Abstract
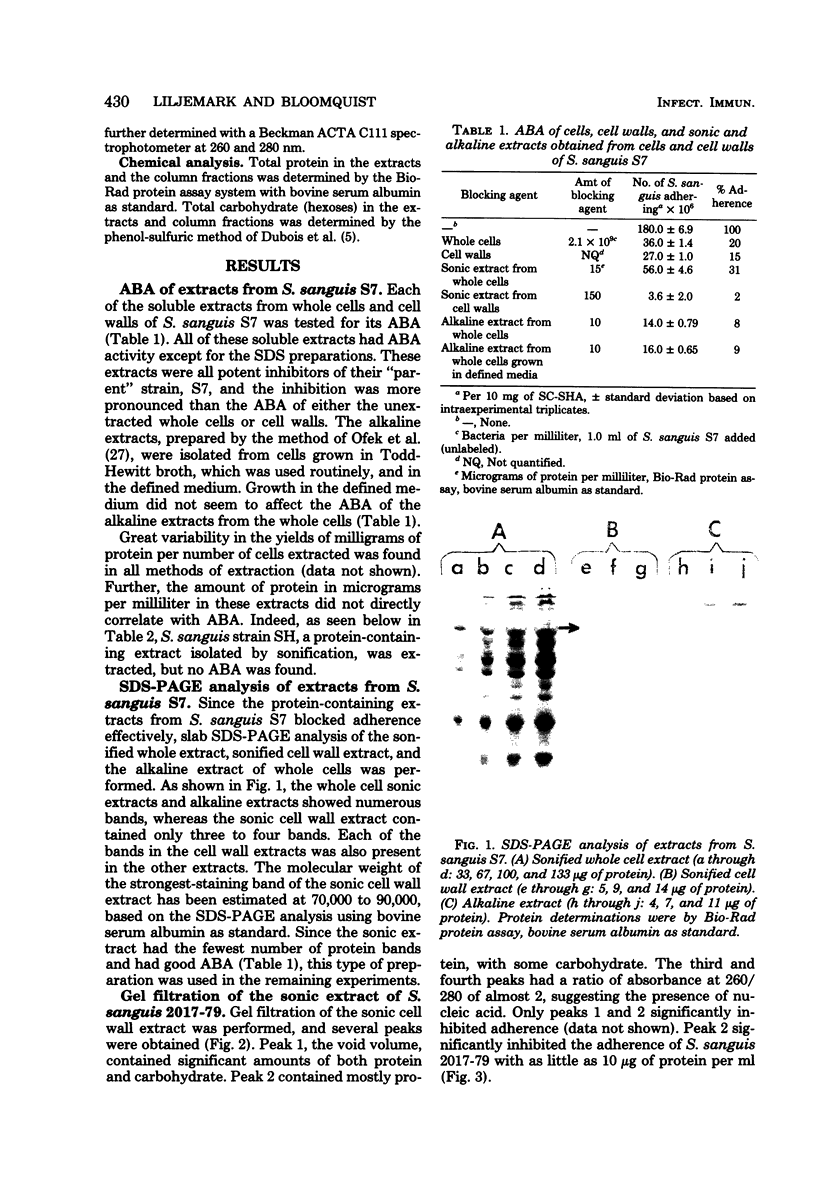
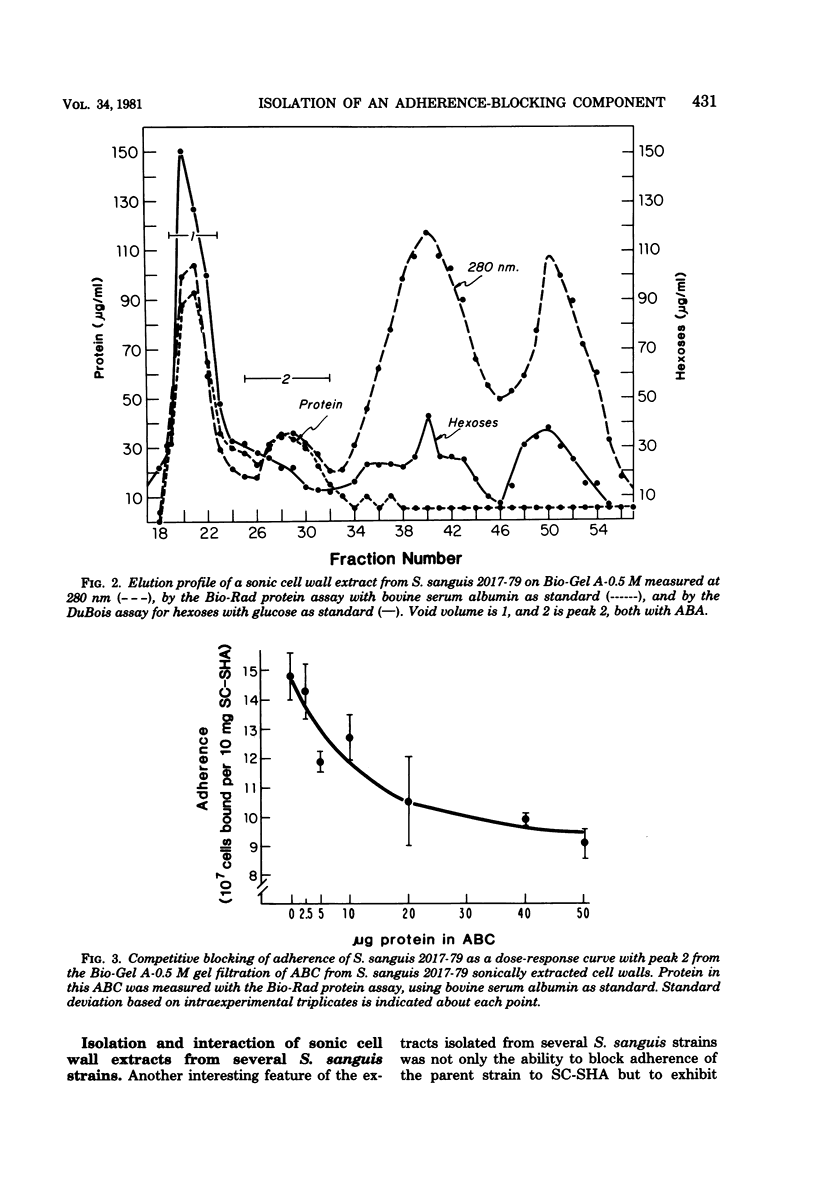
The isolation and partial characterization of a protein-containing cell surface component from Streptococcus sanguis which blocks the adherence of this microbe to saliva-coated hydroxyapatite are described. Several methods of extraction were attempted. Sonication of whole cells and cell walls proved to be the most successful and yielded biologically active adherence-blocking components. The adherence-blocking ability of these components was effective in intraspecies blocking experiments. The extract obtained from cell walls of S. sanguis was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and shown to contain one major and two to three minor bands when stained with Coomassie blue. The molecular weight of the major band was estimated to be 70,000 to 90,000. Gel filtration of the sonified cell wall extract on 10% agarose yielded two active adherence-blocking peaks, the void volume and a second peak.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton R. W. Adherence of oral streptococci to hydroxyapatite in vitro via glycerol-teichoic acid. Arch Oral Biol. 1980;25(2):111–114. doi: 10.1016/0003-9969(80)90085-0. [DOI] [PubMed] [Google Scholar]
- Chiu T. H., Emdur L. I., Platt D. Lipoteichoic acids from Streptococcus sanguis. J Bacteriol. 1974 May;118(2):471–479. doi: 10.1128/jb.118.2.471-479.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Rölla G., Bowen W. H., Reilly J. A. Adsorption of Streptococcus mutans lipoteichoic acid to hydroxyapatite. Scand J Dent Res. 1977 Sep;85(6):387–391. doi: 10.1111/j.1600-0722.1977.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Van Houte J., Liljemark W. F. Parameters that effect the adherence of Streptococcus salivarius to oral epithelial surfaces. J Dent Res. 1972 Mar-Apr;51(2):424–435. doi: 10.1177/00220345720510023101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Havlícek J. M protein of type 12 Strepto coccus pyogenes. Isolation by electrofocusing and some molecular weight-dependent properties. Pathol Microbiol (Basel) 1975;42(3):147–158. [PubMed] [Google Scholar]
- Hay D. I. The interaction of human parotid salivary proteins with hydroxyapatite. Arch Oral Biol. 1973 Dec;18(12):1517–1529. doi: 10.1016/0003-9969(73)90127-1. [DOI] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Ofstehage J. C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979 Dec;26(3):1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Ability of Veillonella and Neisseria species to attach to oral surfaces and their proportions present indigenously. Infect Immun. 1971 Sep;4(3):264–268. doi: 10.1128/iai.4.3.264-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V., Bloomquist C. G. Compounds which affect the adherence of Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. J Dent Res. 1978 Feb;57(2):373–379. doi: 10.1177/00220345780570023901. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Staat R. H., Rosan B., Taylor K. G., Doyle R. J. Association of protein with the cell wall of Streptococcus mutans. Infect Immun. 1980 Apr;28(1):118–126. doi: 10.1128/iai.28.1.118-126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Rosan B. A comparison of the phenol water and Rantz and Randall teichoic acid antigens in group H streptococci. Adv Exp Med Biol. 1978;107:791–802. doi: 10.1007/978-1-4684-3369-2_89. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Determination of muramic acid in automated amino acid analysis. Anal Biochem. 1972 Aug;48(2):624–628. doi: 10.1016/0003-2697(72)90119-4. [DOI] [PubMed] [Google Scholar]
- Rosan B., Lai C. H., Listgarten M. A. Streptococcus sanguis: a model in the application in immunochemical analysis for the in situ localization of bacteria in dental plaque. J Dent Res. 1976 Jan;55:A124–A141. doi: 10.1177/002203457605500105011. [DOI] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Zanders E. D., Bergmeier L. A., Lehner T. Affinity purification and characterization of protease-susceptible antigen I of Streptococcus mutans. Infect Immun. 1980 Sep;29(3):999–1006. doi: 10.1128/iai.29.3.999-1006.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. IV. The composition of the cell walls of some Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1953 Apr;10(4):512–523. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]