Abstract
The molecular mechanism underlying channel opening in response to agonist binding remains a challenging issue in neuroscience. In this regard, many efforts have been recently undertaken in ATP-gated P2X receptors. Among those efforts, we have provided evidence in the P2X2 receptor that tightening of ATP sites upon agonist binding induces opening of the ion channel. Here we extend our analysis to show that the sulfhydryl-reactive ATP analog 8-thiocyano-ATP (NCS-ATP), a potent P2X2 agonist, when covalently labeled in the ATP-binding site at position Leu186 likely favors the tightening mechanism, but not the channel opening mechanism. Our data predict the existence of intermediate or preactivation state(s) trapped by NCS-ATP, in which tightening of the binding site is favored while the channel is still closed. We propose that this (these) intermediate ATP-bound state(s) prime(s) channel gating in the P2X2 receptor.
Keywords: purinergic receptor, gating mechanism, ligand-gated ion channel, neurotransmitter receptor, affinity labeling, zinc-binding sites
Introduction
P2X receptors are a family of ligand-gated cation channels activated by extracellular ATP. They are trimeric assemblies composed of seven distinct subunit subtypes (P2X1–7) that associate to form homomeric or heteromeric channels. Upon ATP binding, a large conformational change opens the transmembrane pore, allowing Na+, K+ and Ca2+ ions to diffuse passively across the membrane. The first crystal structure of the zebrafish (zf) P2X4 receptor resolved in the absence of ATP confirmed in 2009 the unique protein fold of P2X receptors and revealed the conformation of the closed state.1 This structure has aroused a wide interest and motivated several studies as evidenced by extensive efforts to define precisely the agonist-binding site,2-5 to identify conformational changes that follow ATP binding,6,7 and to propose a mechanism of ions conduction through the open pore.8-10 Recently we used the site-directed affinity labeling strategy to trap ATP-binding sites in the rat (r) P2X2 receptor with the sulfhydryl-reactive P2X2 agonist 8-thiocyano-ATP (NCS-ATP).4 NCS-ATP covalently labeled two previously unidentified positions, Asn140 and Leu186, which resulted in distinct functional consequences. ATP-gated currents were inhibited by labeling at N140C but potentiated by labeling at L186C.4
More recently we studied the protein conformational change in the rP2X2 receptor accompanying ion channel activation by successfully engineering receptors with histidines, in which extracellular zinc was able to bridge specifically distant regions that were predicted to come closer to each other during gating.11 Based on these geometrical constrains and on the use of the pore T339S mutation, which produces channels displaying spontaneous openings,12 we concluded that tightening of the ATP sites, shaped like open ‘jaws’ in the closed channel state, induces opening of the ion channel.11 We demonstrated that ATP induces this tightening mechanism, whereas the competitive antagonist 2',3′-O-(2,4,6-trinitrophenyl)-ATP (TNT-ATP) most likely prevents this closure. Outstandingly, this agonist-induced tightening mechanism has been very recently confirmed by a second crystal structure of the zfP2X4 resolved at 2.8 Å in the presence of ATP.13 In this structure, ATP binds to the expected site located at the subunit interface, and indeed promotes closure of the binding cleft.13 Interestingly, the adenine base is deeply buried in the binding pocket and makes hydrophobic interactions with nonpolar residues including Leu191, which is homologous to the P2X2-Leu186 labeled by NCS-ATP.13 To further document the action of agonists on P2X channel gating, we extend our analysis to show that NCS-ATP covalently labeled at L186C likely favors the tightening mechanism, but not the channel opening mechanism. Our data not only confirm the jaw tightening mechanism, but also predict the existence of intermediate or primed ATP bound state(s) associated with jaw tightening, while the channel is still closed.
Results
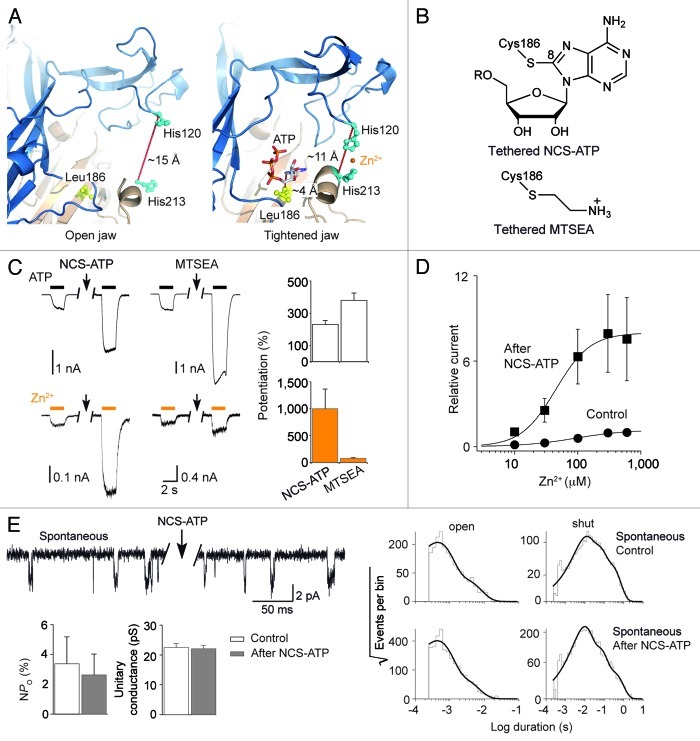
Based on the two recent crystal structures of the zfP2X4 receptor resolved in the absence and presence of ATP,13 we built two homology rP2X2 models, one presumably representing the closed state of the receptor (Fig. 1A, left), and another representing an ATP-bound, open channel state (Fig. 1A, right). Interestingly, we found (1) the adenine moiety of ATP in the rP2X2 model in the open state was in direct contact with the side chain of Leu186 (Fig. 1A, right), a position previously labeled by the agonist NCS-ATP,4 as observed in the zfP2X4 crystal structure with the homologous residue L191;13 and (2) the distance separating the α carbons of the two histidines His120 and His213 shortened by ~4 Å in the open state, resulting in an overall tightening of the binding jaw, as we recently anticipated.11 This allowed us to reconstitute a zinc-binding site in the open state (Fig. 1A, right), consistent with the fact that zinc potentiates ATP-gated currents in the rP2X2 through residues H120 and H213.14 This is also consistent with our recent data showing that zinc activates the T339S mutant through the same histidine residues.11
Figure 1. (A) Structural model of the binding jaw of the rP2X2 receptor built from the X-ray structures of zfP2X4 resolved in the absence (left, open jaw) and presence (right, tightened jaw) of ATP. The distance separating the α-carbons of the two histidines forming the Zn2+-binding site, and the distance between the α-carbon of the Leu186 and position 8 of adenine ring of ATP are indicated. (B) Chemical structures of NCS-ATP and MTSEA covalently tethered to Cys186 in the rP2X2 L186C mutant. R = P3O94-. (C) Representative traces (left) and corresponding pooled data (right, n = 3–5 for each condition) showing the potentiating effect of NCS-ATP (10 µM, 30 sec) or MTSEA (1 mM, 30 sec) application on ATP- (100 µM) or Zn2+- (100 µM) evoked currents on cells expressing the L186C/T339S mutant. Potentiation was defined as the ratio of Zn2+- or ATP-gated currents recorded after exposure to either NCS-ATP or MTSEA to those recorded before exposure. (D) Dose-response curves of Zn2+-evoked currents for the double mutant L186C/T339S before (EC50 = 102 ± 24 µM, nH = 1.2 ± 0.1, n = 4) and after (EC50 = 42 ± 5 µM, nH = 1.7 ± 0.2, n = 4) NCS-ATP (10 µM, 30 sec) treatment. (E) Single channel currents (upperleft) from outside-out patches expressing the double mutant L186C/T339S showing that channels open spontaneously before and after NCS-ATP treatment (10 µM, 12 sec). Corresponding dwell-time distributions (right) of open- and shut-times of channel activities (pooled data from 6 patches containing 3077 events for the control, and 7 patches containing 6872 events for the condition after NCS-ATP treatment). The open-time distribution can be best fit with two components [τ1 = 0.43 ms (87.9%), τ2 = 2.34 ms (12.1%) for control; τ1 = 0.41 ms (88.4%), τ2 = 1.88 ms (11.6%) after NCS-ATP treatment]. The shut-time distribution can be best fit with three components [τ1 = 8.48 ms (42.2%), τ2 = 39.09 ms (37.0%), τ3 = 195.9 ms (20.8%) for control; τ1 = 8.16 ms (58.3%), τ2 = 46.91 ms (31.8%), τ3 = 263.8 ms (9.9%) after NCS-ATP treatment]. Histograms (bottom left) showing the NPo and the single-channel conductance remain unaltered after NCS-ATP treatment.
We thus questioned whether covalently bound ATP at Leu186 (Fig. 1B) was able to favor zinc activation as did non-covalently bound ATP.11 To monitor zinc activation,11 we introduced the L186C mutation into the T339S background. Zn2+ produced robust whole-cell currents in HEK-293 cells expressing the double mutant L186C/T339S (Fig. 1C). These currents were dramatically increased after NCS-ATP exposure, but interestingly they were barely potentiated after exposure to the sulfhydryl-reactive reagent 2-aminoethyl methanethiosulfonate hydrobromide (MTSEA) (Fig. 1C). Notably, covalently bound NCS-ATP in the binding site greatly enhanced both zinc sensitivity and efficacy (Fig. 1D). Consistent with previous data performed on the single mutant L186C,4 NCS-ATP or MTSEA robustly increased ATP-evoked currents (Fig. 1C), suggesting that introduction of the T339S mutation did not perturb the potentiation effects occurring in the ATP-binding sites. These results thus suggest that covalently bound NCS-ATP, but not MTSEA favors Zn2+-induced channel gating.
Single-channel recordings further elucidated the mechanism underlying the potentiation by NCS-ATP of zinc-gated currents. In outside-out patches expressing the L186C/T339S double mutant, spontaneous openings were present, similar to those previously observed in the single mutant T339S.11,12 Furthermore, we found that the nominal open-channel probability (NPo) and unitary conductance remained unchanged after NCS-ATP exposure (Fig. 1E). Detailed analysis of the open- and shut-time distributions (Fig. 1E) showed no change of the mean open-time (0.89 ms and 0.90 ms for control and after NCS-ATP exposure, respectively) and the mean shut-time (47.0 ms and 46.6 ms for control and after NCS-ATP exposure, respectively). These results thus suggest that while tethered NCS-ATP favors Zn2+-induced channel gating, it does not gate spontaneously the ion channel.
Discussion
The mechanism by which agonist binding causes a channel to rapidly open remains a fundamental question in neuroscience. An early attempt to formalize this fundamental process was achieved by del Castillo and Katz who postulated the existence of both inert (closed) and depolarizing (open) channel states.15 These states are in equilibrium and agonists or antagonists displace this equilibrium in favor to opening or closing of the ion channel, respectively.16 Recently this simple theory has been challenged by other studies, which depicted a more complex picture. It has been suggested that additional shut state(s) exist(s) before the opening of the ion channel. For example, in the case of the pentameric channels of the nicotinic receptor family, an earlier conformational change called priming or flipping has been identified to take place while the channel is still shut.17,18 For ATP-gated P2X receptors, the existence of such intermediate shut states remains to be firmly demonstrated, but a detailed kinetic study performed on the rP2X2 receptor suggested that an additional closed state between the resting and active states was necessary to account for the delay in opening at saturating concentration of ATP.19
In this paper we found that covalently bound NCS-ATP in the rP2X2 receptor favors Zn2+-induced channel gating reflecting tightening of the binding site, but not channel opening. However, a major issue of our approach is the relevance of the use of mutants covalently trapped by an ATP analog. For the following reasons, we believe that NCS-ATP trapped intermediate state(s) that is (are) normally populated by ATP in the wild-type receptor. First, NCS-ATP is a potent P2X2 agonist in the wild-type receptor,4 a condition necessary to reach conducting states. Second, the recent crystal structure of zfP2X4 bound to ATP showed that Leu191,13 which is homologous to Leu186 labeled by NCS-ATP in the rP2X2,4 was one of the residues directly involved in the adenine base recognition of ATP. This further validates the affinity labeling approach using cysteine mutants as chemical sensors of agonist binding.20 Third, tethered NCS-ATP at L186C, but not MTSEA, favors jaw tightening of the binding site, as previously suggested for ATP,11 and recently observed in the ATP-bound crystal structure.13 This suggests that only an ATP moiety covalently attached to the binding site produces a mechanism that is similar to that induced by ATP. Taken together, these data strongly suggest that tethered NCS-ATP at L186C is akin to binding an ATP molecule, and thus that NCS-ATP may trap state(s) relevant to activation.
The present study also reports the fact that NCS-ATP attachment did not affect the spontaneous channel activities in receptors bearing the T339S mutation. This further confirms our previous observation that covalently bound NCS-ATP failed to open substantially the channel,4 but also raises the question of how an agonist covalently trapped in its binding site did not lock the receptor in an open channel state, or even, did not modify the spontaneous activities of the T339S mutant. If one admits, as previously proposed, that NCS-ATP labeling occurred in a partially agonist-bound state,4 then a possible explanation may be that partial occupation of the binding sites is not sufficient to alter the equilibrium between closed and open states. This hypothesis, that deserves further experimental testing, might also readily explain why NCS-ATP failed to lock irreversibly the receptor in an open pore conformation. This mechanism would be reminiscent of that found in the cyclic nucleotide-gated channels, in which monoliganded channels open with a very low probability that is not different from spontaneous channel opening.21
On the basis of our results we propose the existence of intermediate closed channel state(s) that follow(s) the resting (or apo) state, but precede(s) an open channel state in the P2X2 receptor. In this model, gating proceeds with two steps. First, following partial occupation of the receptor by the agonist there is a conformational change of the protein confined within the binding sites that primes the tightening of the open jaws. In this intermediate, partially agonist-bound state, the initial conformational change is not sufficient to significantly open the channel, but instead is sufficient to increase agonist binding for unoccupied site(s). Second, following more occupancy of the binding sites due to increased agonist binding, a subsequent and larger conformational change that is spread up to the channel region then opens the ion pore. This implies that opening requires full occupancy of three—or maybe two—sites by agonist to escape from the intermediate state(s), a conclusion that is consistent with a previous detailed single-channel kinetic study performed on the P2X2 receptor, which showed that channels only open after being fully liganded.22 An attractive future direction would be to resolve the structure by X-ray crystallography of this (these) intermediate shut state(s) trapped by NCS-ATP, as recently performed in a pentameric proton-gated ion channel by chemical cross-linking.23
Materials and Methods
Molecular modeling
The all-heavy-atom structures of the zfP2X4 receptor taken from the Protein Data Bank (PDB) ID codes 4DW0 and 4DW1 were taken as templates to construct the rP2X2 models with the program MODELER.24 For the open state model the ATP molecule present in the PDB file was retained and the zinc-binding site was reconstructed by imposing a distance constrain (2.06 Å) between zinc ion and the NE2 atom of histidines.
CDNA construction and site-directed mutagenesis
The pcDNA-based expression plasmids, mutagenesis and sequencing procedure have been described previously.6
Cell culture and transfection
HEK-293 cells were cultured and transiently transfected with the rP2X2 L186C mutant (0.01 μg and 2 μg for single-channel recording and whole-cell recording, respectively) and a green fluorescent protein cDNA construct (0.3 μg). Culture and transfection procedures were followed as described previously.6
Chemicals
MTSEA was purchased from Toronto Research Chemicals. ZnCl2 solution and other drugs were purchased from Sigma. NCS-ATP was synthesized and characterized as described previously.4
Electrophysiology
Whole-cell and single-channel recordings were performed as described previously.11 Following NCS-ATP exposure, cells or patches were always washed by the external solution for at least 15 sec before recordings.
Acknowledgments
This work was funded in part by the ANR (ANR 11 BSV5 001-01), the CNRS (Programme d’incitation à la mobilité d’équipe), ic-FRC (www.icfrc.fr) and the Ministère de la Recherche. R.J. is a recipient of a fellowship from ANR 11 BSV5 001-01.
Glossary
Abbreviations:
- NCS-ATP
8-thiocyano-ATP
- TNP-ATP
2',3'-O-(2,4,6-trinitrophenyl)-ATP
- zfP2X4
zebrafish ATP-gated P2X4
- rP2X2
rat ATP-gated P2X2
- HEK-293
human embryonic kidney cells
- MTSEA
2-aminoethyl methanethiosulfonate hydrobromide
- NPo
nominal channel opening probability
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/21520
References
- 1.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–8. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allsopp RC, El Ajouz S, Schmid R, Evans RJ. Cysteine scanning mutagenesis (residues Glu52-Gly96) of the human P2X1 receptor for ATP: mapping agonist binding and channel gating. J Biol Chem. 2011;286:29207–17. doi: 10.1074/jbc.M111.260364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodnar M, Wang H, Riedel T, Hintze S, Kato E, Fallah G, et al. Amino acid residues constituting the agonist binding site of the human P2X3 receptor. J Biol Chem. 2011;286:2739–49. doi: 10.1074/jbc.M110.167437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang R, Lemoine D, Martz A, Taly A, Gonin S, Prado de Carvalho L, et al. Agonist trapped in ATP-binding sites of the P2X2 receptor. Proc Natl Acad Sci U S A. 2011;108:9066–71. doi: 10.1073/pnas.1102170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lörinczi E, Bhargava Y, Marino SF, Taly A, Kaczmarek-Hájek K, Barrantes-Freer A, et al. Involvement of the cysteine-rich head domain in activation and desensitization of the P2X1 receptor. Proc Natl Acad Sci U S A. 2012;109:11396–401. doi: 10.1073/pnas.1118759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang R, Martz A, Gonin S, Taly A, de Carvalho LP, Grutter T. A putative extracellular salt bridge at the subunit interface contributes to the ion channel function of the ATP-gated P2X2 receptor. J Biol Chem. 2010;285:15805–15. doi: 10.1074/jbc.M110.101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts JA, Allsopp RC, El Ajouz S, Vial C, Schmid R, Young MT, et al. Agonist binding evokes extensive conformational changes in the extracellular domain of the ATP-gated human P2X1 receptor ion channel. Proc Natl Acad Sci U S A. 2012;109:4663–7. doi: 10.1073/pnas.1201872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Kawate T, Silberberg SD, Swartz KJ. Pore-opening mechanism in trimeric P2X receptor channels. Nat Commun. 2010;1:1–7. doi: 10.1038/ncomms1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne LE, Cao L, Broomhead HE, Bragg L, Wilkinson WJ, North RA. P2X receptor channels show threefold symmetry in ionic charge selectivity and unitary conductance. Nat Neurosci. 2011;14:17–8. doi: 10.1038/nn.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kracun S, Chaptal V, Abramson J, Khakh BS. Gated access to the pore of a P2X receptor: structural implications for closed-open transitions. J Biol Chem. 2010;285:10110–21. doi: 10.1074/jbc.M109.089185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang R, Taly A, Lemoine D, Martz A, Cunrath O, Grutter T. Tightening of the ATP-binding sites induces the opening of P2X receptor channels. EMBO J. 2012;31:2134–43. doi: 10.1038/emboj.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao L, Broomhead HE, Young MT, North RA. Polar residues in the second transmembrane domain of the rat P2X2 receptor that affect spontaneous gating, unitary conductance, and rectification. J Neurosci. 2009;29:14257–64. doi: 10.1523/JNEUROSCI.4403-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–12. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaya N, Tittle RK, Saar N, Dellal SS, Hume RI. An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem. 2005;280:25982–93. doi: 10.1074/jbc.M504545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957;146:369–81. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- 16.Changeux JP, Edelstein SJ. Allosteric receptors after 30 years. Neuron. 1998;21:959–80. doi: 10.1016/S0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- 17.Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–7. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhtasimova N, Lee WY, Wang HL, Sine SM. Detection and trapping of intermediate states priming nicotinic receptor channel opening. Nature. 2009;459:451–4. doi: 10.1038/nature07923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffatt L, Hume RI. Responses of rat P2X2 receptors to ultrashort pulses of ATP provide insights into ATP binding and channel gating. J Gen Physiol. 2007;130:183–201. doi: 10.1085/jgp.200709779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foucaud B, Perret P, Grutter T, Goeldner M. Cysteine mutants as chemical sensors for ligand-receptor interactions. Trends Pharmacol Sci. 2001;22:170–3. doi: 10.1016/S0165-6147(00)01674-6. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz ML, Karpen JW. Single cyclic nucleotide-gated channels locked in different ligand-bound states. Nature. 1997;389:389–92. doi: 10.1038/38744. [DOI] [PubMed] [Google Scholar]
- 22.Ding S, Sachs F. Single channel properties of P2X2 purinoceptors. J Gen Physiol. 1999;113:695–720. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevost MS, Sauguet L, Nury H, Van Renterghem C, Huon C, Poitevin F, et al. A locally closed conformation of a bacterial pentameric proton-gated ion channel. Nat Struct Mol Biol. 2012;19:642–9. doi: 10.1038/nsmb.2307. [DOI] [PubMed] [Google Scholar]
- 24.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]