Abstract
Study Design
In vitro experiment using human intervertebral disc (IVD) cells and adenovirus-therapeutic gene constructs.
Objectives
To examine the biologic effect of “cocktail” therapeutic gene transfer to human IVD cells in three dimensional cultures.
Summary of Background Data
. Gene therapy is regarded as a potential option for the treatment of degenerative disc disease. Although various anabolic genes have previously been introduced for this purpose, cocktail gene transfer of anabolic genes to IVD cells has never been attempted.
Materials and Methods
Human IVDs were harvested during surgical disc procedures and cultured. We prepared recombinant adenovirus constructs bearing the TGF-β1 gene (Ad/TGF-β1), the IGF-1 gene (Ad/IGF-1), and the BMP-2 gene (Ad/BMP-2). Transgene expression was detected by luciferase assays, enzyme linked immuno-sorbent assays, and Western blot analysis. Newly synthesized proteoglycan was measured by 35S-sulfate incorporation on Sephadex G-25M in PD 10 columns. Human IVD cells were transduced by single, double, and triple combination of Ad/TGF-β1, Ad/IGF-1, Ad/BMP-2 with an MOI of 75, then cultured three-dimensionally in alginate beads.
Results
Transgene expression was detected at 18 hours after viral transduction. IVD cultures with Ad/TGF-β1, Ad/IGF-1, Ad/BMP-2 (MOI of 75) showed 2.9, 1.8, and 1.9 fold increases, respectively, in proteoglycan synthesis compared to control. Human IVD cultures with double gene combination (MOI of 75) showed 3.2 to 3.9 fold increases of proteoglycan synthesis. Lastly, Human IVD cultures with triple gene combination (TGF-β1+IGF-1+BMP-2 genes with an MOI of 75) transfer demonstrated 4.7 fold increase in proteoglycan synthesis compared control.
Conclusion
Combination or “cocktail” gene therapy offers a promising mechanism for maximizing matrix synthesis with low dose of adenoviral mixtures, circumventing systemic, local toxic effect, and immune response.
Keywords: Gene Therapy, Intervertebral Disc, Proteoglycan
Introduction
Intervertebral disc (IVD) disease accounts for approximately one quarter of all cases of back pain and is a leading source of health care cost, lost wages and patient morbidity. 1 The IVD has two structural components, the nucleus pulposus and the annulus fibrosus. Proteoglycan, a major component of the nucleus pulposus, is known to imbibe water and provide positive swelling pressure for the nucleus pulposus. 2 The IVD provides biomechanical stability that stems from the high positive pressure in the nucleus pulposus combined with the integrity of the tensile annulus fibrosus. 3 Therefore having an appropriate amount of proteoglycan in the nucleus pulposus, together with an intact annulus, is believed to be crucial for the optimal function of the IVD. Although the etiology and pathophysiology of IVD degeneration are largely unknown, some evidence indicates that it results from an imbalance in proteoglycan synthesis and catabolism, apoptosis, and other imbalances in complex self-regulatory mechanisms involving various cytokines and enzymes. 2, 4–9 The loss of proteoglycan may directly affect the biomechanical functions of IVD, altering loading of the facet joint and other structures, causing degenerative changes of the spine. 10
Growth factors such as transforming growth factor-β1 (TGF-β1), insulin like growth factor-1 (IGF-1), osteogenic protein-1 (OP-1), and bone morphogenetic protein-2 (BMP-2) appear to have promising therapeutic properties such as stimulating proteoglycan synthesis and maintaining the chondrogenic phenotype of IVD. 11–14 Nevertheless direct and repetitive injections of growth factor protein(s) to the IVD do not appear feasible for chronic types of disease, such as disc degeneration, that require sustained delivery of growth factor(s) throughout the disease course. An alternative means of delivering exogenous growth factors is the genetic modification of IVD cells through gene transfer such that the cells continuously manufacture the desired growth factors endogenously.
The definition of gene therapy has become quite broad. It no longer refers strictly to the treatment of an existing disorder by replacement of a defective gene with a functional copy by gene transfer. 15 Instead, gene therapy is now defined by use of nucleic acid transfer, either RNA or DNA, to treat or prevent a disease.16 In previous studies, we successfully used an adenovirus-mediated gene transfer technique to directly deliver exogenous genes to rabbit lumbar IVDs in vitro and in vivo.17, 18 Furthermore we demonstrated the susceptibility of human IVD cells to adenovirus-mediated gene transfer.19 Despite the promising effects of gene therapy, the therapeutic transfer of single genes using high doses of viral vector appears hazardous in terms of systemic effects, cytotoxicity, and immune response.21 Combination or cocktail therapy appears to have distinct advantages that enhance therapeutic potential and lessen side effects. To achieve maximal anabolic effect in terms of proteoglycan synthesis, selection of therapeutic genes for cocktail therapy is of prime importance. In literature survey, growth factors i.e., TGF-β1, IGF-1, and BMP-2 appear to be feasible candidates for matrix regeneration of IVD. Hence, therapeutic genes of TGF-β1, IGF-1, and BMP-2 are selected for the experiment. 11, 12, 14 Viral induced toxicity is thought to be critical in the clinical application of gene therapy, especially in adenovirus mediated gene delivery. 21–24 Therapeutic cocktail gene transfer might represent an important tool to enhance the regeneration of the matrix of the IVD while reducing the administered viral dose.
Accordingly, the objectives of this in vitro experimental study were first, to attempt cocktail gene therapy with human IVD cells cultured in three dimensional alginate beads and second, to determine which cocktail or combinations can produce synergistic or additive effect on matrix synthesis.
Materials and Methods
All of the experimental protocols were approved by the human subjects Institutional Review Board.
Patient data and tissue acquisition procedures
Lumbar IVD tissue was obtained from twenty-two patients (Age range 25 to 59 years) during surgical disc procedures performed for herniated disc and spinal stenosis. Classification of the intervertebral disc of each patient was performed based on magnetic resonance images of each disc as described in the literature.24 IVDs with grade III and IV degeneration were exclusively harvested to minimized the effect of IVD degeneration on transgene expression and proteoglycan synthesis. An attempt was made by the operating surgeon (JDK, SHM) to carefully obtain tissue from the central aspect of the disc to optimize harvest of only the nucleus pulposus and the inner annulus fibrosus.
The disc tissue specimens were transported in sterile GBSS to the laboratory, less than 20 minutes following surgical removal and then were washed with Gey’s balanced salt solution (GBSS, GIBCO-BRL, Grand Island, NY) to remove blood and bodily fluid contaminants.
Adenoviral vectors
Four different adenoviral constructs were prepared for this study: Adenovirus luciferase construct (Ad/luciferase) encoding firefly luciferase as a viral control, adenovirus TGF-β1 construct (Ad/TGF-β1), adenovirus IGF-1 construct (Ad/IGF-1), and adenovirus BMP-2 construct (Ad/BMP-2). Each recombinant adenoviral vector originated from replication-deficient type 5 adenovirus lacking the E1 and E3 regions of the genome.15 Each luciferase, TGF- β1, IGF-1, BMP-2 genes were cloned respectively into the E1 region under the control of the human cytomegalovirus early promoter. Recombinant virus was grown in transformed human embryonic kidney 293 cells and underwent CsC1 density gradient purification. Titers were determined by optical density at 260nm (OD260) and a standard plaque assay. 25, 26
Disc cell culture method
Any obvious granulation tissue, dense outer annulus, and cartilaginous endplate tissues were removed carefully from the disc tissue specimens. Disc cells were then isolated from the inner annulus and nucleus pulposus as described previously.27 Briefly, the dissected specimens were minced with a scalpel into fragments approximately two cubic millimeters in volume. Disc tissues from the nucleus pulposus and inner annulus were digested for 60 minutes at 37° C under gentle agitation in a medium composed of equal parts of Ham’s F-12 medium and Dulbecco’s Modified Eagle Medium (F12/DMEM, GIBCO-BRL, Grand Island, NY) containing 5% heat-inactivated fetal bovine serum (FBS, GIBCO-BRL, Grand Island, NY) with 0.2% pronase (Calbiochem, La Jolla, CA) and 0.004% deoxyribonuclease II type IV (DNAse, Sigma, St. Louis, MO). The tissue to solution ratio was 1:5. The tissue was then washed 3 times with F12/DMEM and digested overnight under the same conditions, except that the pronase was replaced with bacterial 0.02% collagenase type II (Sigma, St. Louis, MO). The cells were washed once with F12/DMEM, pelleted by centrifugation at 2000 RPM for 10 minutes, and then resuspended in F12/DMEM. Suspended cells were filtered through a sterile nylon mesh filter (pore size: 75 um) to remove large cell aggregates and debris, and then were counted in a haemocytometer and plated in 24-well plates (Falcon, Franklin Lakes, NJ) at a density of approximately 5×105 cells/ml. Primary cultures were sustained for 3 weeks in F12/DMEM containing 10% FBS, 1% v/v penicillin, streptomycin and nystatin (all antibiotics from GIBCO-BRL, Grand Island, NY) in a 5% CO2 incubator with humidity. Culture medium was changed twice a week.
Cell viability was determined by trypan blue exclusion test. 28 Under microscopic observation, round birefringent cells without blue staining were regarded as viable cells, and the percentage of viable cells to total cells was calculated.
Incorporation of isolated cells into alginate beads
The preparation of intervertebral disc cells in alginate beads was performed as described elsewhere.28 Briefly, isolated cells from primary culture with trysinization were resuspended in sterile 0.15M NaCl containing 1.2% low-viscosity alginate (Kelco, Chicago, IL) at a density of one million cells per milliliter, then slowly expressed through a 22 gauge needle in a dropwise fashion into 102mM CaCl2 solutions. After instaneous gelation, the beads were allowed to polymerize further for a period of 10 minutes in the CaCl2 solution. The beads were then washed once in 10 volume of 0.15M NaCl and three times in 10 volumes of F12/DMEM medium. The beads were finally placed in complete culture medium. Ten beads (containing an average number of cells/bead) were cultured in each well of a 24-well plate.
Depolymerization of alginate bead
To remove cells from the alginate bead, wells were rinsed twice with 0.15M NaCl with gentle pipetting into the well. The rinse solution was incubated for 1 minute and was aspirated off. Three times the volume of alginate in dissolving buffer (55mM sodium citrate and 0.15M NaCl) was added to the wells and plates were incubated at 37 °C for 10 minutes with shaking.
In-vitro transduction of the human disc cells
At full confluence, the disc cell cultures were organized into four groups. In Group 1 (Saline or viral control): treatment of IVD cells in monolayer culture with normal saline or Ad/luciferase and incorporation of treated cells into alginate beads. In Group 2 (Single therapeutic gene transfer): adenoviral transduction with Ad/TGF-β1, Ad/IGF-1, and Ad/BMP-2 respectively in monolayer culture and incorporation of transduced cells into alginate beads, In Group 3 (Double therapeutic gene transfer): adenoviral transduction with double combination of Ad/TGF-β1, Ad/IGF-1, and Ad/BMP-2, i.e., Ad/TGF-β1+Ad/IGF-1, Ad/TGF-β1+Ad/BMP-2, and Ad/IGF-1+Ad/BMP-2. In Group 4 (Triple therapeutic gene transfer): adenoviral transduction with triple combination of Ad/TGF-β1, Ad/IGF-1, and Ad/BMP-2. Viral concentration was calculated as 100 adenoviral particles for 1 plaque-forming unit (PFU) and 1 PFU per IVD cell as 1 multiplicity of infection (MOI). PFU of 5×105 in each culture well rendered 1 MOI of adenoviral transduction since approximately 5×105 IVD cells were cultured in 24 well culture plates. Total viral concentration was adjusted to 75 MOI in viral control, single, double, and triple combination groups. For example, concentration of each adenoviral construct in double combination group was 37.5 MOI and concentration of each adenovirus construct in triple combination group was 25 MOI, therefore the total concentration of adenoviral vector was 75 MOI.
In detail, conditioned culture medium was aspirated and cultures were rinsed three times with GBSS. Then cultures were treated with 50 ul of GBSS containing Ad/luciferase, Ad/TGF-β1, Ad/IGF-1, Ad/BMP-2 in single or combination as described previously. Then cultures were incubated in 5% CO2 at 37° C with humidity for one hour under gentle agitation to facilitate transduction. Afterwards IVD cells were incorporated into alginate beads as described above.
After each transduction procedure, Newman Tytell serumless media (Gibco BRL, Grand Island, NY) was added to cultures to detect transgene expression. Conditioned culture medium was added to each well, and the alginate beads were further incubated in 5% CO2 at 37° C with humidity for 1 week with daily medium change.
Analysis of transgene expressions
Luciferase assay
Luciferase gene expression was assessed using a standard luciferase assay.29 The culture medium was removed and the alginate beads were rinsed twice with PBS. Then beads were depolymerized as described. Cell culture lysis buffer (25 mM Tris-phosphate, pH 7.8, 2 mM dithiothreitol, 2 mM diaminocyclohexane-tetraacetic acid, 10% glycerol, and 1% Triton X-100) was added in each well to cover the cells. After brief incubation, cells were scraped from the culture well and transferred to a microcentrifuge tube (Fisher Scientific, Pittsburgh, PA). After brief centrifugation (12,000g for 5 seconds), the supernatant (cell extract) was transferred to a new tube. Luciferase assay reagent (20 mM Tricine, pH 7.8, 1.07mM (MgCO3)4Mg(OH)2 5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 33.3 mM dithiothreitol, 270 uM coenzyme A, 470 uM D-luciferin, and 530 uM ATP) was mixed with the cell extract. Light production over a period of 30 seconds was measured with luminometer (Autolumat LB 953, EG & G, Gaithersburgh, MI) and expressed as relative light units (RLU).
Enzyme linked immunosorbent assay for TGF-β1 transgene expression
TGF-β1 content was measured by enzyme linked immunosorbent assay (ELISA) (ELISA kit for human TGF -β1; R&D System, Inc., Minneapolis, MN). Because this receptor based ELISA detects only levels of bioactive TGF-β1, serial time course samples of supernatant from IVD cell culture after transduction with Ad/TGF-β1 (150 MOI) were collected and subjected to acidification to preactivate them, thus enabling quantification of the total (active +latent) protein by ELISA.
Western Blot analysis for TGF-β1, IGF-1, and BMP-2 transgene expressions
Transduced human IVD cells were sonicated in 200 ul ice cold lysis buffer (1x PBS, 70mM β-GP, 0.1 mM sodium vanadate, 2 mM MgCl2, 1 mM EGTA, 1 mM DTT, 0.5% triton X-100, 0.2 mM PMSF, 1/1000 v/v protease inhibitor) for 5 sec. Crude lysates were centrifuged at 15,000 rpm for 15 min at 4°C to remove cellular debris. Supernatant was transferred to another tube. Protein concentration was determined by Bradford assay (BioRad) using bovine serum albumin as the standard. Proteins were separated by Tricine-SDS-PAGE gel electrophoresis. Proteins were diluted in 5x gel loading buffer, boiled for 5 min, and loaded onto 13% resolving gel containing a 4% stacking gel. After electrophoresis, proteins were transferred to Hybond-P (Amersham pharmacia) membrane. Western analysis was performed by incubating the membranes with the indicated primary antibody followed by horseradish peroxidase-conjugated secondary antibody according to the rapid detection protocol provided by Amersham Pharmacia. Immune complexes on the membranes were visualized by enhanced chemiluminescence using an ECL kit. Primary antibody was used at a 1:2000-anti-α-tublin, 1:2000- anti-TGF- β1(rabbit IgG, Santa Cruz), 1:500-anti-IGF-1, or 1:2000-anti-BMP-2(mouse IgG, R&D) dilution.
Measurement of newly synthesized proteoglycan
On day 2, cultures were administered with fresh Newman-Tytell serumless medium containing 35S-sulfate (final concentration 20 °Ci/ml). After a period of 4 hours, alginate cultures were depolymerized as described previously and depolymerized cultures with labeling medium were extracted at 4° C for 48 hour by addition of an equal volume of 8M guanidine hydrochloride, 20 mM EDTA and a mixture of proteinase inhibitors.30 For quantitative evaluation of 35S-sulfate labeled proteoglycans, aliquots (200 uL) of the stored extracts were eluted on Sephadex G-25M in PD 10 columns (Pharmacia Biotech, Uppsala, Sweden) under dissociative conditions.30 Then 1 mL of fractions were collected in scintillation vials, mixed with 6 mL of scintillation mixture (Ultima Gold, Packard, Meriden, CT), and counted in a liquid scintillation counter (Packard #1900 TR, Mariden, CT). Scintillation count was normalized by cell number of each experimental group.
Statistical analysis
Data are presented as mean ± the standard deviation. SPSS software was utilized for data analysis (SPSS Inc, Chicago, IL). One-way analysis of variance with Fisher’s protected LSD post-hoc test and Student t-test was conducted to examine difference of proteoglycan synthesis among the experimental groups. Significance level was set at p<0.05.
Results
Disc cell yield and viability
The average yield of cells from the disc tissue was 5×106 cells/gram of harvested tissue Initial cell viability upon isolation was 95% to 100% based on the trypan blue exclusion tests. The transduction and incorporation into alginate beads were found to be innocuous for cell survival, with the cells retaining 90–95% viability immediately after the procedure.
Luciferase gene expression
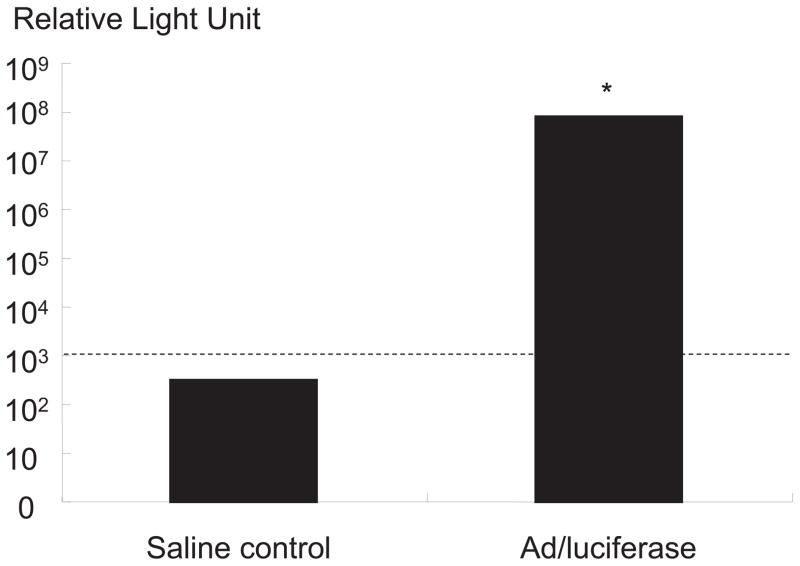
IVD cells with Ad/luciferase with an MOI of 75, cultured in alginate beads, exhibited a marked increase in luciferase activity (8.8 ±0.6) × 107 RLU over saline control (Figure 1). The result of this marker gene study proves highly efficient transduction in monolayer and expression of transduced gene even in alginate beads.
Figure 1.
Transgene expression of luciferase gene in human disc cells with Ad/luciferase with an MOI of 75, cultured in alginate beads. Cultures with Ad/luciferase exhibited marked increase in luciferase expression compared to control culture with saline.
Time course of TGF-β1 transgene expression
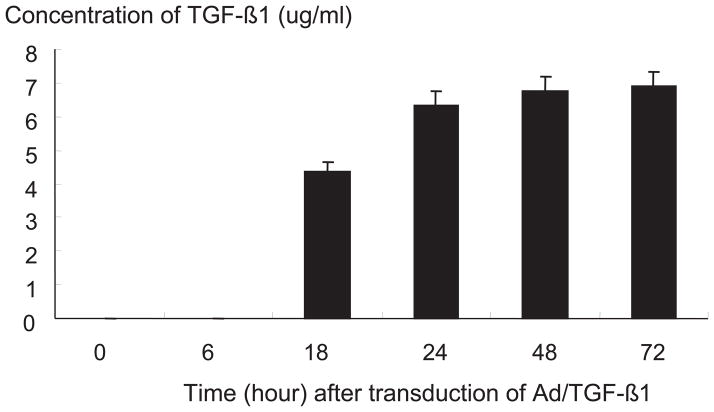
The supernatant from IVD cells with Ad/TGF-β1, cultured in monolayer, exhibited increased production of total TGF-β1 content at 18, 24, 48, 72 hours after viral transduction (p<0.05) (Figure 2)
Figure 2.
Transgene expression of human intervertebral disc cells with Ad/TGF-β1, cultured in monolayer, and measured at 18, 24, 48, 72 hours after adenoviral transduction.
TGF-β1, IGF-1, and BMP-2 transgene expression
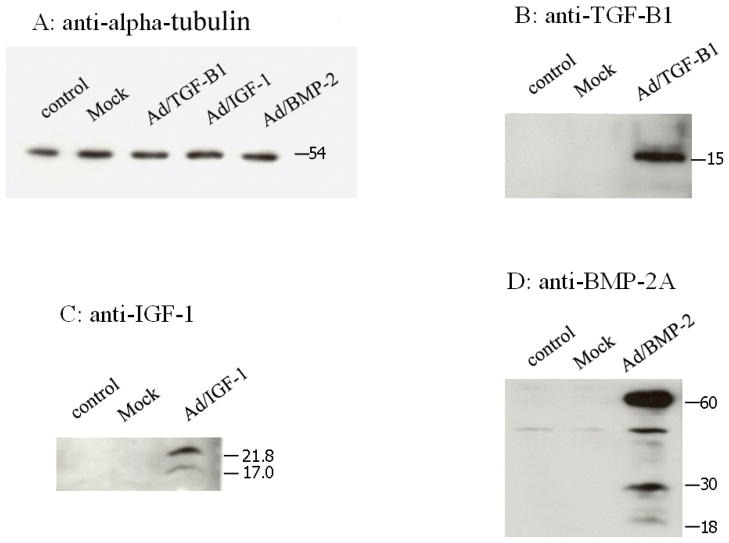
Western analysis demonstrated that IVD cell cultures transduced with Ad/TGF-β1, Ad/BMP-2, Ad/IGF-1 all expressed their respective proteins, while cultures with saline and Ad/luciferase (mock virus) showed no detectable protein expression (Figure 3).
Figure 3.
Western blot analysis for transgene expression for TGF-β1, IGF-1, BMP-2 gene. Alpha-tubulin was utilized as control. Mock denotes Ad/luciferase served as viral control. Anti-TGF- β1, anti-IGF-1, anti-BMP-2A antibody were utilized in the assay.
Proteoglycan synthesis
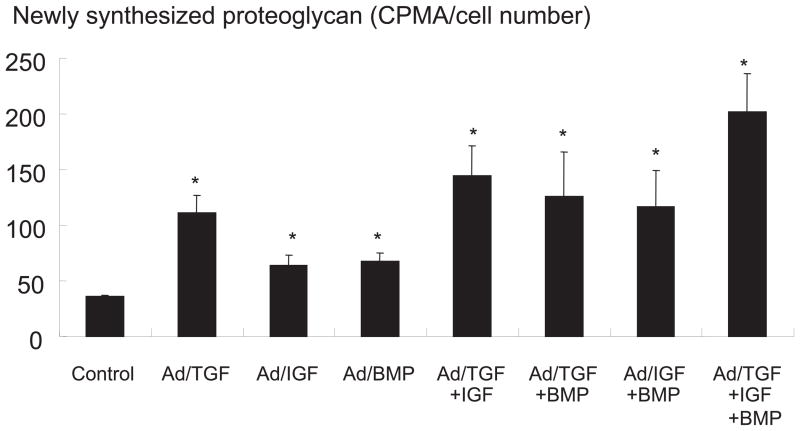
There was no statistically significant increase in newly synthesized proteoglycan in the Ad/luciferase group compared to saline control. In IVD culture, transduced with a single gene, there was a statistically significant 2.9 fold increase of newly synthesized proteoglycan in the Ad/TGF-β1 group, a 1.8 fold increase in the Ad/IGF-1 group, and a 1.9 fold in the Ad/BMP-2 group compared to the saline and viral controls (p<0.05). In cultures transduced with a double combination of therapeutic genes, there was a 3.9 fold increase of newly synthesized proteoglycan in the Ad/TGF-β1+Ad/IGF-1 group, a 3.2 fold increase in Ad/IGF-1+Ad/BMP-2, and a 3.5 fold increase in Ad/TGF-β1+Ad/BMP-2 compared to the saline and viral controls (p<0.05). In culture transduced with a triple combination of therapeutic genes, there was a 4.7 fold increase of newly synthesized proteoglycan in the Ad/TGF-β1+Ad/IGF-1+Ad/BMP-2 group compared to saline and viral controls (p<0.05) (Figure 4).
Figure 4.
Proteoglycan synthesis as measured 35S-sulfate incorporation after transduction of single, double and triple combination of Ad/TGF-β1, Ad/IGF-1, and Ad/BMP-2. Total amount of adenovirus were 75 MOI in all groups. * p<0.05
Discussion
Gene therapy involves the introduction of a nucleic acid sequence encoding a gene of interest into a population of cells such that the cells express that particular gene.15 By altering the genetic complement of a cell, the cell can serve as a “protein producing factory” affecting not only its own metabolism, but the metabolism of adjacent non-genetically altered cells.31 To advocate gene therapy as a treatment for disc degeneration, several steps are needed: first, in vitro and in vivo feasibility of marker gene transfer to confirm susceptibility of disc tissue to gene transfer, second, elucidation of biologic effects of the therapeutic gene in disc tissue, and finally therapeutic gene therapy in an appropriate animal model for disc degeneration. In previous studies, we demonstrated the feasibility of marker gene transfer and the biologic modulatory effect of a therapeutic gene (TGF-β1) in rabbits, and we also demonstrated that human IVD cells are genetically modifiable in vitro by adenovirus-mediated gene transfer.17–19 The feasibility of human in vivo studies is virtually limited because of ethical reasons. Therefore thorough validation of the biologic modulatory effect of gene therapy in an acceptable in vitro system is mandatory before planned clinical trials.
Efficient and reliable methods to deliver exogenous gene(s) to the intervertebral disc have become available with recent developments in molecular biology and vector technology. The optimal combination of exogenous genes for the management of degenerative disc disease, however, is unknown. While the cDNAs of anabolic growth factors as well as catabolic cytokines and enzymes are available, most research efforts to date have focused on enhancing the anabolic aspects of disc metabolism—hence the emphasis has been on anabolic growth factors. The current study has investigated the effect of combination or “cocktail” gene therapy on disc matrix synthesis by human IVD cells cultured in three-dimensional alginate beads. The current study has demonstrated robust anabolic effects on matrix synthesis which seem to be additive, depending upon the combination of genes applied. Enhanced biologic effects with cocktail gene therapy provide a valuable mechanism to reduce the amount of viral vector in gene therapy. For instance, IVD cells with Ad/TGF-β1 with an MOI of 75 showed a 2.9 fold increases in proteoglycan synthesis, while triple gene cocktail (Ad/TGF-β1, Ad/IGF-1, Ad/BMP-2) with the same MOI demonstrated a 4.7 fold increase in proteoglycan synthesis compared to control cultures. To achieve 2.9 fold incrseases of proteoglycan synthesis, approximately 45 MOI of adenoviral vector would be sufficient with triple gene combination, while 75 MOI of adenoviral vector is needed with Ad/TGF-β1. Gene therapy with lower doses of virus has several advantages, firstly, avoiding systemic local toxic effects, secondly, circumventing immune response and minimizing the possible occurrence of replicating virus. 32, 33
The mechanism underlying the enhanced biologic effect of cocktail gene therapy using TGF-β1, IGF-1, and BMP-2 gene is still under investigation. Possible explanations are receptor saturation, negative feedback, and different intracellular signal transduction pathways. TGF-β1 and BMP-2 both function in the TGF-superfamily pathway, nevertheless, after receptor activation, Smad2 and Smad4 for TGF- β1, Smad1 and Smad4 for BMP-2 were activated and form complex with Smad4.34 Furthermore IGF-1 utilizes a different signal transduction pathway than the TGF-superfamily pathway.35 Therefore different signal molecules and pathways of TGF-β1, IGF-1, and BMP-2 enable enhanced biologic responses such as the proteoglycan synthesis induced by cocktail gene therapy.
Extrapolation of the current in vitro results obtained with cultured human disc cells to in vivo clinical situations is, of course, premature and requires further validation of the combination gene therapy approach in animal models. We are also actively characterizing gene expression profiles as a function of the disc degeneration process to better guide the optimal selection of genes for therapeutic/prophylactic intradiscal gene therapy approaches.
In conclusion, combination or “cocktail” gene therapy offers a promising mechanism for maximizing matrix regeneration with a minimal dose of adenoviral mixtures.
References
- 1.Anderson JAD. In: Back pain and occupation. The lumbar Spine and Back Pain. 3. Jayson MIV, editor. London: Chirchill Livingstone; 1987. pp. 2–36. [Google Scholar]
- 2.Lipson SJ, Muir H. Proteoglycan in experimental intervertebral disc degeneration. Spine. 1981;6:194–210. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Palmer EI, Lotz JC. The compressive creep properties of normal and degenerated murine intervertebral discs. J Orthop Res. 2004;22:164–9. doi: 10.1016/S0736-0266(03)00161-X. [DOI] [PubMed] [Google Scholar]
- 4.Goupille P, Jayson MIV, Valat J-P, et al. Matrix metalloproteinases: The clue to intervertebral disc degeneration? Spine. 1998;14:1612–6. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 5.Gronbald M, Virri J, Ronkko S, et al. A controlled biochemical and immunohistochemical study of human synovial-type(group II) phospholipase A2 and inflammatory cells in macroscopically normal, degenerated, and herniated human lumbar disc tissues. Spine. 1996;22:2531–8. doi: 10.1097/00007632-199611150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Kanemoto M, Hukuda S, Komiya Y, et al. Immunohistochemical study of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 in human intervertebral dics. Spine. 1996;21:1–8. doi: 10.1097/00007632-199601010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–7. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Spine. 1997;22:1065–73. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kim KW, Kim YS, Ha KY, et al. An autocrine or paracrine Fas-mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine. 2005;30:1247–51. doi: 10.1097/01.brs.0000164256.72241.75. [DOI] [PubMed] [Google Scholar]
- 10.Butler D, Trafimow JH, Andersson GB, et al. Discs degenerate before facets. Spine. 1990;15:111–3. doi: 10.1097/00007632-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature cannine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Osada R, Oshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, Tsuji H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insuline-like growth factors-1 on proteoglycan synthesis in bovine intervertebral discs. J Ortho Res. 1996;14:690–9. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 13.Takegami K, An HS, Kumano F, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–8. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Kim DJ, Moon SH, Kim H, et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28:2679–84. doi: 10.1097/01.BRS.0000101445.46487.16. [DOI] [PubMed] [Google Scholar]
- 15.Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47. [PubMed] [Google Scholar]
- 16.Crystal RG. Transfer of genes to human: early lessons and obstacles to success. Science. 1995;270:404–10. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 17.Nishida K, Kang JD, Suh J-K, Robbins PD, Evans CH, Gilbertson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells: Implication for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437–43. doi: 10.1097/00007632-199811150-00016. [DOI] [PubMed] [Google Scholar]
- 18.Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–25. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Moon SH, Gilbertson LG, Nishida K, et al. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: implications for the clinical management of intervertebral disc disorders. Spine. 2000;25:2573–9. doi: 10.1097/00007632-200010150-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lozier JN, Csako G, Mondoro TH, et al. Toxicity of a first-generation adenoviral vector in rhesus macaques. Hum Gene Ther. 2002;13:113–24. doi: 10.1089/10430340152712665. [DOI] [PubMed] [Google Scholar]
- 21.Lohr F, Huang Q, Hu K, et al. Systemic vector leakage and transgene expression by intratumorally injected recombinant adenovirus vectors. Clin Cancer Res. 2001;7:3625–8. [PubMed] [Google Scholar]
- 22.Nemunaitis J, Cunningham C, Buchanan A, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–59. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 23.Mazzolini G, Narvaiza I, Perez-Diez A, et al. Genetic heterogeneity in the toxicity to systemic adenoviral gene transfer of interleukin-12. Gene Ther. 2001;8:259–67. doi: 10.1038/sj.gt.3301387. [DOI] [PubMed] [Google Scholar]
- 24.Eyre D, Benya P, Buckwalter J, Frymoyer JW, Gordon SL. New Perspectives in Low Back Pain. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1989. Intervertebral disc: Part B. Basic science perspective; pp. 147–207. [Google Scholar]
- 25.Graham FL, Ajvd Eb. A new technique for assay of infectivity of human adenovirus 5 DNA. Virol. 1973;52:456–7. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 26.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chelberg MK, Banks GM, Geiger DF, et al. Identification of heterogenous cell populations in normal human intervertebral disc. J Anat. 1995;186:43–53. [PMC free article] [PubMed] [Google Scholar]
- 28.Maldonado BA, Oegema TR. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10:677–90. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 29.De Wet JR, Wood KV, Deluca M, et al. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–37. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tiss Res. 1988;18:223–34. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- 31.Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg Am. 1995;77:1103–13. doi: 10.2106/00004623-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Viggeswarapu M, Boden SD, et al. Overcoming the immune response to permit ex vivo gene therapy for spine fusion with human type 5 adenoviral delivery of the LIM mineralization protein-1 cDNA. Spine. 2003;28:219–26. doi: 10.1097/01.BRS.0000042417.37236.3F. [DOI] [PubMed] [Google Scholar]
- 33.Varnavski AN, Calcedo R, Bove M, et al. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12(5):427–36. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- 34.Lai CF, Cheng SL. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J Biol Chem. 2002;277:15514–22. doi: 10.1074/jbc.M200794200. [DOI] [PubMed] [Google Scholar]
- 35.Adams TE, McKern NM, Ward CW. Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor. Growth Factors. 2004;22:89–95. doi: 10.1080/08977190410001700998. [DOI] [PubMed] [Google Scholar]