Abstract
Introduction:
Schizophrenia (Sch) is a complex neurodevelopmental disorder associated with impairment of cognitive function as a central feature, which is confirmed by a number of studies performed on patients suffering from Sch, where clinical symptoms and social functioning of patients are consequences of neurocognitive deficits.
Goal:
The goal of this study was to assess the clinical usability of the Montreal Cognitive Assessment (MoCA) as a screening instrument for cognitive impairment in schizophrenic patients, alone and in correlation with the Mini-Mental State Examination (MMSE).
Material and methods:
This clinical prospective study included 30 patients diagnosed with schizophrenia. Patients were selected from Psychiatric Clinic, Clinical Center University of Sarajevo (CCUS) during 2010. For assessment of cognitive impairment we used Montreal Cognitive Assessment Scale (MoCA) and Mini-Mental State Examination (MMSE).
Results:
From the total number of respondents (n=30), 15/30 (50 %) were males and 15/30 (50 %) were females; age of onset were 23.5±6.69; duration of illness before hospitalization (mean±SD) 32.5±12.9. If we make a comparison of MoCA scale and MMSE under the limit values, then we get that there was 10 true positive, 4 true negative, 14 false positive and 2 false negative. This all leads to sensitivity of MoCA scale again in comparison with the MMSE of 41.7%, specificity 66.7%, positive predictive value of 83.3% and negative predictive value of 22.2%.
Conclusions:
Our findings provide preliminary evidence that MoCA scale performs well in detecting true positive but it is imprecise in the detection of true negative findings.
Key words: schizophrenia, cognitive deficit, MoCA, MMSE.
1. INTRODUCTION
Schizophrenia is a complex neurodevelopmental disorder with impairment of cognitive function as a central feature, which is confirmed by a number of studies performed on patients who suffer from Sch where clinical symptoms and social functioning of patients are consequences of neurocognitive deficits. The cognitive deficit is core aspect of the schizophrenic illness (1, 2). Traditionally, significant cognitive impairment was thought to be evident only in elderly deteriorated patient with schizophrenia. Significant cognitive impairment is common in schizophrenia, affecting up to 75% of patients (3). Only 27% of the patients with schizophrenia were classified as neuropsychopathologically “normal”. This indicates that significant cognitive impairment in schizophrenia is, in fact, the norm. The pathophysiology underlying the cognitive deficits often associated with schizophrenia may be distinct from that causing some of the core clinical features (3). In general, the strongest voices to emerge have been those that claim a disproportionate impairment of memory functioning (4, 5). The cognitive impairment often pre-dates the illness onset. A wide range of cognitive functions are affected, particularly memory, attention, motor skills, executive function and intelligence. Cognitive impairment is related to social and functional outcome (3).
Cognitive impairment in operational memory, declarative memory and attention are the basic symptoms of schizophrenia that contribute to heterogeneity in the phenomenological expression of symptoms and influence on the complexity, diversity course and outcome of the disease. Schizophrenia is a chronic disorder involving approximately 1% of the general population. Prefrontal cortex plays a dominant role in the psychic life of humans, because it integrates information coming directly from the limbic regions, neocortex, brainstem and hypothalamus and indirectly via the thalamus from almost all regions of the brain, so dysfunctional, that certain structural and/or functional changes in this part of the CNS leading to quantitative and qualitative disorders of consciousness, planning, actions implementation, the quantitative and qualitative disorders of vision, concentration, speech, emotion and affect (6). As a group, people with schizophrenia have lower achievements on a wide range of cognitive tests, especially those relating to regulation of the frontal lobe such as attention, the use of strategies and problem solving (7). Numerous scientific studies show that the variance of neurocognitive deficits in schizophrenic patients is between 20-60% and in numerous studies, is closely related to the outcome of the symptoms. Schizophrenia is a chronic mental illness that leads to a high degree of impairment in relation to other psychiatric disorders, based on the significant decline in social, occupational functioning and lower education level. Current estimates indicate that 85% of patients were unemployed at any given time. During the past twenty years, many evidences showed that patients with schizophrenia have a standard deviation of 1-2 damage on various measures of neurocognitive function, including attention, visuospatial/executive function and working memory, language and problem solving in relation to healthy control (8). Presented papers suggest that neurocognitive deficits are explaining 20-60% of the variance in studies of interpersonal problem-solving abilities, social (social and occupational) functioning and the extent of acquiring skills in rehabilitation programs.
To date there are no validated brief screening instruments for the diagnosis and assessment of severity of schizophrenia cognitive deficits. In this study, we compared the Montreal Cognitive Assessment (MoCA), a clinician friendly, validated, brief instrument for the detection of mild cognitive impairment (MCI) with the Mini-Mental State Examination (MMSE) as a screening test for cognitive deficits in schizophrenia.
Since the MoCA assesses multiple cognitive domains, it may be a useful cognitive screening tool for several neurological diseases that affect younger population, such as Parkinson’s disease (5 studies showing the superiority of the MoCA over the MMSE), depression, schizophrenia and heart failure. Cognitive evaluation facilitates the differential diagnosis. Studies show that cognitive functioning is the best single predictor of the need for patients to be admitted to the clinic with patients who are examined as well as emergency cases, even better than the diagnosis which is set (9). Cognitive screening also has significant clinical relevance.
Although the need for screening (rapid testing) has become widely recognized, it is limited by the availability of short tests suitable for the evaluation of the heterogeneous population of hospitalized psychiatric patients with schizophrenia.
2. GOALS
The goals of our study were:
To compare the psychometric properties of two instruments and the characteristics of applications that may be relevant for the clinical application of these instruments.
To examine and compare the degree of cognitive impairment in specific cognitive domains (working memory, declarative memory and/or attention) in patients suffering from schizophrenia using MoCA and the MMSE alone.
Comparison of the degree of sensitivity of the applied instruments (MMSE and MoCA).
3. MATERIAL AND METHODS
The study included inpatients (n=30) who suffering from Schizophrenia (Sch) diagnosed according to tenth revision of the International classification of diseases (ICD-10), treated at the Psychiatric Clinic, Clinical Centre University of Sarajevo (CCUS). The patients were followed during 2010. The study was, for the most part, conducted as a prospective method of clinical research and partly as a descriptive controlled study. The patients were treated with typical antipsychotics (haloperidol and chlorpromazine) (n=15) and atypical antipsychotics (olanzapine, risperidone and clozapine) (n= 15).
To test the statistical significance of observed differences between groups used one-way analysis of variance (ANOVA), while testing the interconnection of the observed variables used Spearman’s correlation coefficient, with significance level of p<0.05 is considered statistically significant.
Instruments
We used MoCA (Montreal Cognitive Assessment Test) and MMSE (Mini Mental State Examination) for the assessment of cognitive deficits.
The MoCA is relatively new cognitive screening test takes 10 to 12 minutes to administer. The Montreal Cognitive Assessment (MoCA) is more sensitive to subtle cognitive deficits in patients with Parkinson’s disease, but in schizophrenia, compared with the conventional Mini-Mental Status Examination (MMSE). The MMSE is the most commonly used screening instrument to detect cognitive dysfunction in schizophrenic patients. Both tests are scored from 0 to 30, with lower scores representing cognitive dysfunction.
4. RESULTS
In the study we included 30 patients diagnosed with schizophrenia, where the age of onset of illness ranged from 17-45 years and the average age of disease onset was 24 years (24.4±6.7; X±SEM). Demographic data (Table 1) showed that number of unemployment was 60%, which indicate the high degree of work incapacity cause by early disease onset (hebephrenic schizophrenia 56.7%), compared to the level of education, with domination of the secondary school education (59%) (Table 2).
Table 1.
Demographic data
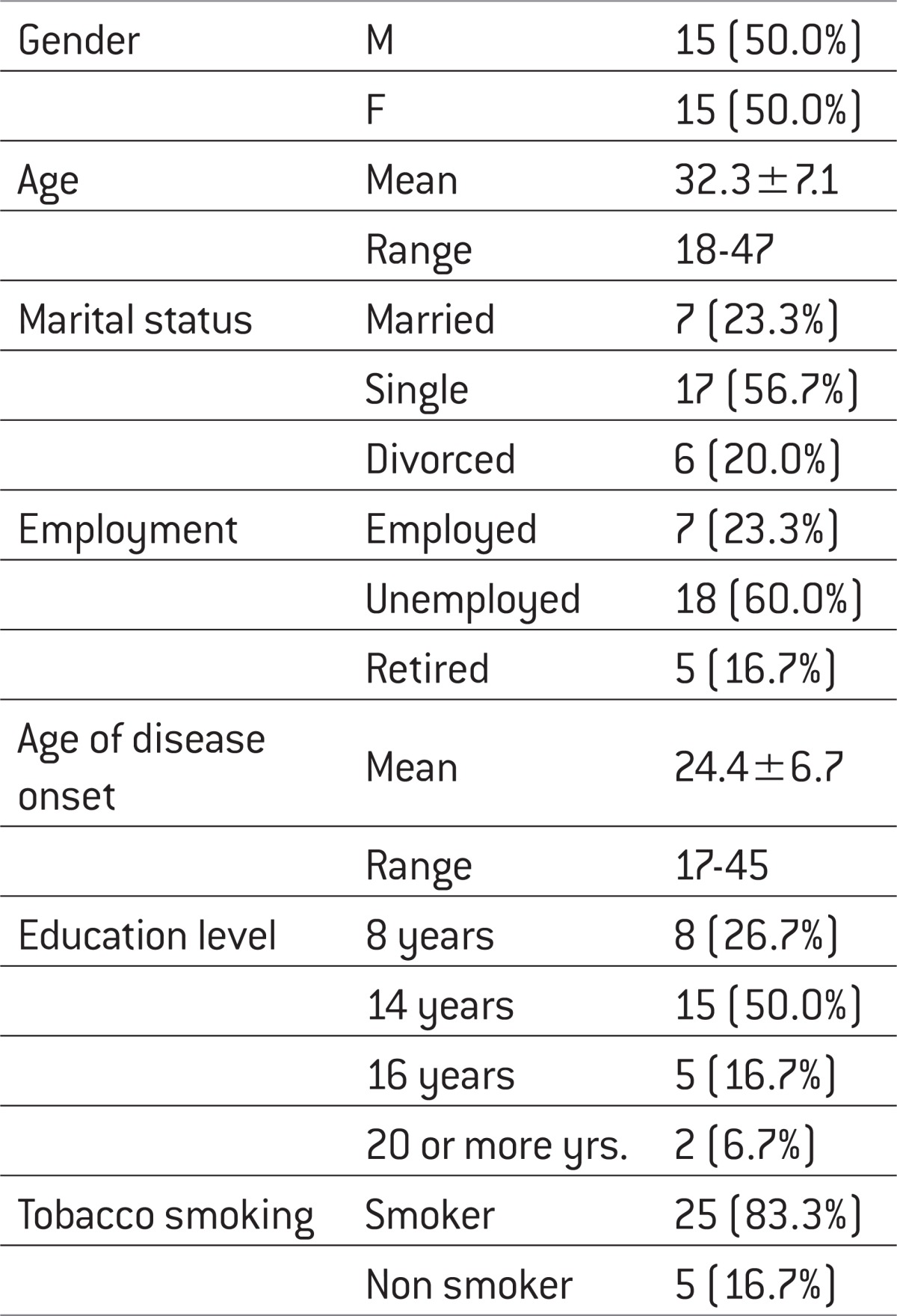 |
Table 2.
Basic data on disease
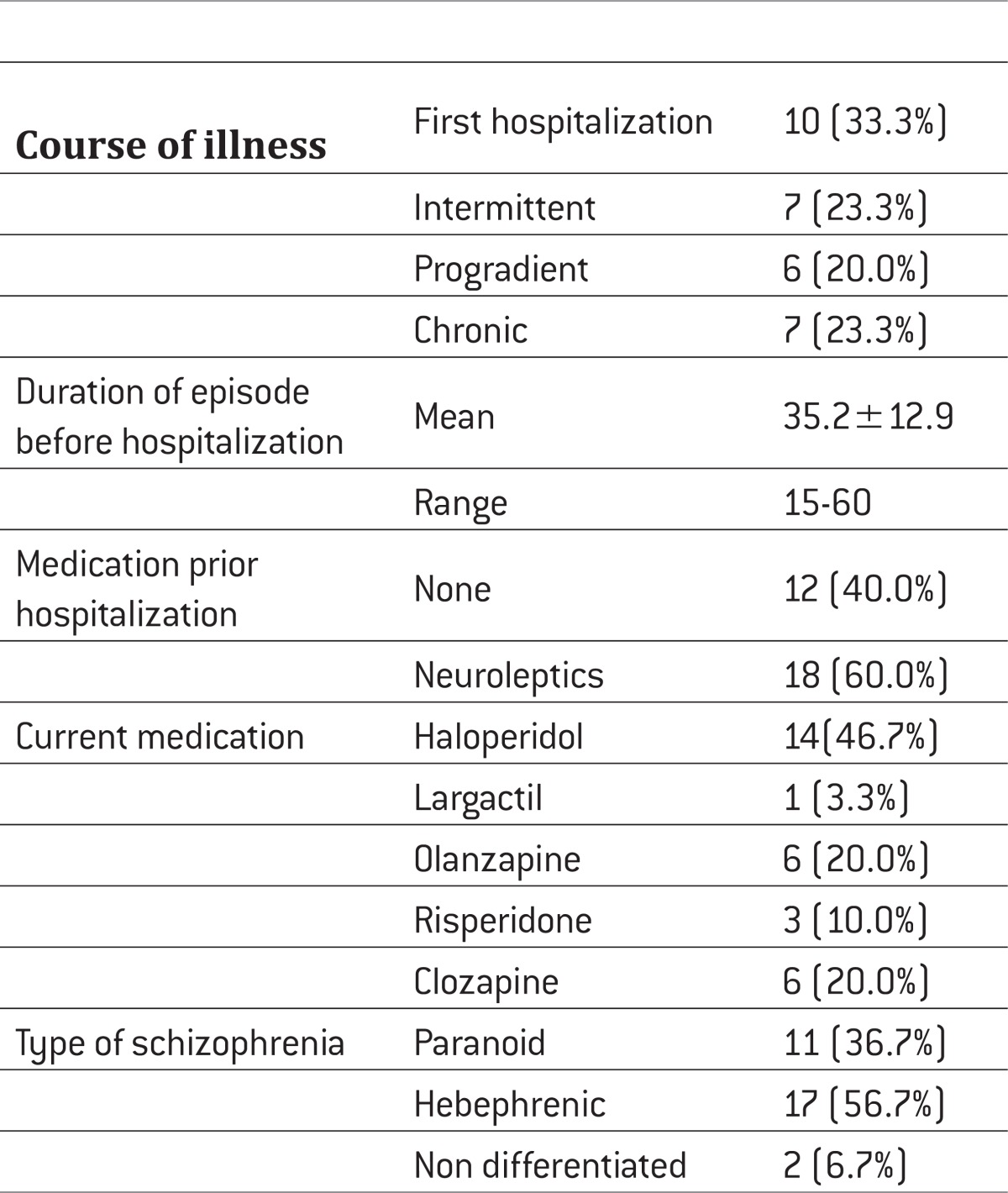 |
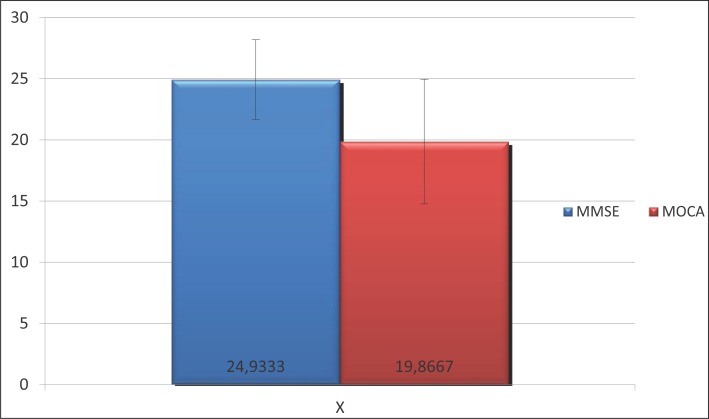
Average scores on the MoCA scale were 19.9±5.1, corresponding to moderate to severe cognitive impairment, while scores on the MMSE scale was 24.9±3.3, corresponding to the result of mild cognitive impairment. (Table 3).
Table 3.
Comparison MMSE : MoCA
 |
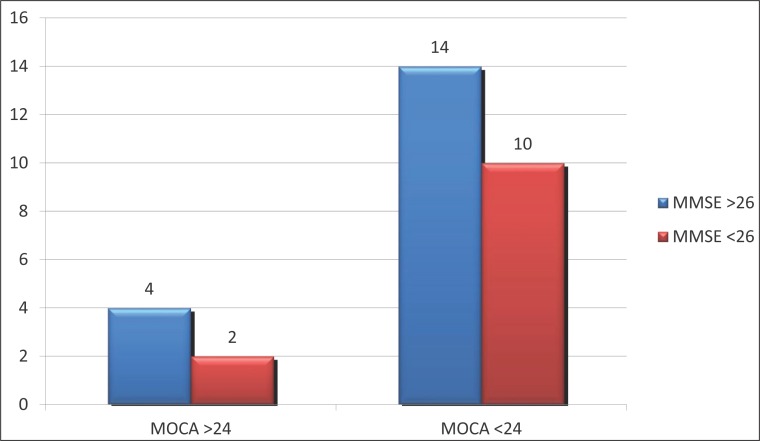
14 patients (77.8%) of those who on MMSE scale had a score greater or equal to 26 (normal range) and the MoCA scale had a normal score (>24), while 2 or 22.2% of patients reported moderate to severe cognitive disability (Table 4).
Table 4.
Mini–Mental State eXaMination *MoCa. Chi-square=0.139; p=0.545
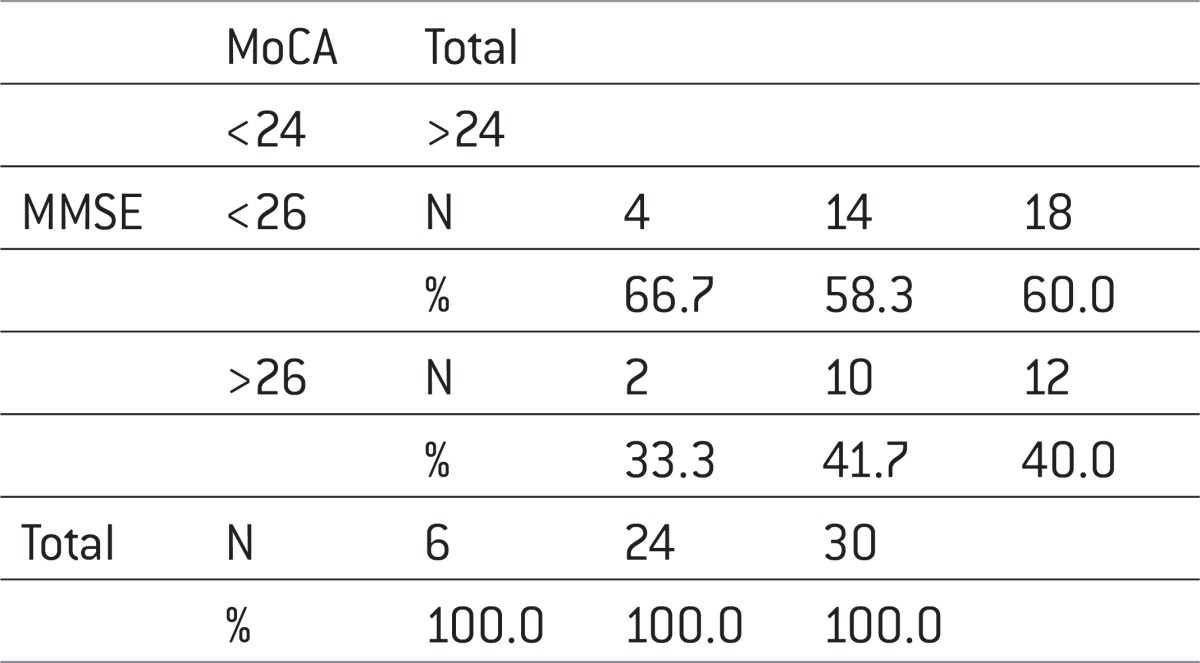 |
Analysis of the correlation coefficient between the total score of MoCA and MMSE scale indicates a statistically significant positive correlation with Pearson rho=0.403 and p=0.027 (p<0.05). Sensitivity was 41.7%, specificity = 66.7%, PPV = 83.3%, NPV = 22.2% (Figure 1).
Figure 1.
Comparison MMSE : MoCA.
If we make a comparison MoCA scale according to the MMSE according to cut-off values then we find that there were 10 true positive, true negative 4, 14 false positive and 2 false negative. (Figure 2) This all leads to sensitivity of MoCA scale, again in comparison with the MMSE of 41.7%, specificity 66.7%, positive predictive value of 83.3% and negative predictive value of 22.2% which indicates that the scale is really good at detecting true positive but have poor performance in the exclusion of false negative findings.
Figure 2.
Mini–Mental State Examination *MoCA.
5. DISCUSSION
The published results of a small number of clinical studies assess the severity of cognitive deficits in schizophrenic patients.
In our clinical study all patients (n=30) underwent the following testing: MoCA and MMSE. The mean MoCA score was, consistent with moderate to severe cognitive impairment (10). Average scores on the MoCA scale were 19.9±5.1, corresponding to moderate to severe cognitive impairment, while scores on the MMSE scale was 24.9±3.3, corresponding to the result of mild cognitive impairment.
These results are in agreement with the results of Preda et al. for MoCA but not for the MMSE scale, which states. The mean MoCA score was 20±4.7, consistent with moderate to severe cognitive impairment. In contrast, the mean MMSE score was 27.2±2, which is within the normal to mild MCI cognition score range.
According to the research of Preda et al. (11) the mean MoCA score was 20± 4.7, consistent with moderate to severe cognitive impairment. In contrast, the mean MMSE score was 27.2±2, which is within the normal to mild MCI cognition score range. Twenty-one patients (84%) of those who scored > or = 26 (normal range) on the MMSE had a MoCA score <26 (MCI range). Twenty-three (85%) of those who scored >or = 24 on the MMSE (MCI range) had a MoCA score <24 (moderate to severe cognitive impairment). 14 patients (77.8%) of those who on the MMSE scale had a score > or = 26 (normal range) also at MoCA scale had a normal score (>24), while in 2 or 22.2% of patients was reported moderate to severe cognitive disability. These findings are in contrast with the findings of Preda et al, where twenty-one patients (84%) of those who scored >or = 26 (normal range) on the MMSE had a MoCA score <26 (MCI range). Twenty-three (85%) of those who scored >or = 24 on the MMSE (MCI range) had a MoCA score <24 (moderate to severe cognitive impairment).
Analysis of the correlation coefficient between the total score of MoCA and the MMSE scale indicates a statistically significant positive correlation with Pearson rho=0.403 and p=0.027 (p<0.05), but this study did not analyze individual subscales as stated in research by Preda et al. and it is difficult to compare with their findings. What arises is the same conclusion that the sensitivity of 41.7%, specificity = 66.7%, PPV = 83.3%, NPV = 22.2%. The MoCA test validation study (9) has shown the MoCA to be a promising tool for detecting Mild Cognitive Impairment (MCI) compared with the well-known Mini-Mental State Examination (MMSE).
During the past few years, an approach that ensures the construct validity of cognitive assessment of various researchers have suggested a recent exploration of the proposal to apply a generalized deficit in order to determine whether multiple performance deficits in schizophrenia are the result of common underlying processes (12).
6. CONCLUSION
There is considerable difficulty is comparison of reliably for assessment scales. To date there are no validated brief screening instruments for the diagnosis and assessment of severity of schizophrenia cognitive deficits. The MoCA has higher sensitivity and specificity to detect cognitive impairment than the MMSE. The MoCA is an easy to administer, useful screening tool for the assessment of cognitive deficits associated with schizophrenia. Our preliminary results also suggest that the MoCA might be a sensitive test for the assessment of some of the core cognitive deficits in schizophrenia such as speeded attention and executive functioning. Further studies validating MoCA against standard neurocognitive testing batteries are recommended. However, it had been established that the MMSE is not well suited for mild cognitive impairment, which raises the question whether it is an adequate „standard“ to compare performance with the MoCA.
Conflict of interest
None declared.
REFERENCES
- 1.Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH. Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study to teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 1987;44(11):1008–1014. doi: 10.1001/archpsyc.1987.01800230088014. [DOI] [PubMed] [Google Scholar]
- 2.Shallice T, Burgess PW, Frith CD. Can the neuropsychological case-study approach be applied to schizophrenia? Psychol Med. 1991;21(3):661–673. doi: 10.1017/s0033291700022303. [DOI] [PubMed] [Google Scholar]
- 3.O’Carroll R. Cognitive impairment in schizophrenia. Advance in Psychiatric Treatment. 2000;6:161–168. [Google Scholar]
- 4.Mc Kanna PJ. Memory, knowledge and delusions. British Journal of Psychiatry. 1991;159(14):36–41. [PubMed] [Google Scholar]
- 5.Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 6.Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neuroognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Goldman RS, Axelrod BN, Taylor SF. Neuropsychological aspects of shizophrenia. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatric disorders. 82nd. New York: Oxford University Press; 1996. [Google Scholar]
- 8.Kurtz MM, Wexler BE, Fujimoto M, Shagan DS, Seltzer JC. Symptoms versus neurocognition as predictors of change in life skills in schizophrenia after outpatient rehabilitation. Schizophr Res. 2008;102(1-3):303–311. doi: 10.1016/j.schres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment (MoCA): A Brief Screening Tool For Mild Cognitive Impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith T, et al. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007 May;52(5):329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 11.Preda A, Adami A, Kemp AS, Nguyen D. MoCA: A screening instrument for the assessment of cognition in schizophrenia. In Abstracts from the 13th International Congress on Schizophrenia Research. Schizophrenia Bulletin. 2011;37(S1):225–226. [Google Scholar]
- 12.Knight RA, Silverstein SM. The role of cognitive psychology in guiding research on cognitive deficits in schizophrenia. In: Lenzenweger MF, Dworkin RH, editors. Origins and development of schizophrenia: Advances in experimental psychopathology. Washington DC: APA Press; 1998. pp. 247–295. [Google Scholar]