Abstract
Recent reports have suggested that SDF (Stromal cell-derived factor)-1α- CXCR4 axis has a direct effect on stem and progenitor cell recruitment in muscle and neural tissue repair after injury. No information is available about SDF-1α or CXCR4 in dental tissues. The aim of this study was to assess the expression of SDF-1α and its receptor, CXCR4, in healthy or inflamed human dental pulp and to evaluate the effects of SDF-1α on dental pulp cells (DPCs) in both proliferation and migration in vitro. Immunohistochemical staining and RT-PCR detected weak expression of SDF-1α and CXCR4 in healthy dental pulp and strong expression of SDF-1a and CXCR4 in inflamed dental pulp. An MTT assay demonstrated that SDF-1α could not promote DPCs proliferation. A transmigration assay, however, indicated that SDF-1α enhanced DPCs migration, and which could be abolished by anti-CXCR4 antibodies. Taken together, these results imply that the SDF-1α-CXCR4 axis may play a role in the recruitment of CXCR4-positive DPCs toward the damaged sites
INTRODUCTION
Reparative dentinogenesis occurs after dental injury (for example, caused by deep cavity preparation) leads to odontoblast death (1). The formation of reparative dentin results from the recruitment and proliferation of pulp stem cells (2). Increasing evidences suggest the presence of resting progenitor or stem cells in the dental pulp (2, 3). Under physiological conditions, these cells are localized to a perivascular area (4). Under pathological conditions, however, they are attracted to an injury site and differentiate into a second-generation of odontoblasts or odontoblast-like cells (5, 6). Migration of these cells toward the site of injury is a critical step in the pulp healing. However, little has been known about the underlying molecular mechanisms of these cells in that process. Signaling molecules expressed by dental pulp cells after injury could play a role in the recruitment of these cells (7).
Recently, much attention has been paid to SDF-1α and its unique receptor CXCR4 for the migration of adult stem cells to the site of injury, which contributes to the impaired tissue repair and regeneration (8). SDF-1α is a small (8–13 kDa) secreted chemokine protein. In many damaged organs, it is up-regulated to attract CXCR4-positive stem cells which are mobilized from their niches in response to the stimulation related to tissue/organ damage (9). CXCR4 has been considered as a new surface marker expressed by stem cells (10). Besides haemopoietic stem cells (HSCs), CXCR4 has also been expressed by other tissue specific stem cells, such as skeletal muscle satellite cells, which are responsible for the migration of satellite cells toward an SDF-1 gradient (11).
However, SDF-1α and CXCR4 expression is currently unknown in dental pulp. We hypothesize that SDF-1α-CXCR4 axis may play a crucial role in recruiting stem cells or progenitor cells to the injured site and results in reparative dentin formation. In the present study, the SDF-1α/CXCR4 expression in healthy and inflamed pulp was examined. And the role of SDF-1α-CXCR4 axis in DPCs proliferation and migration in vitro was investigated. This is the first report regarding the expression and the roles of SDF-1α and CXCR4 in dental tissues.
MATERIALS & METHODS
Preparation of Tissue Sectioned Specimens
The healthy teeth were obtained from the extracted third molars or premolars for orthodontic reasons of 19 adults aged from 18 to 29 years old. An informed consent was obtained from each patient before the extractions. The Ninth People’s Hospital Ethics Committee approved the procedure and the research protocol for tooth extractions.
Whole tooth specimens for demineraliation
Four healthy teeth were demineralized in solution of 3.4% sodium formate/17% formic acid for 21 days after the fixation in 4% of paraformaldahyde for 48 hours at 4°C. Then the teeth were processed through a series of graded alcohols and xylene, and finally embedded in paraffin wax.
Pulp specimens from healthy and inflamed teeth
Healthy pulps were obtained from the health teeth, which were slit immediately after the extraction. Inflamed pulps were extracted before the root canal therapy from patients diagnosed with irreversible pulpitis. Diagnosis was made on the following criteria: the teeth with spontaneous pain or prolonged pain to thermal stimuli, without sensitivity to percussion, and radiographic examination showing absence of periapical lesions(12). Eleven healthy and eight inflamed pulpal tissues were sent to Department of Oral Pathology for frozen sections within 20 minutes. Three healthy and three inflamed pulpal tissues for RT-PCR were soaked in sample protector (Takara, JAP) at −40°C.
Culture of Dental Pulp Cells
Immediately after the extraction, the third molar was soaked in prechill phosphate-buffered saline (PBS, 0.01 M, pH 7.4) solution at 4°C. Under sterile conditions, a tooth was washed with sterile PBS and cut around the cementum-enamel junction using sterilized dental fissure burs to reveal the pulp chamber. The pulp tissue was gently separated from the crown and the root and cut into several pieces until its size was not more than 1mm3. These tissue fragments were covered by plastic coverslips (Thermanox Coverslip, NUNC, USA), which were adhered on base wall of culture dish with sterile vaseline, and incubated with medium in 100-mm-diameter culture dishes at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. The culture medium was DMEM (Gibco, USA) supplemented with 2mM glutamine (Sigma, USA), 100IU/ml penicillin-100g/ml streptomycin (Gibco, USA), 0.25g/ml amphotericin B (Fungizone, Gibco, USA), and 20% fetal bovine serum (FBS, Gibco, USA). The culture medium was changed at 5-day intervals. Confluent cultures were collected by trypsinization (0.2% trypsin and 0.02% EDTA, Gibco, USA) and subcultured with DMEM plus 10% FBS, hereinafter referred to as the complete medium (CM).
Immunohistochemistry
Secondary passage DPCs grown on coverslips
Secondary passage of DPCs was seeded on the common coverslips and cultured with CM. DPCs were fixed with 4% paraformaldahyde for 20 minutes when they grew to 60% confluent. Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide for 20 minutes at the room temperature and washed with PBS. The antibodies used in this study included mouse monoclonal antibody against human SDF-1 or CXCR4 (R&D, USA). Primary antibodies were diluted in PBS containing 0.1% bovine serum albumin (BSA, Sigma, USA). The incubation with the primary antibodies at a concentration of 10µg/ml was performed for 3 hours at the room temperature. The immunostaining was revealed using the EnVision™ Detection kit (Dako, Denmark) according to the manufacturer’s instructions. The primary antibodies were replaced with PBS for the negative controls. It has been reported that the squamous cell carcinoma of tongue expressed CXCR4 (13). So we utilized squamous cell carcinoma of tongue Tca-8113 cell line (a human squamous cell carcinoma cell line, was established in Ninth People’s Hospital, School of Medicine, Shanghai Jiao Tong University) as the positive control.
Frozen sections from the healthy and inflamed pulpal tissues
Pulpal tissues were immediately embedded in the O.C.T. compound (Miles, USA). The specimens were cut into 6µm sections using a cryostat (Shandon, Germany) and collected on poly L-lysine-coated slides (14). After the sections were fixed by pre-chill 95% acetone for 10 minutes, a similar procedure to the previous one was used for the incubation with the primary antibodies and the immunostaining.
Paraffin sections of the pulpal tissues from demineralized healthy teeth
Six µm paraffin sections were dewaxed and microwave treated at 500W for 5 minutes in 10 mM sodium citrate (pH 6.0). The rest of the steps were performed as the description in immunohistochemistry of DPCs grown on coverslips.
RT-PCR Detect mRNA of SDF-1α and CXCR4
Total RNA was extracted from tissues using the Trizol (Invitrogen, USA). The cDNA was synthesized using the RT Reagent Kit (Takara, JAP) and the Ex Taq HS (Takara, JAP) were used for amplification according to the manufacture’s instructions. The SDF-1α primer sequences were 5’-GCATTGACCCGAAGCTAAAG-3’ (forward) and 5’-ATGGCAAAGTGTCAAAACA-3’ (reverse) and the product size is 209bp. The CXCR4 primer sequences were 5’-CCGTGGCAAACTGGTACTTT-3’ (forward) and 5’-GACGCCAACATAGACCACCT-3’(reverse) and the product size is 188bp. RT-PCR amplification was performed under the following conditions: 95°C for 5min; 35 cycles at 95°C for 40sec, 60°C for 40sec, and 72°C for 40sec. The β-actin(307bp) was amplified under the same conditions as a control (5’-CCTGTGGCATCCACGAAACT-3’ forward, and 5’-GAAGCATTTGCGGTGGACGA-3’reverse).
MTT Assay for Cell Proliferation
DPCs proliferation was evaluated using methylthiazol tetrazolium (MTT, Amresco, USA) assay, as described previously by Guigand et al., with modifications (15). DPCs from the secondary passage were seeded at a concentration of 6×103/well in a 96-well plate in CM. Cells were divided into 5 groups: the control group and four SDF-1α (R&D, USA) treated groups (i.e., 25, 50, 100, 200 ng/ml). After 24 hour culturing, the cells were replaced with 200µl of fresh CM with the indicated concentrations of SDF-1α, and the control group was given CM only without SDF-1α. After the inoculation for 2, 4, 6, 8, and 10 days, respectively, 20µl of MTT was added into each well. Four hours later, the medium with MTT was removed and replaced by 150µl of dimethyl sulfoxide (DMSO, Sigma, USA) to dissolve the formazan crystals by gentle shaking of the plate. The optical density (OD) was detected with an ELISE microplate reader (Labsystems, Finland) at 490 nm.
Transmigration Assay
Transwell inserts (5-µm pore, Corning, USA) in 24-well plates were coated with fibronectin (Chemicon, USA). DPCs (1×105 cells/well) were seeded into the upper chamber, and 600µl CM with various concentrations (50, 100, 200 and 400 ng/ml) of SDF-1α was added to the lower chamber. Some of the migration experiments with 100ng/ml of SDF-1α were performed using DPCs preincubated with anti-CXCR4 (10µg/ml) for 30 minutes at 37°C. The CM without SDF-1α was used as a negative control. Incubation was stopped after 48 hours, and cells adhering on the polycarbonate membrane of transwell inserts were immediately fixed by 4% paraformaldahyde for 20 minutes. Upper cells were scraped off carefully with cotton wool, and the relative number of transmigrated cells was measured with a modified crystal violet staining method (16). After cells were incubated with 0.1% crystal violet at the room temperature for 10 minutes, the lower side of the membrane was rinsed with distilled water and air-dried. Then, cells were eluted in 10% acetic acid and the OD of the final eluate was determined at 630 nm, using 10% acetic acid as the blank. By analyzing the OD630 of the final elute, we are able to determine the cell migration extent.
Statistical Analysis
All data were presented as mean ± SD. Data analysis was performed using SAS6.12. Statistical significance between groups was determined by using one way ANOVA. A P-value of less than 0.05 (P<0.05) was considered significant.
RESULTS
Immunohistochemical localization of SDF-1α and CXCR4
DPCs from secondary passage grown on coverslips
Using anti-SDF-1α monoclonal antibodies, SDF-1α was not detected in the secondary passage DPCs (Fig. 1A). Using anti-CXCR4 monoclonal antibodies, little DPCs were positive staining (Fig. 1B). All Tca8113 cells expressed CXCR4 (Fig. 1C). All the cells in negative contol had no staining.
Figure 1. Immunostaining of SDF-1α/CXCR4 in DPCs and human dental pulp.
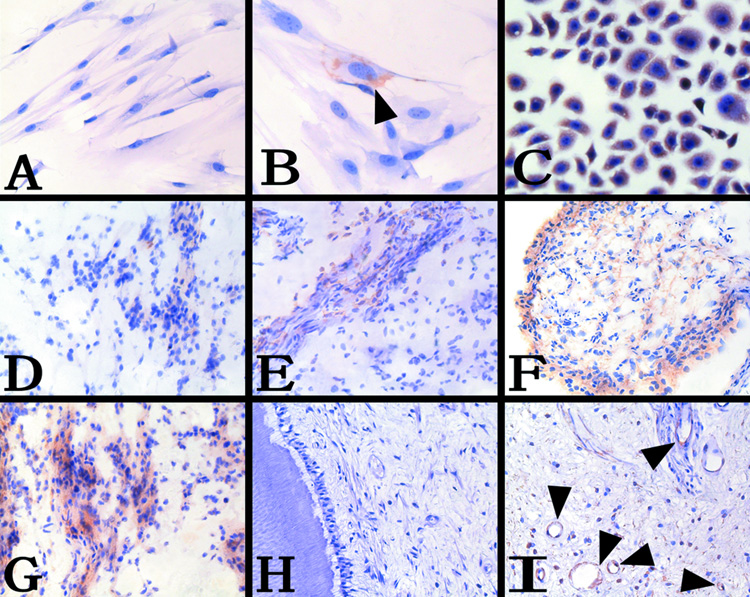
A, DPCs were stained with anti-SDF-1α MoAb, no staining was observed, 100x. B, DPCs were stained with anti-CXCR4 MoAb, CXCR4-positive DPCs (arrowheads), 400x. C, Tca8113 cell line were stained with anti-CXCR4 MoAb as positive control, 200x. D, Weak expression of SDF-1α in the frozen section of health pulpal tissue, 100x. E, Weak expression of CXCR4 in the frozen section of health pulpal tissue, 100x. F, Intense expression of SDF-1α in the outermost layer of the dental pulp in the frozen section of inflamed pulpal tissue, 100x. G, CXCR4-positive DPCs were detected in the dental pulp in the frozen section of inflamed pulpal tissue, 100x. H, No obvious expression of SDF-1α in the paraffin section of health pulpal tissue, 100x. I, Immunolocalization of the CXCR4-positive DPCs to blood vessel walls in the paraffin section of health pulpal tissue (arrowheads), 200x.
Frozen sections from the healthy and inflamed pulpal tissues
Immunohistochemical analyses with anti-SDF-1α antibodies and anti-CXCR4 antibodies respectively, showed that SDF-1α and CXCR4 were weakly expressed in the health pulp (Fig. 1D, E). In contrast, SDF-1α and CXCR4 were intensively expressed in the inflamed pulp (Fig. 1F, G). Either healthy or inflamed dental pulp was not stained in negative control.
Paraffin section of the pulpal tissues from the demineralized healthy teeth
In the paraffin sections of the pulpal tissues from the demineralized healthy teeth, no SDF-1α staining was detected with anti-SDF-1α antibodies (Fig. 1H). Using anti-CXCR4 antibodies, positive staining was detected predominantly in DPCs adjacent to the blood vessel walls in the health pulp tissues (Fig. 1I). The negative control was not stained, and the image was similar to fig. 1H
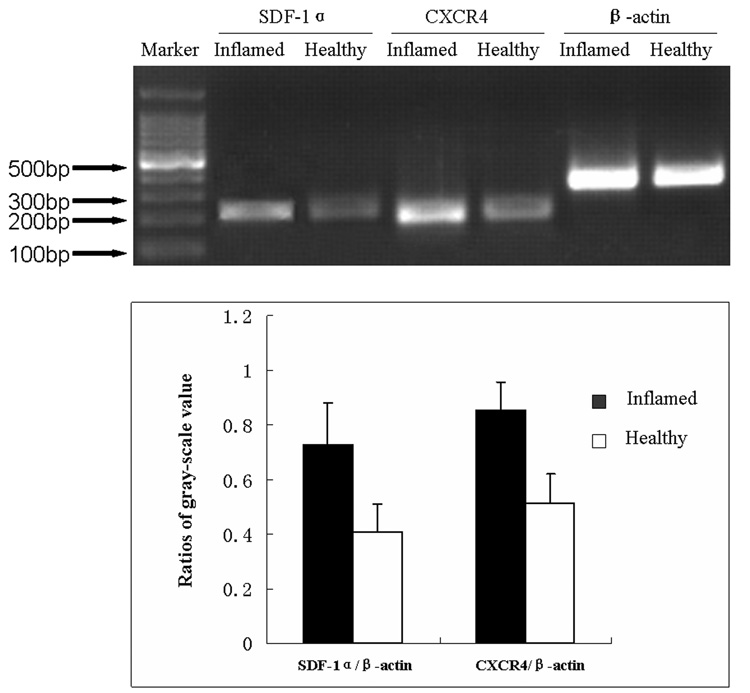
The mRNA of SDF-1α and CXCR4 Expressed in Healthy and Inflamed Pulps
The mRNA of SDF-1α and CXCR4 in healthy and inflamed pulps was detected by RT-PCR. In inflamed pulp tissue, the product of SDF-1α and CXCR4 was more manifest (Fig. 2).
Figure 2. The mRNA of SDF-1α and CXCR4 expression in healthy and inflamed dental pulp.
Expression of SDF1α, CXCR4 and β-actin from the same inflamed sample (Inflamed), and the same healthy dental pulp (Healthy). Ratios of data are mean ±SD. Experiment is repeated three times.
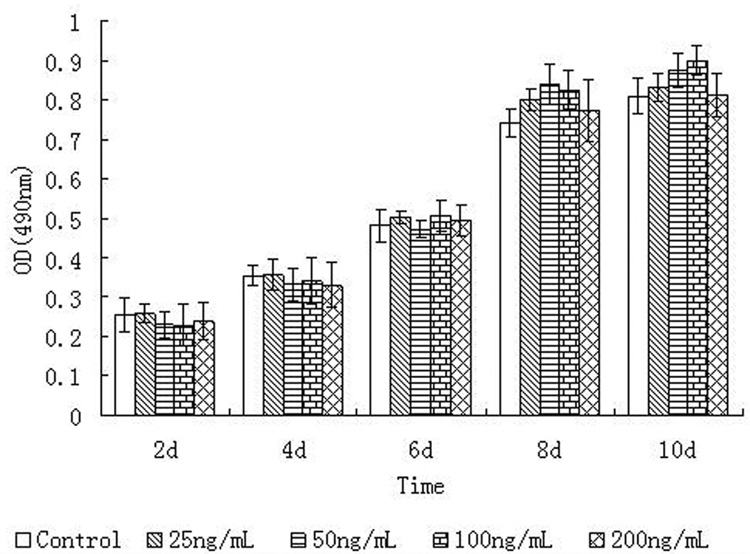
SDF-1α has no Effect on Cell Proliferation
Results from the MTT assays showed that the growing phase of the control cells (i.e., without adding SDF-1α) was from 4 day to 8 day. Between 8d and 10 d, the cell growth reached a plateau. At each time point, for example at the 6d time point, there was no statistical difference (P>0.05) in cell proliferation between the control and the various SDF-1α concentration groups (i.e., 25 ng/ml, 50 ng/ml, 100 ng/ml and 200 ng/ml). Similarly, at 4d, 8d and 10d, no difference in cell proliferation between the control and the SDF-1α treated groups as illustrated in Figure 3. In summary, SDF-1α did not affect the cell number and the cell viability.
Figure 3. Effects of different concentrations of SDF-1α on DPC proliferation.
The OD490 values represent the mean ±SD. At each time point, the proliferation values (OD490) of various groups have no significant difference.
SDF-1α Affects Cell Migration
Transmigration assays were performed on the 24-well plates to determine the effect of SDF-1α in the lower chamber on DPCs migration across the micropores. If SDF-1α is able to affect cell migration, we expect that in the presence of SDF-1α, DPCs would cross the 5 µm pores on the transwell insert, and migrate to the other side of the insert. Under the inverted phase contrast microscopy as shown in Figure 4 (the crystal violet staining of transmigrated cells), in SDF-1α treated groups, more DPCs (Fig. 4C, D) adhered on the lower side of the polycarbonate membrane, than those in the control group (Fig. 4A, B).
Figure 4. Transmigration of DPCs to the lower side of the transwell inserts.
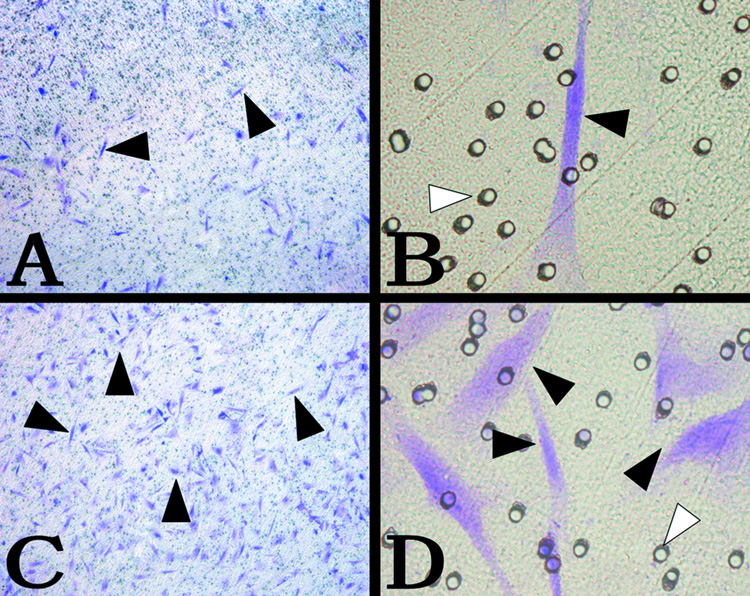
The cells were stained with crystal violet. A, B, DPCs transmigrated through the polycarbonate membrane in the control group. C, D, DPCs transmigrated through the polycarbonate membrane with 100ng/ml SDF-1α in the lower chamber. (A, C 50x, B, D 400x) (black arrow, adhered DPCs; white arrow, 5-µm pore).
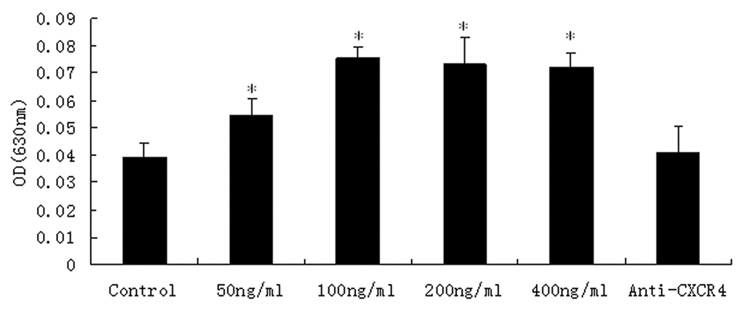
The transmigrated DPCs stained with crystal violet were then eluted with 10% acetic acid and the OD630 of the elutes from various SDF-1α treated groups were measured. Figure 4 showed the effects of different concentrations of SDF-1α on DPC migration. The OD630 values from various elutes were correlated to the number of transmigrate cells. As illustrated in Figure 4, the OD630 values from 50, 100, 200, 400 ng/ml SDF-1α groups were higher than that of the control group (P<0.05). In the 100ng/ml group, the quantity of the transmigrate cells reached its peak. The result of the crystal violet stain experiments demonstrated that SDF-1α at various concentrations in the lower chamber served as a chemoattraction factor to significantly increase the DPCs migration through the membrane micropores. And this chemoattraction effect could be abolished by anti-CXCR4 antibodies (Fig. 5).
Figure 5. Effects of different concentrations of SDF-1α on the migration of DPCs.
The data represent the mean ± SD. The OD of 50ng/ml SDF-1α was higher than the control group. In 100ng/ml, the OD reached the highest point. The OD of anti-CXCR4 group has not significant difference with the control. *P<0.05 as compared with the control group.
DISCUSSION
It was well established that chemokines and their receptors are responsible for the leukocyte movement and mediate inflammation (17). SDF-1 and its receptor CXCR4 were identified in monocytes, resting T cells and dendritic cells. Some chemokine receptors are also expressed on nonhematopoietic cells such as neurons, astrocytes, epithelial cells and endothelial cells. During organ developments, SDF-1α and CXCR4 show dynamic and complementary expression patterns in the developing neuronal, cardicac, vascular, hematopoietic and craniofacial systems (18). Furthermore, it was reported that muscle satellite cells expressed CXCR4 and muscle-derived fibroblasts secreted SDF-1 (11). SDF-1α and CXCR4 were expressed by human gingival fibroblasts in periodontal disease (14). Up to date, no information was available regarding SDF-1α and CXCR4 expression in dental pulp tissues and their roles in dental pulp injury.
This is the first report to establish the expression of SDF-1α and CXCR4 in dental pulp cells. As shown in Figure 1, we detected weak SDF-1α and CXCR4 expression in the frozen sections of healthy teeth, although no positive signal of SDF-1α in the paraffin section of healthy pulpal tissue was detected in our study. This could be due to the conservation of antigenicity in the frozen sections. Interestingly, SDF-1α and CXCR4 expression were up-regulated in the inflamed dental pulp. The explanation for these results is that the inflammation responding to injury could produce many factors, such as interleukin-1, tumor-necrosis factor-α, hypoxia-inducible factor-1, etc., which might stimulate the up-regulated expression of SDF-1α (19). And the increased numbers of CXCR4-Positive cells in the inflamed pulp could be due to chemotactic attraction towards the sources of the chemokine.
SDF-1 is also named “proliferation of B-cell progenitor stimulation factor” (PBSF), that belongs to the CXC group of chemokines. It stimulates proliferation of B-cell progenitors in vitro and is constitutively expressed in bone-marrowed drived stromal cells (20). Imitola reported that exposure of SDF-1α to quiescent neural stem cells (NSCs) enhances proliferation and promotes chain migration (9). In our study, SDF-1α was up-regulated in the inflamed dental pulp and intense CXCR4 expression was also detected in the inflamed dental pulp. Could the high number of CXCR4-Positive cells in the inflamed dental pulp be attributed to SDF-1α induced chemotactic migration of stem cells to the injured tissue? Or could it be attributed to the SDF-1α effect on the proliferation of CXCR4-Positive cells? Therefore MTT assays were performed to test the SDF-1α effect on DPC proliferation. Our MTT assays of DPCs showed that SDF-1α itself did not affect DPCs proliferation. Thus the increased CXCR4-Positive cells observed in the frozen section of the inflamed dental pulp is not likely caused by the increased level of SDF-1α expression at the injured site. SDF-1α was also reported to have no effect on osteoblast proliferation (21). Although SDF-1α was reported to promote B-cell and neural stem cell proliferation, SDF-1α did not affect the proliferation in dental pulp cells and osteoblast lineage cells.
The SDF-1α -CXCR4 axis has been postulated to be involved in chemoattracting CXCR4-Positive muscle stem/progenitor cells and CXCR4-Positive hematopoietic cells to both muscle and bone marrow tissue (11). Ceradini reported that the recruitment of CXCR4-Positive progenitor cells to regenerating tissues is mediated by hypoxic gradients via HIF-1 (hypoxia-inducible factor-1) induced SDF-1 (19). Neural stem cells were directed to sites of CNS (central nervous system) injury by the SDF-1α-CXCR4 pathway (9). Pivotal role of the SDF-1-CXCR4 axis was reported to be involved in normal hematopoietic stem cell trafficking/homing and retention in bone marrow as well as in nonhematopoietic stem/progenitor cell trafficking and homing (8). In our study, SDF-1α was found to significantly increase the DPCs transmigration. And this chemoattraction effect could be abolished by anti-CXCR4 antibodies. It was interesting that DPCs migration ability peaked at the 100ng/ml SDF-1α group. Even when the SDF-1α concentration was increased to 200 and 400 ng/ml, no further effect on cell migration was observed. The possible reason for this phenomenon is that the total number of the CXCR4-positive DPCs is very limited that not enough CXCR4-positive DPCs respond to SDF-1α signal. Our immunohistochemical staining proved that limited presence of CXCR4-Positive DPCs were in the dental pulp.
Dental pulp stem cells (DPSCs) reside in perivascular niche (4). It is generally believed that the injuries promote progenitor/stem cell proliferation at the injured tissues and CXCR4-Positive cells locally increase. Téclès’ study clearly demonstrated that the pulpal injury stimulated the proliferation of progenitor/stem cells. These proliferated progenitor/stem cells migrated to the pulpal injury site (22). In our study, the immunohistochemical staining demonstrated that CXCR4-positive DPCs were also localized to the perivascular area. So we consider that CXCR4 may be one of the surface markers of DPSCs.
Taken together, our data imply that the SDF-1α-CXCR4 axis may play a role in the recruitment of CXCR4-positive DPCs toward the damaged sites. We can hypothesize: during the pulp tissue injury, DPCs at the injured site are stimulated to actively synthesize SDF-1α., and the CXCR4-positive DPCs, which reside in the microvasculature of the pulp tissues, are induced to proliferate by inflammation mediators. Then the newly proliferated CXCR4-positive DPCs are subsequently chemoattracted to the damaged site with a high level of SDF1-α via the SDF-1α-CXCR4 axis and result in reparative dentin formation. Further studies are needed to isolate the CXCR4-positive DPCs and to understand the proliferation and differentiation of these CXCR-4 positive cells.
ACKNOWLEDGEMENTS
This work was completed in Shanghai Key Laboratory of Stomatology and Department of Oral Pathology, Ninth People’s Hospital, School of Medicine, Shanghai Jiao Tong University. We would like to thank Xiu-Li Zhang, Han-Bing Fu and Qing-Shen Hu for their excellent technical support. This work was supported by Shanghai rising-star program 01QB14025 (to Ya-Qin Zhu) and NIH DE11442 (to HHR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mitsiadis TA, Rahiotis C. Parallels between tooth development and repair: conserved molecular mechanisms following carious and dental injury. J Dent Res. 2004;83:896–902. doi: 10.1177/154405910408301202. [DOI] [PubMed] [Google Scholar]
- 2.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 5.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med. 2001;12:425–437. doi: 10.1177/10454411010120050501. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 8.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 9.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1 alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majka M, Kucia M, Ratajczak MZ. Stem cell biology - a never ending quest for understanding. Acta Biochim Pol. 2005;52:353–358. [PubMed] [Google Scholar]
- 11.Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 12.Pezelj-Ribaric S, Anic I, Brekalo I, Miletic I, Hasan M, Simunovic-Soskic M. Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps. Arch Med Res. 2002;33:482–484. doi: 10.1016/s0188-4409(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 13.Delilbasi CB, Okura M, Iida S, Kogo M. Investigation of CXCR4 in squamous cell carcinoma of the tongue. Oral Oncol. 2004;40:154–157. doi: 10.1016/s1368-8375(03)00144-1. [DOI] [PubMed] [Google Scholar]
- 14.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Murakami K, Miyake Y, et al. CXCL12 and CXCR4 expression by human gingival fibroblasts in periodontal disease. Clin Exp Immunol. 2005;141:467–474. doi: 10.1111/j.1365-2249.2005.02852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guigand M, Pellen-Mussi P, Le Goff A, Vulcain JM, Bonnaure-Mallet M. Evaluation of the cytocompatibility of three endodontic materials. J Endod. 1999;25:419–423. doi: 10.1016/s0099-2399(99)80270-2. [DOI] [PubMed] [Google Scholar]
- 16.Velázquez E, Ruiz-Albusac JM, Blázquez E. Glucagon-like peptide-2 stimulates the proliferation of cultured rat astrocytes. Eur J Biochem. 2003;270:3001–3009. doi: 10.1046/j.1432-1033.2003.03677.x. [DOI] [PubMed] [Google Scholar]
- 17.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 18.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 19.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 20.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 21.Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Téclès O, Laurent P, Zygouritsas S, Burger AS, Camps J, Dejou J, et al. Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol. 2005;50:103–108. doi: 10.1016/j.archoralbio.2004.11.009. [DOI] [PubMed] [Google Scholar]