Abstract
For nearly 350 years, veterinary medicine and human medicine have been separate entities, with one geared toward the diagnosis and treatment in animals and the other toward parallel goals in the owners. However, that model no longer fits, since research on diseases of humans and companion animals has coalesced.1–4 The catalyst for this union has been the completion of the human genome sequence, coupled with draft sequence assemblies of genomes for companion animals.5,6 Here, we summarize the critical events in canine genetics and genomics that have led to this development, review major applications in canine health that will be of interest to human caregivers, and discuss expectations for the future.
HUMAN AND CANINE GENOMICS
In 2001, two independent draft versions of the human genome sequence and the concomitant identification of approximately 30,000 genes were the seminal events that defined completion of the Human Genome Project.7,8 The genome was officially declared to be finished in 2004, with sequencing reported to include 99% of transcribing DNA.9 By comparison, the genome of the domestic dog, Canis lupus familiaris, was sequenced twice, once to 1.5× density (i.e., covering the genome, in theory, 1.5 times) and once to 7.8× density (providing sequencing for more than 95% of base pairs) in the standard poodle and boxer, respectively.5,10 Subsequent contributions to the canine genome have focused on better annotation to locate missing genes,11 understanding chromosome structure,12 studying linkage disequilibrium,5,13 identifying copy-number variants,14–16 and mapping the transcriptome.17
The use of the canine genome to understand the genetic underpinning of disorders that are difficult to disentangle in humans has been on the rise for nearly two decades.1,2,18 The reason relates back to the domestication of dogs from gray wolves (C. lupus), an event that began at least 30,000 years ago.19–21 Since their domestication, dogs have undergone continual artificial selection at varying levels of intensity, leading to the development of isolated populations or breeds5,22,23 (Fig. 1). Many breeds were developed during Victorian times24 and have been in existence for only a few hundred years, a drop in the evolutionary bucket.25 Most breeds are descended from small numbers of founders and feature so-called popular sires (dogs that have performed well at dog shows and therefore sire a large number of litters). Thus, the genetic character of such founders is overrepresented in the population.25,26 These facts, coupled with breeding programs that exert strong selection for particular physical traits, mean that recessive diseases are common in purebred dogs,22,27,28 and many breeds are at increased risk for specific disorders.2,29 We, and others, have chosen to take advantage of this fact in order to identify genes of interest for human and canine health.
Figure 1. The Diversity of Dog Breeds.
Breeds vary according to many traits, including size, leg length, pelage (coat), color, and skull shape. Shown are borzoi (Panel A), basset hound (Panel B), Chihuahua (Panel C), giant schnauzer (Panel D), bichon frise (Panel E), collie (Panel F), French bulldog (Panel G), dachshund (Panel H), German shorthaired pointer (Panel I), papillon (Panel J), and Neapolitan mastiff (Panel K). (Images courtesy of Mary Bloom, American Kennel Club.)
THE GENETIC POWER OF CANINE FAMILIES
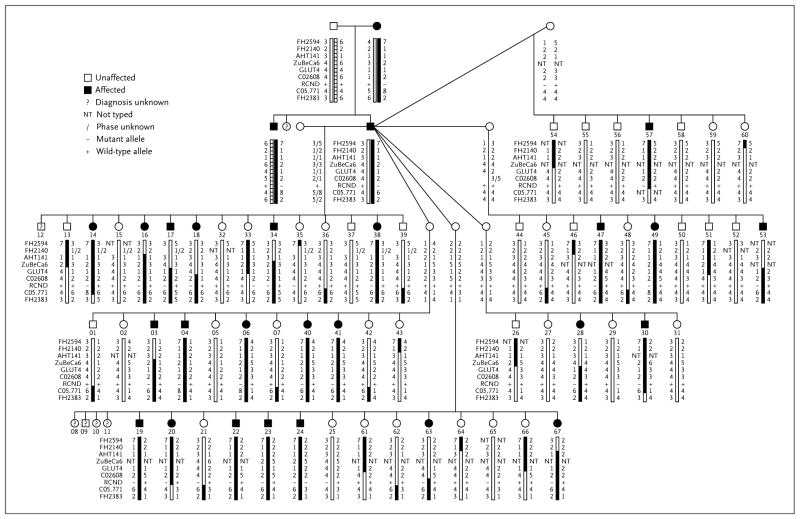
One of the most striking features of canine families is their large size, which makes them amenable to conventional linkage mapping. This fact was particularly well illustrated in the search for the canine gene for hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis (RCND) in German shepherds.30 Although rare, RCND is a naturally occurring inherited cancer syndrome that includes bilateral, multifocal tumors in kidneys and numerous, dense collagen-based nodules in the skin,31 a disorder that is similar to the Birt–Hogg–Dubé syndrome (BHD) in humans.32 In dogs, the disease allele is highly penetrant and transmitted in an autosomal dominant fashion. The dog pedigree that was used for mapping the disease included one affected founder male who sired several litters (Fig. 2). With DNA available from nearly all dogs, this single pedigree had sufficient power to localize the disease gene to canine chromosome 5q12 with a logarithm of odds (LOD) score of 4.6, giving odds of more than 10,000 to 1 that the mapping was correct.30
Figure 2. Mapping Pedigree for Canine Renal Cystadenocarcinoma and Nodular Dermatofibrosis (RCND).
A single affected male dog carrying an autosomal dominant allele for RCND sired five litters of pups with five unique and unaffected females. Affected dogs are shown in black, and unaffected dogs in white. Squares indicate males, circles females, and lines relationships. The portion of canine chromosome 5q14 showing linkage is indicated as a rectangle below each square or circle. Black bars indicate the portion of the affected parental chromosome inherited by each offspring from the affected father, and white bars indicate the portion inherited from the normal chromosome of the father. Alleles for each marker are indicated as numbers. Breakpoints allow the disease gene to be localized to a region adjacent to marker ZuBeCa6. Reprinted from Jónasdóttir et al.,30 with the permission of the publisher.
After the localization of RCND, the human BHD locus was mapped to human chromosome 17p12q11,33 which corresponds to canine chromosome 5q12. Both affected dogs and humans were found to carry mutations in the same gene encoding tumor-suppressor protein folliculin,34,35 which is hypothesized to interact with the energy and nutrient-sensing signaling pathway consisting of AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR).36
Three issues about this example are striking. First, the single, large dog pedigree was collected and genotyped in a fraction of the time it took to collect and characterize the many necessary human pedigrees. Second, BHD is associated with substantial variability in disease presentation in humans and may be hard to distinguish from similar disorders.37 In the case of the large extended dog family, phenotyping was easy, since every dog had the same genetic background and the disease presentation was highly uniform. Also, the dog locus was found before the human locus. Other disease genes that were first mapped in dogs for which there is a close human proxy include narcolepsy,38 copper toxicosis,39,40 neuronal ceroid lipofuscinosis,41 and ichthyosis,42 to name a few.
Each of such stories is illuminating in its own way. In the case of narcolepsy in the Doberman pinscher, the identification of a mutation in the gene encoding hypocretin receptor 2 suggested a newly recognized pathway that is involved in the molecular biology of sleep. Another example is canine neuronal ceroid lipofuscinosis, a late-onset disorder of American Staffordshire terriers with symptoms that are similar to a human adult-onset form of the disorder known as Kuf’s disease. In American Staffordshire terriers, neuronal ceroid lipofuscinosis is caused by an R99H mutation in exon 2 of the gene encoding arylsulfatase G (ARSG), leading to a 75% decrease in sulfatase activity. This study, therefore, both identified a new gene for consideration in human neuronal ceroid lipofuscinosis and provided new information regarding sulfatase deficiency and pathogenesis of the disease.
BREED STRUCTURE AND GENETIC COMPLEXITY SIMPLIFIED
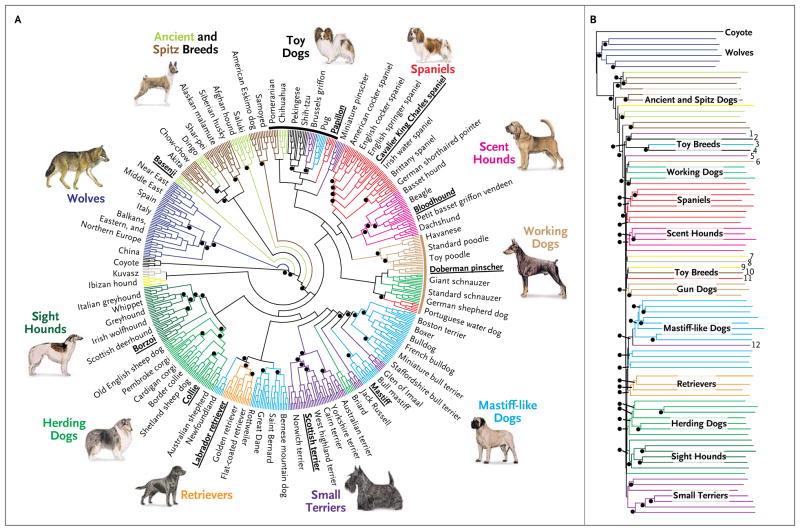
A recurring theme in the gene mapping of canine diseases is the power of the breed structure (Fig. 3). To be a registered member of a breed, the dog’s ancestors must have been registered members as well.26 In 2011, the American Kennel Club (www.akc.org) recognized 173 distinct dog breeds, with European clubs taking the number of established breeds to more than 400.24,43
Figure 3. Neighbor-Joining Tree of Domestic Dogs.
On average, 10 to 12 dogs were genotyped for each of approximately 80 breeds. Trees were constructed with the use of data from each genotyped dog individually or by grouping the data from each member of a breed together, so each breed is represented as a single data entry. Data were also analyzed in two ways: by considering adjacent 10 single-nucleotide-polymorphism (SNP) windows or haplotypes or by considering each SNP alone. The two analytic methods provided similar results. Panel A shows the relationships among the various dog breeds. The color groupings indicate breeds that probably share common founders. Panel B shows the historical relationship of the breeds with the same color coding used in Panel A. In each case, breeds that share either common behaviors or morphologic traits are grouped together on the basis of DNA analysis, indicating that they probably share common ancestors. A black dot indicates at least 95% bootstrap support (a measure of the likelihood that an evolutionary split occurred in a given location in an evolutionary tree) after the performance of 1000 replicates. Reprinted from vonHoldt et al.23 with permission of the publisher.
Dog breeds offer the same advantage of reducing locus heterogeneity that is gained by studying humans from geographically isolated countries such as Finland or Iceland.29 For any given complex disease, a small number of genes and deleterious alleles will dominate the breed,3 much as the 999del5 BRCA2 mutation does in Icelandic women with hereditary breast cancer.44
Epilepsy is a good example, since this disease has been difficult to disentangle genetically in humans because of indistinct clinical phenotypes and a high degree of locus heterogeneity. The disease affects 5% of dogs and is reported in dozens of breeds. Remitting focal epilepsy in the Lagotto Romagnolo breed45 is caused by variants in LGI2, a homologue of the human epilepsy LGI1 gene. In contrast, miniature wire-haired dachshunds have a form of epilepsy reminiscent of the progressive myoclonic disease known as Lafora’s disease, which in humans is the most severe form of teenage-onset epilepsy. The similar disease in dachshunds is caused by an unusual expansion of a dodecamer repeat46 within the gene encoding malin (EPM2B) that modulates gene expression by a factor of nearly 900. The presentation of epilepsy is expectedly unique in other breeds.47 Thus, one way to disentangle complex diseases like epilepsy is to study the disorder in different dog breeds.
BREED STRUCTURE AND REDUCING REGIONS OF LINKAGE DISEQUILIBRIUM
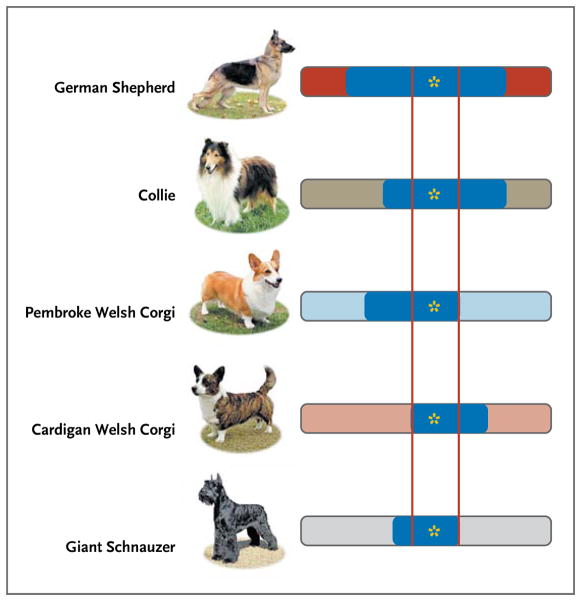
The second way in which breed structure offers unique advantages to genetic mapping is that when used judiciously, it allows researchers to move quickly from linked or associated markers to genes. In humans, linkage disequilibrium typically extends on the order of kilobases, whereas within dog breeds it can extend for megabases.5,13 Long linkage disequilibrium means that although only a modest number of single-nucleotide polymorphisms (SNPs) are needed for an initial mapping study, subsequent identification of the disease mutation can be difficult. This task is facilitated by leveraging interbreed relatedness. Haplotypes in the region of interest can be compared in related breeds with the same disorder, with the goal of identifying a segment that is shared by all affected dogs but absent in those lacking the trait (Fig. 4).
Figure 4. Comparing Haplotypes as a Method for Reducing a Region of Association for a Given Mutation.
The mutation causing a hypothetical disease is indicated by a yellow star. The various breeds with the disease are shown on the left; the chromosome responsible for the disease is indicated by a horizontal bar. Within each breed, meiotic breakpoints are indicated by the start and finish of the blue bar for each breed. When all breeds are considered together, the minimal associated region where the mutation must lie is between the red vertical lines.
Among the many investigators who have demonstrated this principle are Goldstein et al.,48,49 who had previously mapped a form of canine progressive retinal atrophy called progressive rod–cone degeneration to a 30-mb region. Progressive retinal atrophy is analogous to human retinitis pigmentosa, for which there are many forms and causative genes. Although progressive rod–cone degeneration was initially mapped in miniature and toy poodles, the disorder appears in more than a dozen breeds and is phenotypically similar to one form of human adult-onset, autosomal recessive retinitis pigmentosa. Analysis of additional SNPs allowed the investigators to reduce the disease locus to a 106-kb haplotype that is shared by affected dogs from 14 breeds. A mutation in a novel gene was ultimately determined to cause the disease.50 Had there not been 14 affected breeds sharing the founder mutation, which allowed the haplotype to be significantly reduced, only next-generation sequencing could have ultimately localized the disease gene.
Although researchers could have correctly guessed a subset of the breeds that shared the same mutation at the causative locus for progressive rod–cone degeneration by knowing about their shared heritage, common geographic origin, or shared morphologic features, in many cases the relationship among the breeds is too ancient to be obvious. With the use of both cluster analysis51,52 and neighbor-joining trees,23 a clear picture is emerging regarding how breeds are related to one another genetically (Fig. 3). This type of information highlights groups of breeds that probably share common founders (and hence the same disease alleles) and facilitates experimental design.
MORPHOLOGIC FEATURES AND GENETIC VARIATION
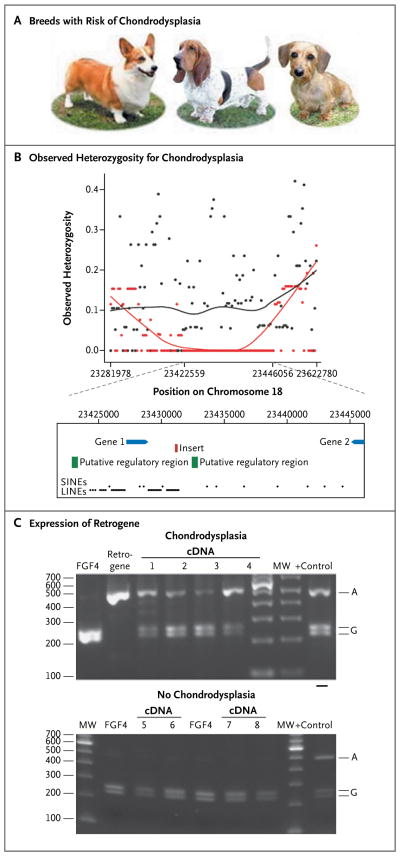
The examples discussed thus far have focused on disease phenotypes. However, canine morphologic studies have been informative for both discovering new ways of perturbing the genome and suggesting candidate genes for related diseases. For instance, chondrodysplasia is a fixed trait for more than 20 breeds with disproportionately short legs recognized by the American Kennel Club, including the dachshund, corgi, and basset hound (Fig. 5).53
Figure 5. Mapping the Breed-Fixed Trait of Chondrodysplasia.
Panel A shows examples of breeds that are associated with chondrodysplasia, including the corgi, basset hound, and wire-haired dachshund. Panel B shows observed heterozygosity for breeds that are at increased risk for chondrodysplasia (red) and those that are not at increased risk (black) within the associated 34-kb region on canine chromosome 18. The x axis indicates the chromosomal position of association, and the y axis indicates observed heterozygosity The red and black lines indicate trends and highlight a 24-kb region with low heterozygosity in the dogs at risk for chondrodysplasia that is absent in dogs that are not at increased risk. Gene 1 is a pseudogene, a defective segment of DNA that resembles a gene but cannot be transcribed, called txndc1 (similar to the gene encoding thioredoxin-related transmembrane protein 1), and gene 2 marks the 33 end of the gene encoding semaphorin 3C (SEMA3C). The green boxes are conserved in both sequence and context in all mammals for which data are available. A 5-kb insertion (red rectangle), which was observed only in dogs with an association with chondrodysplasia and was found between the two putative regulatory elements, contains an fg f4 retrogene. LINE denotes long interspersed nuclear element, and SINE short interspersed nuclear element. Panel C shows expression studies indicating that the fg f4 retrogene is expressed in articular cartilage from the distal and proximal humerus isolated from a 4-week-old dog with chondrodysplasia. The retrogene and source gene are distinguished by a single-nucleotide polymorphism, which is cut by restriction enzyme BsrB1 in complementary DNA (cDNA) produced from the source gene, resulting in two bands on a 2% agarose gel, but uncut in the cDNA from the retrogene that is present in dogs with chondrodysplasia, resulting in only one band. MW denotes molecular weight marker. The source of control material is DNA isolated from the testes of a dog with chondrodysplasia. Modified from Parker et al.,51 with the permission of the publisher.
A genomewide association study comparing 95 dogs from eight chondrodysplastic breeds with 702 dogs from 64 breeds lacking the trait identified a single strong association (P = 1.0×10−102) with canine chromosome 18. Although this very low P value is probably exaggerated because of the population structure, such a strong association is not unusual when breeds sharing a trait from a common founder are compared with a large number of unrelated control breeds. In this case, the trait is caused by expression of an fgf4 retrogene. This retrogene encodes fibroblast growth factor 4 in which all fgf4 exons are present, but introns and regulatory signals are missing (Fig. 5). The spliced copy of the gene is located a large distance away from the source gene. Although such an arrangement is common in insects, this was the first report of an expressed retrogene that alters a mammalian trait.53 Expression studies showed that the fgf4 retrogene was expressed in the long bones of 4-week-old puppies, suggesting that mistimed expression, incorrect RNA levels, or mislocalization of the retrogene product caused premature closure of the growth plates in the long bones of the carrier breeds. It will be interesting to see whether this gene, or this method of mutating mammalian genomes, turns out to be important in similar human diseases.
Other canine morphologic traits that include such characteristics as body size, leg width, and coat color have been mapped.22,28,54–58 Not surprisingly, loci that control both a morphologic trait and a disease have been identified. This may be a result of strong selection by breeders to propagate dogs of a certain appearance, which results in piggybacking of disease alleles, or in some cases, diseases are associated with the same genetic variants that create a morphologic effect. This is best illustrated by dermoid sinus, a neural-tube defect in the ridgeback breed that is caused by the same copy-number variant that produces the hair ridge characteristic of the Rhodesian ridgeback.59
MAPPING MULTIGENIC TRAITS
When the dog genome sequence was published in 2005, Lindblad-Toh et al.5 hypothesized that breed structure would enable mapping of simple recessive traits in dogs with a genomewide association study of no more than 20 cases and controls each. They further reasoned that complex traits that are controlled by, for instance, five genes could be mapped with 97% certainty on the basis of just 100 cases and 100 controls. This was a bold prediction, since most genomewide association studies of complex human disorders require thousands of samples. But the investigators’ prediction proved to be correct, and many genomewide association studies in dogs have successfully mapped complex traits on the basis of no more than 50,000 SNPs and fewer than 200 dogs.
Recent work by Wilbe et al.60 that identifies genes for systemic lupus erythematosus (SLE)–related disease complex illustrates this point. Nova Scotia duck-tolling retrievers have an abnormally high rate of autoimmune diseases, including SLE.61 The breed is descended from a small number of founders that survived two major outbreaks of canine distemper virus in the early 1900s.62 It has been hypothesized that autoimmune disorders develop in these dogs because they have a particularly strong or reactive immune system, which helped them to survive the distemper outbreaks. In an analysis of 81 cases and 57 controls in a genomewide association study of 22,000 SNPs, investigators found five associated loci, three of which have already been validated.60 Candidate genes of particular interest include those associated with T-cell activation such as PPP3CA, BANK1, and DAPPI.
DOGS AND CANCER
Of all the disorders for which dogs are likely to inform human health, canine cancer is likely to have the greatest effect.63 Cancers are the most frequent cause of disease-associated death in dogs, and naturally occurring cancers are well described in several breeds.3,64,65 Although considerable effort has gone into the study of common cancers, the dog has also served as a model for studies of rare tumors, including histiocytic sarcomas, which are highly aggressive, lethal, dendritic-cell neoplasms.66 In dogs, two forms exist: a localized variant, in which skin and sub-cutical tumors develop in a leg and metastasize to lymph nodes and blood vessels, and a disseminated multisystem form, in which tumors affect the spleen, liver, and lungs.67 Histiocytic sarcomas will develop in approximately 20% of Bernese mountain dogs,68 and the condition is invariably fatal.69 In humans, similar disorders such as Langerhans’-cell histiocytosis have been well characterized clinically, but the underlying cause is unknown.70
Recently, a genomewide association study for histiocytic sarcoma was undertaken in dogs.71 Because the disorder occurs in so few breeds, Bernese mountain dogs from France, the United States, and the Netherlands were included, with the idea that these independently propagating lines would offer the same advantages for reducing a region of association that distinct, but related, dog breeds provide.72 For this breed, this assumption proved to be true, and two loci were identified, one on chromosome 18. Fine mapping and sequencing narrowed the locus to a single risk-associated haplotype that spans the MTAP gene and contains one or more variants that alter the expression of the nearby INK4A–ARF–INK4B locus but do not affect expression of MTAP itself.
Although 40% of a random sample of Bernese mountain dogs in the United States are homozygous for the disease haplotype, histiocytic sarcoma develops in only about 20% of these dogs. However, more than 60% of Bernese mountain dogs eventually die of cancer. The disease-associated portion of chromosome 11 corresponds to human chromosome 9p21, which has been associated with several types of cancer.73–75 We have hypothesized that multiple distinct cancers in Bernese mountain dogs may be related to variants within the MTAP–CDKN2A region and the associated canine locus. Thus, studies of this naturally occurring dog model not only illuminate a causative locus but also suggest a biologic model for the study of germline variation in this important cancer-susceptibility locus.
DOG GENETICS AND GENE THERAPHY
Although I have focused largely on the role of dogs in the identification of genes that are associated with disease, dogs have also served an important role in the development of treatments. One form of progressive retinal atrophy called Leber’s congenital amaurosis type 2 is a disease of dogs and humans that is caused by a loss of the RPE65 protein owing to mutations in RPE65, causing blindness shortly after birth. In a landmark study in 2001, Acland et al.76 used a recombinant adeno-associated virus carrying wild-type RPE65 to restore vision in a dog that was homozygous for the RPE65 mutation. Replication was successful,77 and treated dogs maintained stable vision for at least 3 years.78 Humans with Leber’s congenital amaurosis are now being successfully treated for the disorder.79,80 Progressive retinal atrophy occurs in more than 100 breeds of dogs, suggesting dozens of naturally occurring models for additional study. So far, 18 genes for canine retinal diseases have been found.81
DOG GENETICS AND BEHAVIOR
The canine system is valuable for mapping behaviors that are specific to both breed82 and species.23 Abnormal behaviors, including separation anxiety, dominance aggression, and obsessive–compulsive disorder, are most amenable to genetic studies.83 Partial success has been achieved with obsessive–compulsive disorder in bull terriers and Doberman pinschers.84,85 In Dobermans, the disease presents as flank or blanket sucking and was recently mapped to a 1.7-Mb region of chromosome 7 near the CDH2 gene. CDH2 mediates synaptic activity-regulated neuronal adhesion, but to date no functional studies have illuminated these findings and no mutation has been reported.85
SUMMARY
What we most wish to understand about dog health is the very same thing we wish to know about ourselves. When will we, or they, get sick? How is the illness best treated? And what is the likely outcome? Each half of a pet–human pair wants to know what to expect from the other end of the leash and how to prolong the relationship. Finally, as the end of life approaches, we seek to make both our canine companions and ourselves comfortable, settled in the knowledge that a full life has been achieved. When considered in that frame, we are not so different from our canine companions. As the scientific advances coalesce, joining us ever closer to the one family member we actually get to choose, it is worth bearing in mind that though our methods may be different, our goals are the same: a healthy life well spent in the best of company.
Biography
Franklin H. Epstein, M.D., served the New England Journal of Medicine for more than 20 years. A keen clinician, accomplished researcher, and outstanding teacher, Dr. Epstein was Chair and Professor of Medicine at Beth Israel Deaconess Medical Center, Boston, where the Franklin H. Epstein, M.D., Memorial Lectureship in Mechanisms of Disease has been established in his memory.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Tsai KL, Clark LA, Murphy KE. Understanding hereditary diseases using the dog and human as companion model systems. Mamm Genome. 2007;18:444–51. doi: 10.1007/s00335-007-9037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson EK, Lindblad-Toh K. Leader of the pack: gene mapping in dogs and other model organisms. Nat Rev Genet. 2008;9:713–25. doi: 10.1038/nrg2382. [DOI] [PubMed] [Google Scholar]
- 3.Shearin AL, Ostrander EA. Leading the way: canine models of genomics and disease. Dis Model Mech. 2010;3:27–34. doi: 10.1242/dmm.004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lequarré A-S, Andersson L, André C, et al. LUPA: a European initiative taking advantage of the canine genome architecture for unravelling complex disorders in both human and dogs. Vet J. 2011;189:155–9. doi: 10.1016/j.tvjl.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–19. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 6.Pontius JU, Mullikin JC, Smith DR, et al. Initial sequence and comparative analysis of the cat genome. Genome Res. 2007;17:1675–89. doi: 10.1101/gr.6380007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [Erratum, Nature 2001;412:565.] [DOI] [PubMed] [Google Scholar]
- 8.Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [Erratum, Science 2001;292:1838.] [DOI] [PubMed] [Google Scholar]
- 9.International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–45. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 10.Kirkness EF, Bafna V, Halpern AL, et al. The dog genome: survey sequencing and comparative analysis. Science. 2003;301:1898–903. doi: 10.1126/science.1086432. [DOI] [PubMed] [Google Scholar]
- 11.Derrien T, Vaysse A, André C, Hitte C. Annotation of the domestic dog genome sequence: finding the missing genes. Mamm Genome. 2012;23:124–31. doi: 10.1007/s00335-011-9372-0. [DOI] [PubMed] [Google Scholar]
- 12.Breen M. Canine cytogenetics — from band to basepair. Cytogenet Genome Res. 2008;120:50–60. doi: 10.1159/000118740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutter NB, Eberle MA, Parker HG, et al. Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome Res. 2004;14:2388–96. doi: 10.1101/gr.3147604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas TJ, Baker C, Eichler E, Akey JM. A high-resolution integrated map of copy number polymorphisms within and between breeds of the modern domesticated dog. BMC Genomics. 2011;12:414. doi: 10.1186/1471-2164-12-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen WK, Swartz JD, Rush LJ, Alvarez CE. Mapping DNA structural variation in dogs. Genome Res. 2009;19:500–9. doi: 10.1101/gr.083741.108. [Erratum, Genome Res 2009;19:690.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholas TJ, Cheng Z, Ventura M, Mealey K, Eichler EE, Akey JM. The genomic architecture of segmental duplications and associated copy number variants in dogs. Genome Res. 2009;19:491–9. doi: 10.1101/gr.084715.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briggs J, Paoloni M, Chen Q-R, Wen X, Khan J, Khanna C. A compendium of canine normal tissue gene expression. PLoS One. 2011;6(5):e17107. doi: 10.1371/journal.pone.0017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostrander EA, Giniger E. Semper fidelis: what man’s best friend can teach us about human biology and disease. Am J Hum Genet. 1997;61:475–80. doi: 10.1086/515522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilà C, Savolainen P, Maldonado JE, et al. Multiple and ancient origins of the domestic dog. Science. 1997;276:1687–9. doi: 10.1126/science.276.5319.1687. [DOI] [PubMed] [Google Scholar]
- 20.Savolainen P, Zhang YP, Luo J, Lundeberg J, Leitner T. Genetic evidence for an East Asian origin of domestic dogs. Science. 2002;298:1610–3. doi: 10.1126/science.1073906. [DOI] [PubMed] [Google Scholar]
- 21.Sablin MV, Khlopachev GA. The earliest Ice Age dogs: evidence from Eliseevichi 1. Curr Anthropol. 2002;43:795–9. [Google Scholar]
- 22.Boyko AR, Quignon P, Li L, et al. A simple genetic architecture underlies morphological variation in dogs. PLoS Biol. 2010;8(8):e1000451. doi: 10.1371/journal.pbio.1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.vonHoldt BM, Pollinger JP, Lohmueller KE, et al. Genome-wide SNP and haplo-type analyses reveal a rich history underlying dog domestication. Nature. 2010;464:898–902. doi: 10.1038/nature08837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogle B. Encyclopedia of the dog. London: DK Publishing; 1995. [Google Scholar]
- 25.Parker HG. Genomic analyses of modern dog breeds. Mamm Genome. 2012;23:19–27. doi: 10.1007/s00335-011-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Kennel Club. The complete dog book. 19. New York: Howell Book House; 1998. rev. [Google Scholar]
- 27.Patterson DF. Companion animal medicine in the age of medical genetics. J Vet Intern Med. 2000;14:1–9. [PubMed] [Google Scholar]
- 28.Vaysse A, Ratnakumar A, Derrien T, et al. Identification of genomic regions associated with phenotypic variation between dog breeds using selection mapping. PLoS Genet. 2011;7(10):e1002316. doi: 10.1371/journal.pgen.1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrander EA, Kruglyak L. Unleashing the canine genome. Genome Res. 2000;10:1271–4. doi: 10.1101/gr.155900. [DOI] [PubMed] [Google Scholar]
- 30.Jónasdóttir TJ, Mellersh CS, Moe L, et al. Genetic mapping of a naturally occurring hereditary renal cancer syndrome in dogs. Proc Natl Acad Sci U S A. 2000;97:4132–7. doi: 10.1073/pnas.070053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moe L, Gamlem H, Jónasdóttir TJ, Lingaas F. Renal microcystic tubular lesions in two 1-year-old-dogs — an early sign of hereditary renal cystadenocarcinoma? J Comp Pathol. 2000;123:218–21. doi: 10.1053/jcpa.2000.0408. [DOI] [PubMed] [Google Scholar]
- 32.Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–7. [PubMed] [Google Scholar]
- 33.Schmidt LS, Warren MB, Nickerson ML, et al. Birt-Hogg-Dubé syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11. 2. Am J Hum Genet. 2001;69:876–82. doi: 10.1086/323744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–64. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 35.Lingaas F, Comstock KE, Kirkness EF, et al. A mutation in the canine BHD gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in the German Shepherd dog. Hum Mol Genet. 2003;12:3043–53. doi: 10.1093/hmg/ddg336. [DOI] [PubMed] [Google Scholar]
- 36.Hasumi Y, Baba M, Ajima R, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc Natl Acad Sci U S A. 2009;106:18722–7. doi: 10.1073/pnas.0908853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smit DL, Mensenkamp AR, Badeloe S, et al. Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin Genet. 2011;79:49–59. doi: 10.1111/j.1399-0004.2010.01486.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 39.Yuzbasiyan-Gurkan V, Blanton SH, Cao V, et al. Linkage of a microsatellite marker to the canine copper toxicosis locus in Bedlington terriers. Am J Vet Res. 1997;58:23–7. [PubMed] [Google Scholar]
- 40.van De Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11:165–73. doi: 10.1093/hmg/11.2.165. [DOI] [PubMed] [Google Scholar]
- 41.Abitbol M, Thibaud JL, Olby NJ, et al. A canine Arylsulfatase G (ARSG) mutation leading to a sulfatase deficiency is associated with neuronal ceroid lipofuscinosis. Proc Natl Acad Sci U S A. 2010;107:14775–80. doi: 10.1073/pnas.0914206107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grall A, Guaguère E, Planchais S, et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat Genet. 2012;44:140–7. doi: 10.1038/ng.1056. [DOI] [PubMed] [Google Scholar]
- 43.Wilcox B, Walkowicz C. The atlas of dog breeds of the world. 5. Neptune City, NJ: TFH Publications; 1995. [Google Scholar]
- 44.Tulinius H, Olafsdottir GH, Sigvaldason H, et al. The effect of a single BRCA2 mutation on cancer in Iceland. J Med Genet. 2002;39:457–62. doi: 10.1136/jmg.39.7.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seppälä EH, Jokinen TS, Fukata M, et al. LGI2 truncation causes a remitting focal epilepsy in dogs. PLoS Genet. 2011;7(7):e1002194. doi: 10.1371/journal.pgen.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohi H, Young EJ, Fitzmaurice SN, et al. Expanded repeat in canine epilepsy. Science. 2005;307:81. doi: 10.1126/science.1102832. [DOI] [PubMed] [Google Scholar]
- 47.Ekenstedt KJ, Patterson EE, Mickelson JR. Canine epilepsy genetics. Mamm Genome. 2012;23:28–39. doi: 10.1007/s00335-011-9362-2. [DOI] [PubMed] [Google Scholar]
- 48.Acland GM, Ray K, Mellersh CS, et al. Linkage analysis and comparative mapping of canine progressive rod-cone degeneration (prcd) establishes potential locus homology with retinitis pigmentosa (RP17) in humans. Proc Natl Acad Sci U S A. 1998;95:3048–53. doi: 10.1073/pnas.95.6.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein O, Zangerl B, Pearce-Kelling S, et al. Linkage disequilibrium mapping in domestic dog breeds narrows the progressive rod-cone degeneration interval and identifies ancestral disease-transmitting chromosome. Genomics. 2006;88:541–50. doi: 10.1016/j.ygeno.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zangerl B, Goldstein O, Philp AR, et al. Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics. 2006;88:551–63. doi: 10.1016/j.ygeno.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parker HG, Kim LV, Sutter NB, et al. Genetic structure of the purebred domestic dog. Science. 2004;304:1160–4. doi: 10.1126/science.1097406. [DOI] [PubMed] [Google Scholar]
- 52.Parker HG, Kukekova AV, Akey DT, et al. Breed relationships facilitate fine mapping studies: a 7. 8-kb deletion cosegregates with Collie eye anomaly across multiple dog breeds. Genome Res. 2007;17:1562–71. doi: 10.1101/gr.6772807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker HG, Ostrander EA. Canine genomics and genetics: running with the pack. PLoS Genet. 2005;1(5):e58. doi: 10.1371/journal.pgen.0010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutter NB, Bustamante CD, Chase K, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–5. doi: 10.1126/science.1137045. [Erratum, Science 2007; 316:1284.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cadieu E, Neff M, Quignon P, et al. Coat variation in the domestic dog is governed by variants in three genes. Science. 2009;326:150–3. doi: 10.1126/science.1177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quignon P, Schoenebeck JJ, Chase K, et al. Fine mapping a locus controlling leg morphology in the domestic dog. Cold Spring Harb Symp Quant Biol. 2009;74:327–33. doi: 10.1101/sqb.2009.74.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karlsson EK, Baranowska I, Wade CM, et al. Efficient mapping of mendelian traits in dogs through genome-wide association. Nat Genet. 2007;39:1321–8. doi: 10.1038/ng.2007.10. [DOI] [PubMed] [Google Scholar]
- 58.Schmutz SM, Berryere TG. Genes affecting coat colour and pattern in domestic dogs: a review. Anim Genet. 2007;38:539–49. doi: 10.1111/j.1365-2052.2007.01664.x. [DOI] [PubMed] [Google Scholar]
- 59.Salmon-Hillbertz NHC, Isaksson M, Karlsson EK, et al. Duplication of FGF3, FGF4, FGF19 and ORAOV1 causes hair ridge and predisposition to dermoid sinus in Ridgeback dogs. Nat Genet. 2007;39:1318–20. doi: 10.1038/ng.2007.4. [DOI] [PubMed] [Google Scholar]
- 60.Wilbe M, Jokinen P, Truvé K, et al. Genome-wide association mapping identifies multiple loci for a canine SLE-related disease complex. Nat Genet. 2010;42:250–4. doi: 10.1038/ng.525. [DOI] [PubMed] [Google Scholar]
- 61.Hansson-Hamlin H, Lilliehöök I. A possible systemic rheumatic disorder in the Nova Scotia duck tolling retriever. Acta Vet Scand. 2009;51:16. doi: 10.1186/1751-0147-51-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strang A, MacMillan G. The Nova Scotia duck tolling retriever. Loveland, CO: Alpine Publications; 1996. [Google Scholar]
- 63.Khanna C, Lindblad-Toh K, Vail D, et al. The dog as a cancer model. Nat Biotechnol. 2006;24:1065–6. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- 64.Bronson RT. Variation in age at death of dogs of different sexes and breeds. Am J Vet Res. 1982;43:2057–9. [PubMed] [Google Scholar]
- 65.Maquat LE. Defects in RNA splicing and the consequence of shortened translational reading frames. Am J Hum Genet. 1996;59:279–86. [PMC free article] [PubMed] [Google Scholar]
- 66.Moore PF, Affolter VK, Vernau W. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Vet Pathol. 2006;43:632–45. doi: 10.1354/vp.43-5-632. [DOI] [PubMed] [Google Scholar]
- 67.Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74–83. doi: 10.1354/vp.39-1-74. [DOI] [PubMed] [Google Scholar]
- 68.Moore PF, Rosin A. Malignant histiocytosis of Bernese mountain dogs. Vet Pathol. 1986;23:1–10. doi: 10.1177/030098588602300101. [DOI] [PubMed] [Google Scholar]
- 69.Fulmer AK, Mauldin GE. Canine histiocytic neoplasia: an overview. Can Vet J. 2007;48:1041–50. [PMC free article] [PubMed] [Google Scholar]
- 70.Grogan TM, Pileri SA, Chan JKC, Weiss LM, Fletcher CDM. Histiocytic and dendritic cell neoplasms. 4. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 71.Shearin AL, Hedan B, Cadieu E, et al. The MTAP-CDKN2A locus confers susceptibility to a naturally occurring canine cancer. Cancer Epidemiol Biomarkers Prev. 2012 May 23; doi: 10.1158/1055-9965.EPI-12-0190-T. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quignon P, Herbin L, Cadieu E, et al. Canine population structure: assessment and impact of intra-breed stratification on SNP-based association studies. PLoS One. 2007;2(12):e1324. doi: 10.1371/journal.pone.0001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Debniak T, Górski B, Huzarski T, et al. A common variant of CDKN2A (p16) predisposes to breast cancer. J Med Genet. 2005;42:763–5. doi: 10.1136/jmg.2005.031476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41:905–8. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bishop DT, Demenais F, Iles MM, et al. Genome-wide association study identifies three loci associated with melanoma risk. Nat Genet. 2009;41:920–5. doi: 10.1038/ng.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–5. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 77.Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–65. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–82. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaplan J. Leber congenital amaurosis: from darkness to spotlight. Ophthalmic Genet. 2008;29:92–8. doi: 10.1080/13816810802232768. [DOI] [PubMed] [Google Scholar]
- 81.Miyadera K, Acland GM, Aguirre GD. Genetic and phenotypic variations of inherited retinal diseases in dogs: the power of within- and across-breed studies. Mamm Genome. 2012;23:40–61. doi: 10.1007/s00335-011-9361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spady TC, Ostrander EA. Canine behavioral genetics: pointing out the phenotypes and herding up the genes. Am J Hum Genet. 2008;82:10–8. doi: 10.1016/j.ajhg.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Overall KL. Natural animal models of human psychiatric conditions: assessment of mechanism and validity. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:727–76. doi: 10.1016/s0278-5846(00)00104-4. [DOI] [PubMed] [Google Scholar]
- 84.Moon-Fanelli AA, Dodman NH. Description and development of compulsive tail chasing in terriers and response to clomipramine treatment. J Am Vet Med Assoc. 1998;212:1252–7. [PubMed] [Google Scholar]
- 85.Dodman NH, Karlsson EK, Moon-Fanelli A, et al. A canine chromosome 7 locus confers compulsive disorder susceptibility. Mol Psychiatry. 2010;15:8–10. doi: 10.1038/mp.2009.111. [DOI] [PubMed] [Google Scholar]