consists of two instinct bundles; anteromedial (AM) and posterolateral (PL) bundle can been seen, separated by a septum.
Traditional ACL reconstruction procedures have been performed using a single graft bundle, without attempting to recreate the native double-bundle ACL anatomy. The tunnel placement techniques commonly employed, e.g. trans-tibial drilling of the femoral tunnels and/or the “o’clock” method for drill orientation, also failed to reliably place the graft within the native ACL footprint.[36,37] These single-bundle, non-anatomical procedures may eliminate anterior/posterior (AP) laxity and successfully restore normal AP translation, but fail to restore rotational stability [38,39]. Numerous in-vivo kinematic studies utilizing a variety of loading conditions have confirmed that these procedures fail to restore normal dynamic knee function. Logan et al., using open-access MRI, reported that ACL reconstruction reduced sagittal laxity to within normal limits but did not restore normal tibiofemoral kinematics during static weightbearing.[40] Georgoulis et al. examined ACL-deficient individuals before and after bone-patellar tendon-bone ACL reconstruction during walking using video-motion analysis [41]. The ACL-deficient patients demonstrated greater tibial internal rotation, which decreased closer to normal levels after ACL reconstruction. In a subsequent investigation with higher-demand activities (stair descent and pivoting), tibial rotation was significantly larger in the ACL reconstructed knees compared to the contralateral, intact legs [42]. Kinematics after ACL reconstruction have also been investigated using radiographic techniques to analyze in-vivo knee kinematics without errors from skin motion artifacts. Brandsson et al. found that tibial rotation and AP translation were not restored by ACL reconstruction (using bone-patella tendon-bone autografts) in nine unilateral ACL patients 1 year after surgery using continuous radiostereometric analysis [43]. Papannagari et al. reported that although anterior laxity was restored according to KT-1000 arthrometer testing, ACL reconstruction did not restore normal knee kinematics under weight-bearing conditions when measured using a dual-orthogonal fluoroscopic system [44]. Studies of more physically demanding tests require specialized high-speed radiographic imaging systems. Further evidence of rotational instability following ACL reconstruction was provided by Tashman et al. who used a 250 frame/s dynamic stereo x-ray (DSX) system to evaluate in vivo kinematics of the knee during downhill running for patients who underwent traditional, nonanatomic single-bundle reconstruction [45,46]. This traditional single-bundle ACL reconstruction restored normal AP translation, but the reconstructed knees were more externally rotated (mean 4°) and more adducted (mean 3°) relative to the contralateral, uninjured knees. These rotational changes were associated with shifts in the areas of joint contact as well as a reduction in medial-compartment joint space under dynamic loading. Thus, there is substantial and growing evidence from in vivo knee kinematics studies that non-anatomical single-bundle ACL reconstruction fails to restore pre-injury knee function under functional loading conditions.
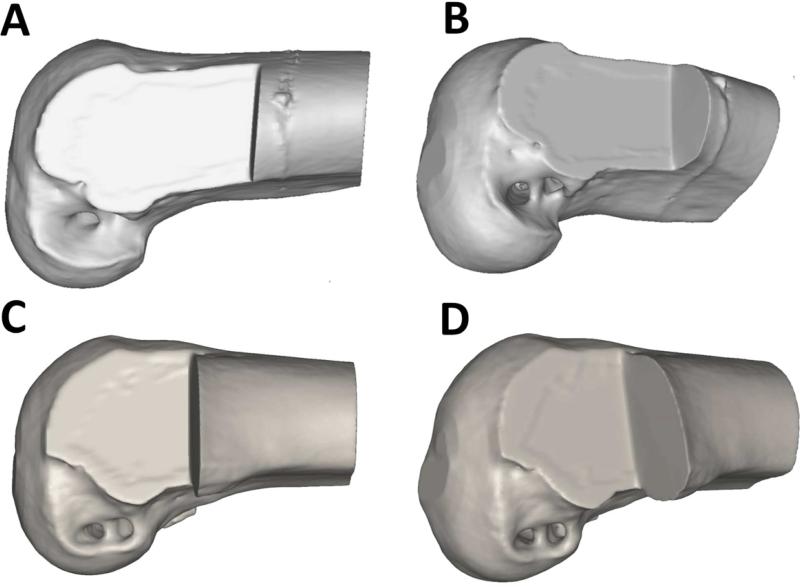
This evidence, along with increasing knowledge of ACL anatomy, led to greater interest in double-bundle ACL reconstruction techniques. Several in vitro biomechanical studies have suggested that double-bundle ACL reconstruction might improve anterior tibial translational and rotational stability [47,48]. Conversely, other studies have reported no significant differences in clinical outcome between single-bundle and double-bundle ACL reconstruction procedures [49,50]. Yasuda et al. performed a prospective comparative cohort study of 72 patients evaluated 2 years after surgery to compare the clinical outcomes of anatomic double-bundle ACL reconstruction with those of non-anatomic single- and double-bundle reconstructions. They observed no significant differences in the range of motion, the muscle torque and International Knee Documentation Committee (IKDC) evaluation scores. However, side-to-side KT-2000 measurements and pivot shift examination of anatomic double-bundle ACL reconstruction showed significantly better results than in the single-bundle procedure [51]. Since “double bundle” does not necessarily imply “anatomical”, interpretation of these findings is complicated by uncertainty as to whether the double-bundle procedures in some studies were actually performed anatomically (i.e. with tunnels drilled in the footprints of the native ACL bundles). Non-anatomic and anatomic tunnel placements are shown in Figure 2. There is insufficient high-quality data in the literature to adequately assess whether non-anatomic double-bundle ACL reconstruction is kinematically superior to non-anatomic single-bundle procedures.
Figure 2.
Non-anatomic tunnel placement in double-bundle ACL reconstruction (A, B). Anatomic tunnel placement in double bundle ACL reconstruction (C, D).
Dynamic knee function: Anatomical ACL Reconstruction
In the last decade, anatomic placement of ACL grafts has become a more widely accepted principle for ligament reconstruction. Anatomic ACL reconstruction techniques aim to better restore the normal anatomy and biomechanics of the knee, and are hypothesized to potentially decrease the incidence of OA after ACL reconstruction. Cadaver studies have shown mixed results comparing anatomical single-bundle ACL reconstruction procedures to anatomic double-bundle procedures, with some reporting superior stability for double-bundle [52] and others reporting little difference for anatomic double-bundle compared to centrally placed single-bundle reconstructions.[53] Though differences in the outcomes of single-bundle and double-bundle ACL reconstruction comprise a topic of ongoing discussion, it is generally believed that both methods benefit from anatomical tunnel placement. [38,54,55]
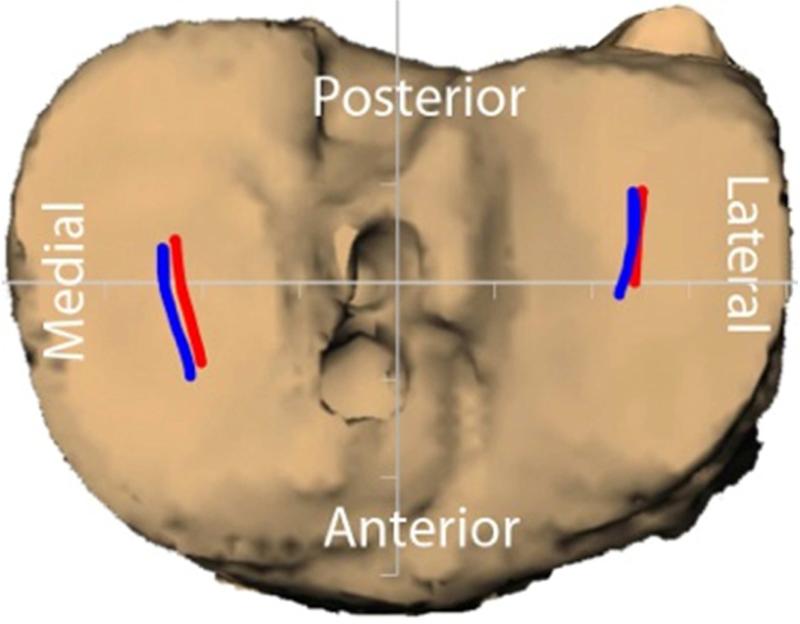
Controversy over the possible merits of both anatomical and double-bundle techniques for restoring knee function has motivated a number of in vivo kinematics studies over the last several years. Abebe et al., using biplanar fluoroscopy and MR imaging, reported that anatomical femoral placement of the graft in single-bundle reconstruction resulted in a more stable knee (Figure 2). [56] While subjects with non-anatomical graft placement had up to 3.4 mm more anterior tibial translation, 1.1 mm more medial tibial translation and 3.7° more internal tibial rotation compared to the contralateral side, subjects with anatomic graft placement had motion that more closely replicated that of the intact knee (Figure 3). The additional benefit of double-bundle (vs. single-bundle) anatomic reconstruction for restoring kinematics has, however, yet to be established. Several video-motion analysis studies have found no differences in knee kinematics and rotational stability between double-bundle and single-bundle ACL-reconstructed knees during gait, high-demand pivoting activities and other dynamic movement tasks (Figure 4). [57,58,59,60] These studies concluded that both techniques were able to restore tibial rotational excursion when compared with the contralateral knees and/or with control knees from uninjured subjects. However, these studies cannot definitively address differences between single and double-bundle anatomic ACL reconstruction, due to concerns regarding the ability of surface marker based techniques for assessing small (but potentially important) differences in transverse and coronal-plane rotations or assessing shifts in tibiofemoral contact locations.
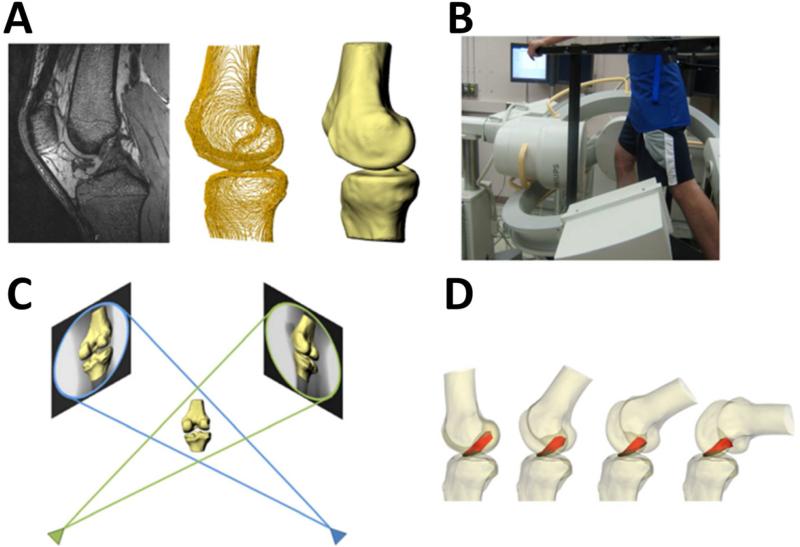
Figure 3.
Multi-planar, high resolution MR imaging was used to create 3D models of the knee, including the attachment sites of the ACL and graft (A). Biplanar fluoroscopy was used to record each subject's knee motion during a single leg lunge (B). Fluoroscopic images and 3D models were used to reproduce the motion of the each subject's knees during the lunge (C). From these models, the length and orientation of the ACL and graft were measured (D). (Reprinted with permission from Abebe, E. S., G. M. Utturkar, et al. (2011). “The effects of femoral graft placement on in vivo knee kinematics after anterior cruciate ligament reconstruction.” J Biomech 44(5): 924-929.)
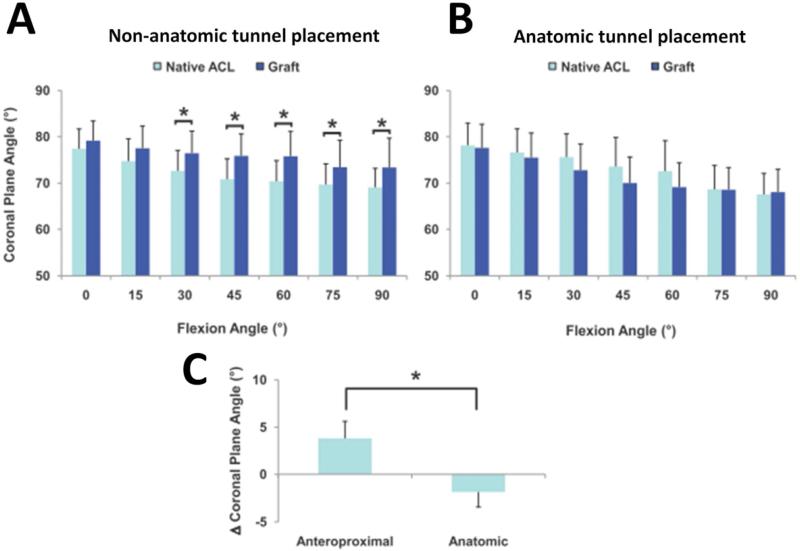
Figure 4.
In the sagittal plane, anteroproximal grafts were more vertical than the native ACL (A), while the anatomic grafts (B) more closely restored the sagittal plane orientation of the native ACL. When their respective differences from native were averaged across all flexion angles, anteroproximal grafts were more vertical compared to the anatomic grafts (C) (*: p < 0.05). (Reprinted with permission from Abebe, E. S., G. M. Utturkar, D. C. Taylor, et al.: The Effects of Femoral Graft Placement on in Vivo Knee Kinematics after Anterior Cruciate Ligament Reconstruction. J Biomech. 44(5): 924-929 (2011).)
Radiographic studies reporting dynamic knee function during high-demand activities after anatomical reconstruction (single-bundle or double-bundle) have yet to appear in the peer-reviewed literature. A pilot study was performed at the University of Pittsburgh Biodynamics Laboratory to evaluate the effectiveness of anatomical double-bundle ACL reconstruction for restoring normal knee kinematics. Eight subjects with isolated ACL ruptures were tested during downhill running approximately 6 months after ACL reconstruction, using methods similar to those employed for previous studies. [46] An anatomical double-bundle surgical procedure was employed to place graft tunnels within the native footprints of the AM and PL bundles. Biplane x-ray images were collected at 150 frames/s during the early to mid-stance phase of running, and a model-based tracking method was employed to determine tibiofemoral kinematics [28]. Tibio-femoral rotations and translations were determined and compared for the reconstructed and uninvolved limbs. No statistically (or clinically) significant differences were found between reconstructed and contralateral limbs for any kinematic variables after anatomical double-bundle reconstruction; mean between-limb differences were 0.4° for external rotation, 0.1° for abduction and 0.7 mm for A/P translation. These preliminary results suggest that anatomical double-bundle reconstruction may be more effective in restoring pre-injury knee function than more traditional techniques (Fig. 6). A larger, randomized study is currently underway to rigorously evaluate this hypothesis. (Irrgang, Fu and Tashman, In Press)
Figure 6.
Estimated contact paths during running (foot strike to mid-stance) for ACL-reconstructed (anatomical double-bundle, red traces) vs. contralateral, uninjured joints (Blue traces). There were no significant differences.
Discussion
Several methods for the objective assessment of in vivo motion have been developed. The most common methods utilize high-speed cameras or skin marker based video-motion capture systems.[61] The advantages of these systems are their ease of application, safety and quick data processing. However, the accuracy of these methods is limited (to several mm at best) by the relative motion between the markers and the skin,[62] and may be inadequate to detect meaningful differences between similar procedures. Dual fluoroscopy (for relatively low-demand tasks) [25] and dynamic stereo-radiography (providing short imaging times and high frames rates for more strenuous activities) [27] are more complex, expensive and invasive (due to radiation exposure), but provide reliable, high-precision assessment of tibio-femoral kinematics (typically ±0.1-0.3 mm accuracy; an order of magnitude or more better than surface-marker techniques, even when employing artifact reduction approaches). These radiographic methods are becoming more widely available, and are likely over the next few years to provide definitive answers regarding the relative merits of different surgical procedures for restoring dynamic joint function and stability.
In vitro biomechanics studies can focus on very specific aspects of ligament properties and behavior, but are insufficient to establish clinical efficacy. For example, the mechanical properties of different graft materials have been well characterized using tensile testing machines. However, the relationship between pre-implantation graft properties and actual graft function after months of healing, loading and remodeling has not been established. Conversely, in vivo human studies have direct clinical relevance, but may be confounded by uncontrolled factors that can cloud interpretation of results. Thus, basic/cadaver research studies are well suited for establishing a scientific basis for developing improved ACL reconstruction procedures. However, these studies must be followed by carefully designed and controlled clinical investigations with appropriate outcome measures.
The majority of ACL-reconstructed knees are clinically stable, regardless of surgical technique. Differences in functional stability have been identified between ACL-reconstructed and contralateral knees, but these differences are subtle and require sensitive outcome measures to detect. Rotational instability has been discussed as a potentially important indicator of surgical outcome, though there are no standardized approaches for assessing rotational laxity. A measurement of tibial rotation in response to an applied torque was insufficient to detect the difference between single- and double-bundle reconstructions,[53,63] whereas the dynamic evaluation of the clinically performed pivot shift test found that double-bundle reconstruction led to improved rotational stability.[39,64,65] Since simple load-displacement types of laxity measurement appear to be relatively insensitive to surgical procedure and do not reliably correlate with clinical outcomes, tests that better replicate functional knee loading are necessary to evaluate knee function after reconstruction. Previous in vivo dynamic studies demonstrated residual rotational instability in ACL reconstructed patients with normal anterior stability but non-anatomically placed grafts.[41,45,66] Similar studies are underway and required to evaluate the potential benefits of anatomical reconstruction techniques.
Conclusion
Concerns about the high rates of OA after ACL reconstruction, along with recent evidence of inadequate restoration of joint kinematics in reconstructed knees, have lead to a reevaluation of surgical procedures with the goals of better restoring both native ACL anatomy and dynamic knee function. While consensus is growing concerning the benefits of more anatomical graft placement, the merits of double-bundle reconstruction for further improving knee function have yet to be firmly established. Well-designed studies, using state-of-the art tools to assess knee kinematics under in vivo, dynamic, high-loading conditions, are necessary to evaluate the relative performance of different procedures for restoring normal joint motion. Additionally, long-term studies are required to establish whether better restoration of knee kinematics is associated with superior clinical results and reduced incidence of OA after ACL injury.
Key Points [DE PLEASE HAVE AUTHOR REVISE THESE...].
Current treatment approaches are moderately successful for restoring function, with most individuals able to return to their pre-injury level of sports activity
While there are many factors that may contribute to joint degeneration, persistent abnormal knee mechanics are often implicated in the initiation and progression of osteoarthritis (OA) in the ACL-injured/reconstructed knee
Concerns about the high rates of OA are largely responsible for the increased interest over the last several years on more anatomical approaches to ACL repair and reconstruction.
Figure 5.
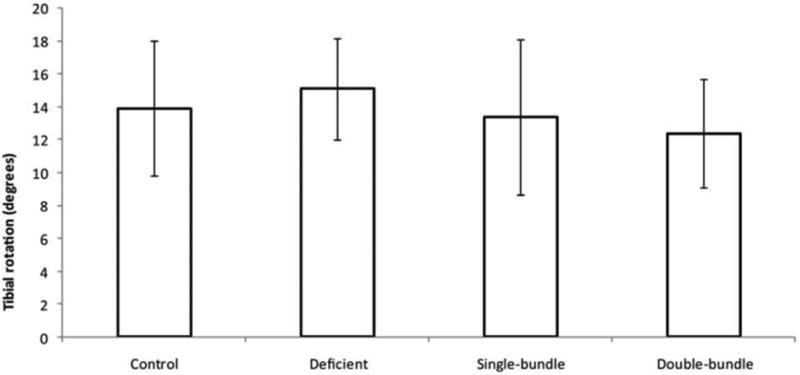
Tibial rotation (mean±SD) of examined groups. There was no significant difference in tibial rotation either between the 4 groups or between sides. The mean knee rotation for the single- and double-bundle groups was lower than the control group. (Reprinted with permission from Tsarouhas, A., M. Iosifidis, D. Kotzamitelos, et al.: Three-Dimensional Kinematic and Kinetic Analysis of Knee Rotational Stability after Single- and Double-Bundle Anterior Cruciate Ligament Reconstruction. Arthroscopy. 26(7): 885-893 (2010).)
Acknowledgments
Funding sources:
Dr. Tashman: NIH, Arthritis Foundation
Dr. Araki: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Conflict of Interest:
Dr. Tashman: None.
Dr. Araki: None
References
- 1.Biau DJ, Tournoux C, Katsahian S, et al. Acl Reconstruction: A Meta-Analysis of Functional Scores. Clin Orthop Relat Res. 2007;458:180–187. doi: 10.1097/BLO.0b013e31803dcd6b. [DOI] [PubMed] [Google Scholar]
- 2.Freedman KB, D'Amato MJ, Nedeff DD, et al. Arthroscopic Anterior Cruciate Ligament Reconstruction: A Metaanalysis Comparing Patellar Tendon and Hamstring Tendon Autografts. Am J Sports Med. 2003;31(1):2–11. doi: 10.1177/03635465030310011501. [DOI] [PubMed] [Google Scholar]
- 3.Daniel DM, Stone ML, Dobson BE, et al. Fate of the Acl-Injured Patient: A Prospective Outcome Study. Am. J. Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 4.Kessler MA, Behrend H, Henz S, et al. Function, Osteoarthritis and Activity after Acl-Rupture: 11 Years Follow-up Results of Conservative Versus Reconstructive Treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Ostenberg A, Englund M, et al. High Prevalence of Knee Osteoarthritis, Pain, and Functional Limitations in Female Soccer Players Twelve Years after Anterior Cruciate Ligament Injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 6.Andriacchi TP, Mundermann A, Smith RL, et al. A Framework for the in Vivo Pathomechanics of Osteoarthritis at the Knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 7.Tochigi Y, Vaseenon T, Heiner AD, et al. Instability Dependency of Osteoarthritis Development in a Rabbit Model of Graded Anterior Cruciate Ligament Transection. J Bone Joint Surg AM. 2011;93(7):640–647. doi: 10.2106/JBJS.J.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye SF. The Knee as a Biologic Transmission with an Envelope of Function: A Theory. Clin Orthop. 1996;(325):10–18. doi: 10.1097/00003086-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Musahl V, Hoshino Y, Becker R, et al. Rotatory Knee Laxity and the Pivot Shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):601–602. doi: 10.1007/s00167-011-1844-y. [DOI] [PubMed] [Google Scholar]
- 10.Snyder-Mackler L, Fitzgerald GK, Bartolozzi AR, et al. The Relationship between Passive Joint Laxity and Functional Outcome after Anterior Cruciate Ligament Injury. Am J Sports Med. 1997;25(2):191–195. doi: 10.1177/036354659702500209. [DOI] [PubMed] [Google Scholar]
- 11.Harter RA, Osternig LR, Singer KM. Long-Term Evaluation of Knee Stability and Function Following Surgical Reconstruction for Anterior Cruciate Ligament Insufficiency. Am J Sports Med. 1988;16:434–443. doi: 10.1177/036354658801600502. [DOI] [PubMed] [Google Scholar]
- 12.Barber SD, Noyes FR, Mangine RE, et al. Quantitative Assessment of Functional Limitations in Normal and Anterior Cruciate Ligament-Deficient Knees. Clin Orthop Rel Research. 1990;255:204–214. [PubMed] [Google Scholar]
- 13.Seto JL, Orofino AS, Morrissey MC. Assessment of Quadriceps/Hamstring Strength, Knee Ligament Stability, Functional and Sports Activity Levels Five Years after Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 1988;16:170–180. doi: 10.1177/036354658801600215. [DOI] [PubMed] [Google Scholar]
- 14.Cross MJ, Wootton JR, Bokor DJ. Acute Repair of Injury to the Anterior Cruciate Ligament: A Long-Term Followup. Am J Sports Med. 1993;21:128–131. doi: 10.1177/036354659302100121. [DOI] [PubMed] [Google Scholar]
- 15.Andriacchi TP, Dyrby CO. Interactions between Kinematics and Loading During Walking for the Normal and Acl Deficient Knee. Journal of Biomechanics. 2005;38(2):293–298. doi: 10.1016/j.jbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Moro-oka TA, Hamai S, Miura H, et al. Dynamic Activity Dependence of in Vivo Normal Knee Kinematics. J Orthop Res. 2008;26(4):428–434. doi: 10.1002/jor.20488. [DOI] [PubMed] [Google Scholar]
- 17.van Dommelen JA, Jolandan MM, Ivarsson BJ, et al. Nonlinear Viscoelastic Behavior of Human Knee Ligaments Subjected to Complex Loading Histories. Ann Biomed Eng. 2006;34(6):1008–1018. doi: 10.1007/s10439-006-9100-1. [DOI] [PubMed] [Google Scholar]
- 18.Dortmans L, Jans H, Sauren A, et al. Nonlinear Dynamic Behavior of the Human Knee Joint--Part Ii: Time-Domain Analyses: Effects of Structural Damage in Postmortem Experiments. J Biomech Eng. 1991;113(4):392–396. doi: 10.1115/1.2895417. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini A, Gill TJ, Li G. In Vivo Anterior Cruciate Ligament Elongation in Response to Axial Tibial Loads. J Orthop Sci. 2009;14(3):298–306. doi: 10.1007/s00776-009-1325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu JL, Hosseini A, Kozanek M, et al. Kinematics of the Anterior Cruciate Ligament During Gait. Am J Sports Med. 2010;38(7):1475–1482. doi: 10.1177/0363546510364240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beynnon BD, Fleming BC. Anterior Cruciate Ligament Strain in-Vivo: A Review of Previous Work. Journal of Biomechanics. 1998;31(6):519–525. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 22.Gao B, Zheng NN. Investigation of Soft Tissue Movement During Level Walking: Translations and Rotations of Skin Markers. J Biomech. 2008;41(15):3189–3195. doi: 10.1016/j.jbiomech.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Holden JP, Orsini JA, Siegel KL, et al. Surface Movement Errors in Shank Kinematics and Knee Kinetics During Gait. Gait & Posture. 1997;5:217–227. [Google Scholar]
- 24.Manal K, Davis IM, Galinat B, et al. The Accuracy of Estimating Proximal Tibial Translation During Natural Cadence Walking: Bone Vs. Skin Mounted Targets. Clinical Biomechanics. 2003;18(2):126–131. doi: 10.1016/s0268-0033(02)00176-6. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Van de Velde SK, Bingham JT. Validation of a Non-Invasive Fluoroscopic Imaging Technique for the Measurement of Dynamic Knee Joint Motion. J Biomech. 2008;41(7):1616–1622. doi: 10.1016/j.jbiomech.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Tashman S. Comments on “Validation of a Non-Invasive Fluoroscopic Imaging Technique for the Measurement of Dynamic Knee Joint Motion”. J Biomech. 2008;41(15):3290–3291. doi: 10.1016/j.jbiomech.2008.07.038. author reply 3292-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tashman S, Bey M, Anderst WJ, et al. Model-Based Tracking of Knee Kinematics from Biplane Radiographs: In-Vivo Validation. Orthopaedic Research Society; Chicago, IL: 2006. [Google Scholar]
- 28.Anderst W, Zauel R, Bishop J, et al. Validation of Three-Dimensional Model-Based Tibio-Femoral Tracking During Running. Med Eng Phys. 2009;31(1):10–16. doi: 10.1016/j.medengphy.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miranda DL, Schwartz JB, Loomis AC, et al. Static and Dynamic Error of a Biplanar Videoradiography System Using Marker-Based and Markerless Tracking Techniques. J Biomech Eng. 2011;133(12):121002. doi: 10.1115/1.4005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girgis FG, Marshall JL, Monajem A. The Cruciate Ligaments of the Knee Joint. Anatomical, Functional and Experimental Analysis. Clin Orthop Relat Res. 1975;(106):216–231. doi: 10.1097/00003086-197501000-00033. [DOI] [PubMed] [Google Scholar]
- 31.Odensten M, Hamberg P, Nordin M, et al. Surgical or Conservative Treatment of the Acutely Torn Anterior Cruciate Ligament. Clin. Orthop. Rel. Res. 1985;198:87–93. [PubMed] [Google Scholar]
- 32.Chhabra A, Starman JS, Ferretti M, et al. Anatomic, Radiographic, Biomechanical, and Kinematic Evaluation of the Anterior Cruciate Ligament and Its Two Functional Bundles. J Bone Joint Surg Am. 2006;88(Suppl 4):2–10. doi: 10.2106/JBJS.F.00616. [DOI] [PubMed] [Google Scholar]
- 33.Ferretti A, Monaco E, Labianca L, et al. Double Bundle or Single Bundle Plus Extra-Articular Tenodesis in Acl Reconstruction? A Caos Study. Knee Surg Sports Traumatol Arthrosc. 2008;16(1):98. doi: 10.1007/s00167-007-0446-1. [DOI] [PubMed] [Google Scholar]
- 34.Gabriel MT, Wong EK, Woo SL, et al. Distribution of in Situ Forces in the Anterior Cruciate Ligament in Response to Rotatory Loads. J Orthop Res. 2004;22(1):85–89. doi: 10.1016/S0736-0266(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 35.Tischer T, Ronga M, Tsai A, et al. Biomechanics of the Goat Three Bundle Anterior Cruciate Ligament. Knee Surg Sports Traumatol Arthrosc. 2009;17(8):935–940. doi: 10.1007/s00167-009-0784-2. [DOI] [PubMed] [Google Scholar]
- 36.Abebe ES, Moorman CT, 3rd, Dziedzic TS, et al. Femoral Tunnel Placement During Anterior Cruciate Ligament Reconstruction: An in Vivo Imaging Analysis Comparing Transtibial and 2-Incision Tibial Tunnel-Independent Techniques. Am J Sports Med. 2009;37(10):1904–1911. doi: 10.1177/0363546509340768. [DOI] [PubMed] [Google Scholar]
- 37.Kopf S, Forsythe B, Wong AK, et al. Nonanatomic Tunnel Position in Traditional Transtibial Single-Bundle Anterior Cruciate Ligament Reconstruction Evaluated by Three-Dimensional Computed Tomography. J Bone Joint Surg AM. 2010;92(6):1427–1431. doi: 10.2106/JBJS.I.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araki D, Kuroda R, Kubo S, et al. A Prospective Randomised Study of Anatomical Single-Bundle Versus Double-Bundle Anterior Cruciate Ligament Reconstruction: Quantitative Evaluation Using an Electromagnetic Measurement System. Int Orthop. 2011;35(3):439–446. doi: 10.1007/s00264-010-1110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yagi M, Kuroda R, Nagamune K, et al. Double-Bundle Acl Reconstruction Can Improve Rotational Stability. Clin Orthop Relat Res. 2007;454:100–107. doi: 10.1097/BLO.0b013e31802ba45c. [DOI] [PubMed] [Google Scholar]
- 40.Logan M, Dunstan E, Robinson J, et al. Tibiofemoral Kinematics of the Anterior Cruciate Ligament (Acl)-Deficient Weightbearing, Living Knee Employing Vertical Access Open “Interventional” Multiple Resonance Imaging. Am J Sports Med. 2004;32(3):720–726. doi: 10.1177/0095399703258771. [DOI] [PubMed] [Google Scholar]
- 41.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, et al. Three-Dimensional Tibiofemoral Kinematics of the Anterior Cruciate Ligament-Deficient and Reconstructed Knee During Walking. Am J Sports Med. 2003;31(1):75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 42.Ristanis S, Giakas G, Papageorgiou CD, et al. The Effects of Anterior Cruciate Ligament Reconstruction on Tibial Rotation During Pivoting after Descending Stairs. Knee Surg Sports Traumatol Arthrosc. 2003;11(6):360–365. doi: 10.1007/s00167-003-0428-x. [DOI] [PubMed] [Google Scholar]
- 43.Brandsson S, Karlsson J, Sward L, et al. Kinematics and Laxity of the Knee Joint after Anterior Cruciate Ligament Reconstruction: Pre- and Postoperative Radiostereometric Studies. Am J Sports Med. 2002;30(3):361–367. doi: 10.1177/03635465020300031001. [DOI] [PubMed] [Google Scholar]
- 44.Papannagari R, Gill TJ, Defrate LE, et al. In Vivo Kinematics of the Knee after Anterior Cruciate Ligament Reconstruction: A Clinical and Functional Evaluation. Am J Sports Med. 2006 doi: 10.1177/0363546506290403. [DOI] [PubMed] [Google Scholar]
- 45.Tashman S. Abnormal Rotational Knee Motion During Running after Anterior Cruciate Ligament Reconstruction. American Journal of Sports Medicine. 2004;32(4):975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 46.Tashman S, Kolowich P, Collon D, et al. Dynamic Function of the Acl-Reconstructed Knee During Running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 47.Mae T, Shino K, Miyama T, et al. Single- Versus Two-Femoral Socket Anterior Cruciate Ligament Reconstruction Technique: Biomechanical Analysis Using a Robotic Simulator. Arthroscopy. 2001;17(7):708–716. doi: 10.1053/jars.2001.25250. [DOI] [PubMed] [Google Scholar]
- 48.Yagi M, Wong EK, Kanamori A, et al. Biomechanical Analysis of an Anatomic Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2002;30(5):660–666. doi: 10.1177/03635465020300050501. [DOI] [PubMed] [Google Scholar]
- 49.Hamada M, Shino K, Horibe S, et al. Single- Versus Bi-Socket Anterior Cruciate Ligament Reconstruction Using Autogenous Multiple-Stranded Hamstring Tendons with Endobutton Femoral Fixation: A Prospective Study. Arthroscopy. 2001;17(8):801–807. doi: 10.1016/s0749-8063(01)90002-7. [DOI] [PubMed] [Google Scholar]
- 50.Adachi N, Ochi M, Uchio Y, et al. Reconstruction of the Anterior Cruciate Ligament. Single- Versus Double-Bundle Multistranded Hamstring Tendons. J Bone Joint Surg Br. 2004;86(4):515–520. [PubMed] [Google Scholar]
- 51.Yasuda K, Kondo E, Ichiyama H, et al. Clinical Evaluation of Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction Procedure Using Hamstring Tendon Grafts: Comparisons among 3 Different Procedures. Arthroscopy. 2006;22(3):240–251. doi: 10.1016/j.arthro.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto Y, Hsu WH, Woo SL, et al. Knee Stability and Graft Function after Anterior Cruciate Ligament Reconstruction: A Comparison of a Lateral and an Anatomical Femoral Tunnel Placement. Am J Sports Med. 2004;32(8):1825–1832. doi: 10.1177/0363546504263947. [DOI] [PubMed] [Google Scholar]
- 53.Ho JY, Gardiner A, Shah V, et al. Equal Kinematics between Central Anatomic Single-Bundle and Double-Bundle Anterior Cruciate Ligament Reconstructions. Arthroscopy. 2009;25(5):464–472. doi: 10.1016/j.arthro.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Irrgang JJ, Bost JE, Fu FH. Re: Outcome of Single-Bundle Versus Double-Bundle Reconstruction of the Anterior Cruciate Ligament: A Meta-Analysis. Am J Sports Med. 2009;37(2):421–422. doi: 10.1177/0363546508327555. author reply 422. [DOI] [PubMed] [Google Scholar]
- 55.Meredick RB, Vance KJ, Appleby D, et al. Outcome of Single-Bundle Versus Double-Bundle Reconstruction of the Anterior Cruciate Ligament: A Meta-Analysis. Am J Sports Med. 2008;36(7):1414–1421. doi: 10.1177/0363546508317964. [DOI] [PubMed] [Google Scholar]
- 56.Abebe ES, Utturkar GM, Taylor DC, et al. The Effects of Femoral Graft Placement on in Vivo Knee Kinematics after Anterior Cruciate Ligament Reconstruction. J Biomech. 2011;44(5):924–929. doi: 10.1016/j.jbiomech.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Claes S, Neven E, Callewaert B, et al. Tibial Rotation in Single- and Double-Bundle Acl Reconstruction: A Kinematic 3-D in Vivo Analysis. Knee Surg Sports Traumatol Arthrosc. 2011;19(Suppl 1):S115–121. doi: 10.1007/s00167-011-1568-z. [DOI] [PubMed] [Google Scholar]
- 58.Ristanis S, Stergiou N, Siarava E, et al. Effect of Femoral Tunnel Placement for Reconstruction of the Anterior Cruciate Ligament on Tibial Rotation. The Journal of Bone and Joint Surgery. 2009;91(9):2151–2158. doi: 10.2106/JBJS.H.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsarouhas A, Iosifidis M, Kotzamitelos D, et al. Three-Dimensional Kinematic and Kinetic Analysis of Knee Rotational Stability after Single- and Double-Bundle Anterior Cruciate Ligament Reconstruction. Arthroscopy. 2010;26(7):885–893. doi: 10.1016/j.arthro.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Misonoo G, Kanamori A, Ida H, et al. Evaluation of Tibial Rotational Stability of Single-Bundle Vs. Anatomical Double-Bundle Anterior Cruciate Ligament Reconstruction During a High-Demand Activity - a Quasi-Randomized Trial. Knee. 2012;19(2):87–93. doi: 10.1016/j.knee.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Pappas E, Zampeli F, Xergia SA, et al. Lessons Learned from the Last 20 Years of Acl-Related in Vivo-Biomechanics Research of the Knee Joint. Knee Surg Sports Traumatol Arthrosc. 2012 doi: 10.1007/s00167-012-1955-0. [DOI] [PubMed] [Google Scholar]
- 62.Garling EH, Kaptein BL, Mertens B, et al. Soft-Tissue Artefact Assessment During Step-up Using Fluoroscopy and Skin-Mounted Markers. J Biomech. 2007;40(Suppl 1):S18–24. doi: 10.1016/j.jbiomech.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Lorenz S, Tashman S, Fu FH. Failed Exploration of Rotational Instability in Single- and Double-Bundle Acl Reconstruction. Arthroscopy. 2009;25(9):949. doi: 10.1016/j.arthro.2009.06.019. author reply 949-950. [DOI] [PubMed] [Google Scholar]
- 64.Hoshino Y, Kuroda R, Nagamune K, et al. In Vivo Measurement of the Pivot-Shift Test in the Anterior Cruciate Ligament-Deficient Knee Using an Electromagnetic Device. Am J Sports Med. 2007;35(7):1098–1104. doi: 10.1177/0363546507299447. [DOI] [PubMed] [Google Scholar]
- 65.Araki D, Kuroda R, Kubo S, et al. The Use of an Electromagnetic Measurement System for Anterior Tibial Displacement During the Lachman Test. Arthroscopy. 2011;27(6):792–802. doi: 10.1016/j.arthro.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 66.Georgoulis AD, Ristanis S, Chouliaras V, et al. Tibial Rotation Is Not Restored after Acl Reconstruction with a Hamstring Graft. Clin Orthop Relat Res. 2007;454:89–94. doi: 10.1097/BLO.0b013e31802b4a0a. [DOI] [PubMed] [Google Scholar]