Abstract
Material properties of lipid bilayers, including thickness, intrinsic curvature and compressibility regulate the function of mechanosensitive (MS) channels. This regulation is dependent on phospholipid composition, lateral packing and organization within the membrane. Therefore, a more complete framework to understand the functioning of MS channels requires insights into bilayer structure, thermodynamics and phospholipid structure, as well as lipid-protein interactions. Phospholipids and MS channels interact with each other mainly through electrostatic forces and hydrophobic matching, which are also crucial for antimicrobial peptides. They are excellent models for studying the formation and stabilization of membrane pores. Importantly, they perform equivalent responses as MS channels: (1) tilting in response to tension and (2) dissipation of osmotic gradients. Lessons learned from pore forming peptides could enrich our knowledge of mechanisms of action and evolution of these channels. Here, the current state of the art is presented and general principles of membrane regulation of mechanosensitive function are discussed.
Keywords: phospholipid membranes, membrane tension, lipid interdigitation, MscL and MscS channels, transmembrane helices, periplasmic loop, hydrophobic mismatch, snorkeling effect, peptide tilting, pore forming peptides, peptidic pores
Introduction
Mechanosensitive (MS) ion channels have developed the capability to undergo conformational changes to form aqueous pores in response to increases in membrane tension. They have been found in membranes of organisms in all three domains of life. As has been frequently noted, “the finding of MS channels in prokaryotes suggests that these membrane proteins were among the first macromolecules that evolved to facilitate transport of solutes in membranes of protocells.”1 Thus, since MS channels are widespread and can be considered universal to cells, the osmotic adaptation probably started with the first microbes and this kind of regulation could have been one of the first mechanisms to evolve. The interaction of these proteins with the surrounding lipid matrix is crucial for the fulfillment of its important function. In general, interactions between proteins and lipids are considered specific in close proximity to the protein surface, but indirect lipid-mediated forces are also particularly important for activating these proteins. Indeed, the direct activation of MscL and MscS channel homologs by membrane tension is well established.1-3 Hence, studying issues such as lipid composition, degree of unsaturation in lipid tails, headgroup structure, area per lipid, viscosity, lipid mobilities and tail order may also contribute to uncovering important aspects regarding how the lipid environment influences the response of MS channels to increases in membrane tension. Therefore, the structural and mechanical properties of membranes are expected to have a significant impact on the activities of these proteins. From this perspective, it is conceivable that biological membranes have evolved to maintain gradients and to establish potentials across them, while also preserving the important property of transducing a mechanical stimulus to the proteins embedded in them.
MS channels have been described in prokaryotes as well as in eukaryotic cells, showing a high molecular diversity.1,3 Bacterial channels MscL and MscS have been extensively studied since they represent proteins with a dual role: sensors of membrane perturbations and effectors by forming aqueous pores that allow the release of osmolytes. This relieves potentially harmful osmotic stresses in prokaryotic cells.4,5 The MscL channel is a 3-nS homopentameric protein basically found in prokaryotes, whereas the small conductance MscS channel is a 1-nS homoheptameric protein, first found in Escherichia coli and with homologs in walled organisms, including bacteria, protists, fungi and plants, but not animals.3 Importantly, the MscL channel family comprises proteins that can exist also in different oligomeric states, ranging from tetramers to hexamers.6,7 However, these arrangements apparently result from a detergent-specific effect and have no physiological significance.7 This intriguing finding is interesting from an evolutionary point of view since it could be indicative of some basic plasticity in this protein. Thus, an important question is how these proteins have evolved. To address this issue, comparative and structural biology can be used as theoretical and experimental tools. The crystal structure of a C-terminal truncated mutant of SaMscL, (from the facultative anaerobic Gram-positive bacterium Staphylococcus aureus), shows that the channel can be arranged as a tetramer, performing an activity comparable to EcoMscL (from E. coli).6 This observation is very suggestive, given the apparent existence of naturally truncated homologs in Archaea in this region.8
MscL homologs have two transmembrane (TM) helices: TM1 and TM2, where TM1 forms a lumen and TM2 is in direct contact with lipids.9,10 In the crystal structure of TbMscL (from Mycobacterium tuberculosis), the main contact with the surrounding lipid bilayer is made by residues in TM2, but some TM1 residues also face outward toward the membrane.10 Thus, MscL channels form a well conserved family of MS channels, but homologs are frequently diverse regarding the length of the protein and the open probability (Po) in response to increases in lateral tension.8,11 Clearly, the lipidic context in which these proteins are embedded clearly has an important effect on their activation. For example, when TbMscL is assayed in E. coli spheroplasts, the gating tension requirement to obtain a comparable Po is at least double in comparison to the same channel reconstituted in azolectin liposomes.12 It is reasonable to interpret this observation as an adaptation to the native lipid environment in Mycobacteria, where a significant portion of molecules form a chemically dense network of complex lipids and sugars existing at extremely low fluidity.13
In MscL, TM1 and TM2 are separated by a periplasmic loop (P-loop), which prevents excessive pore expansion and promotes its closure after opening. The cleavage of such a loop by enzymatic means preserves channel functionality with a substantially lower gating threshold.14 Consistent with this, separate expression of the N-terminal segment including TM1 can form MS channels very sensitive to changes in membrane tension whereas the expression of the C-terminal portion including the TM2 shows no channel activity.15 Thus, at least for MscL, the role of the periplasmic loop appears to be to act as a molecular spring regulating the opening of the helix bundle.16 In contrast, not all the members of MscS-like superfamily have three TM domains, as in EcoMscS. This molecular diversity has led to the interesting proposal that they are members of a superfamily of ion channels with associated domains that gate the channels by different stimuli (e.g., cAMP) with an intrinsic mechanosensitivity.17 Overall, these observations suggest that MS channels have evolved responding to a common stimulus at the level of membrane mechanical properties. In this context, the remarkable in vitro chemical synthesis and oligomerization of bacterial MscL proteins into functional channels18-20 opens attractive new research perspectives. In addition, the use of pore-forming peptides has provided important information for this approach. Antimicrobial peptides form aqueous pores in lipid bilayers when a critical molar peptide/lipid (P/L*) value has been reached and, under specific conditions, they can form stable pores instead of provoking membrane disruption.21,22
In an attempt to identify general principles on the intimate effect of lipid environment on MS channels and to investigate their primordial evolution, this article has been written with the aim of discussing three significant aspects concerning the function of MS channels in separate sections: (1) composition-dependent material properties of lipid bilayers and the implications for MS channel activation, (2) lipid-protein interactions in contemporary bacterial MscL and MscS channels and (3) the use of pore-forming peptides as experimental tools to inquire into MS channel evolution. The essential gating stimulus for activating MS channels, membrane tension, has attracted an important research effort, but a more detailed quantification of their effects on membranes and embedded channels is beyond the scope of this review. Similarly, a more detailed quantification of material parameters, such as compressibility, bending, intrinsic curvature and lateral pressure of bilayers, will not be included herein. On the other hand, the lipid-protein interactions in MscL and MscS channels have been well documented in excellent, recent reviews.23-26 In this work, a brief discussion of such interactions will be provided and some relevant aspects will be highlighted, but it is recommended that interested readers consult the aforementioned references and their bibliographies. With this in mind, the present review aims to contribute to a better understanding of MS channel function and the design of future research.
Mechanical Properties of Lipid Bilayers
As a matrix, lipid bilayers exhibit several material properties depending on their chemical composition and their adaptation to physical variables, such as temperature, pressure, pH or intrinsic tension by differential packing. Importantly, upon heating, lipids undergo a main chain-melting energetic reorganization, a gel-to-liquid crystalline phase transition, which is accompanied by a large entropic change. As is well-known, in aqueous milieu, model and natural lipids tend to self-assemble and form several types of lipid bilayers. They are packed in dynamic three-dimensional lattices and because of their chemical diversity each lipid mixture shows different thermotropic phase behaviors, as well as compressibility and intrinsic bending.27 Furthermore, as can be deduced from thermodynamics, an increase in surface tension reduces the temperature at which phase transition occurs. This reported behavior can be explained in terms of an increase in tension at constant pressure, which induces an increment in the area-per-lipid ratio and consequently a lower temperature is required to achieve higher ordering of the lipid tails.28
Thermotropic phase behavior of phospholipids and their effect on MS channel activity
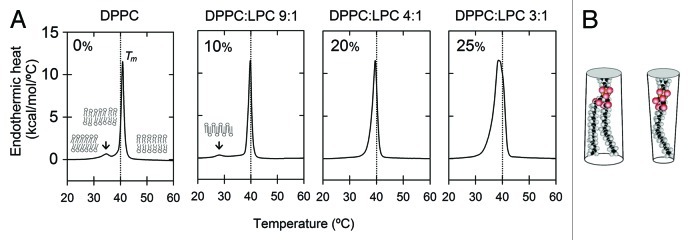
Dipalmitoyl-phosphatidylcholine (DPPC) is a saturated lipid that has been extensively studied as a model lipid because its thermotropic behavior shows several endothermic transitions and the corresponding phase changes have been deciphered.27 Up to three discernible transitions and four lamellar phases have been described and designated as Lc (lamellar crystalline subgel or solid), Lo or Lβ' (liquid-ordered or tilted gel), Ld or Pβ' (liquid-disordered or ripple gel) and Lα (liquid-crystalline or fluid) within the temperature range of 10 to 50°C. For DPPC, the main transition is around + 41.5°C (~315 K); below this temperature, the headgroups are dynamically disordered but acyl chains are packed together closely and tilted. However, as temperature rises toward the mid-point transition temperature (Tm) value, the tilted acyl chains abruptly lose their tight packing and the main phospholipid gel-to-fluid thermotropic transition occurs. As a result, a conformational change takes place in the lipid acyl chains, due to a rotational trans-to-gauche isomerization of methyl groups and the average orientation of the chains becomes almost perpendicular to the plane of the bilayer. Thus, at low temperatures (< Tm), lipid bilayers formed with DPPC are rigid and extremely compact (Lβ' phase) because lipids are arranged on a tilted lattice in the plane of the bilayer. In this configuration, the hydrocarbon chains are in an all-trans configuration and lateral diffusion is very low. When the temperature reaches 34–36°C a pretransition occurs, resulting in a change to the rippled phase in which headgroups are crowded, arranged in a periodic rippled bilayer, and probably some lipids are tilted, whereas others are melted, disordered and fully interdigitated.27,29 Then, at Tm, the rippled phase melts into the Lα phase (Fig. 1A).
Figure 1. The effect of LPC on the phase transition (change in heat capacity) of DPPC MLVs as measured by differential scanning calorimetry (DSC). (A) DPPC thermograms in the absence (0%) or presence of 10, 20 or 25 mol% LPC at the indicated temperatures. Arrows show the displacement of the pretransition temperature (Tp) which defines the ripple phase (Pβ') in pure DPPC. A new pretransition appears at lower temperatures (probably indicating an interdigitated gel phase, LβI), then it vanishes at high doses of LPC. In (B), the structure factor for cylindrical DPPC (Si = 1.11) and inverted cone-shaped LPC (Si = 0.78) are schematized (Lipid structures taken from ref. 40).
Importantly, lipid bilayers in ripple and fluid phases are subject to an additional transition under tension, which induces thinning and interleaflet interdigitation, whereas in the gel phase the two lamellae remain well separated, with no reduction in thickness.30 Additionally, tension reduces lipid acyl chain packing order, as well as the electrostatic potential barrier of the bilayer.31 Therefore, a critical concept in the study of MS channels is the physical state of the membrane that allows or restricts protein conformational changes. In this way, the fluid phase of lipids facilitates any molecular movement and protein-protein or lipid-protein interactions. Other structural and material properties of lipid bilayers that are affected by the thermotropic behavior of phospholipids include the area compressibility (KA) and bending (KC) moduli, measurements of the cohesion between lipids and the elastic bending of the bilayer, respectively; the hydrophobic thickness (h), the lateral pressure profile (P), and the intrinsic curvature (c0), which relates the tendency of the type of lipid to form curved surfaces.32-34
It has been demonstrated that many biophysical properties of K+ channels, including the unitary conductance, Po and dwell times of KcsA, are finely tuned by a change in the physical state of the lipid bilayer through the gel-to-fluid phase transition.35 Besides, the Kv1.2 channel with a voltage sensor paddle from Kv2.1 (the “paddle chimera”) and the Shaker channel also show mechanical sensitivity depending on the lipid composition and the physical state of the bilayer.36 These observations, per se, raise interesting questions and expand the group of mechano-dependent membrane proteins. In MS channels, studies in relation to changes in the phase-transition of lipids are also scarce. However, the effect of Gd3+ on MS channels has been amply reported in the literature.1,37,38 The work of Sukharev et al. has shown that MscL channels are blocked by micromolar Gd3+ in a lipid-compositional manner (negatively charged phosphatidylserine, PS, at 30 mol% in phosphatidylcholine, PC). In these conditions, Gd3+ induces lipid compression and a solid-like phase of PS domains that avoid the expansion of the channel protein to form the open pore, even when a strong negative pressure is applied (−200 mmHg). What’s more, these authors confirm, by isothermal titration calorimetry (ITC), that binding of Gd3+ to dimyristoil-phosphatidylserine (DMPS) liposomes in the gel phase is endothermic, whereas binding to liposomes in the liquid-crystalline phase is exothermic, which is consistent with the isothermal liquid-to-gel phase transition induced by this trivalent cation.37
Geometrical properties of phospholipids and their effect in lipid bilayers
The packing of lipids is directly related to their intrinsic shape. In general, phospholipids with two saturated acyl chains tend to be cylindrical, keeping a spatial relation between headgroup and hydrocarbon tail that induces null spontaneous curvature in membranes, favoring lamellar phases. If this relation changes and the headgroup is significantly smaller than the section area of the tail, a cone-shaped lipid results, forming membranes with negative curvature (hexagonal type II, HII). Lipids with unsaturated acyl chains favor these structures.33,34 On the other hand, inverted cones result when the headgroup area is larger than its acyl chain section area. This is the case for lysolipids, whose packing in membranes induces positive curvature (hexagonal type I, HI).33,34,39 The generation of local positive (convex) curvatures by lysophosphatidylcholine (LPC) addition into lamellar phases, is caused by its lipid-headgroup area (60Å2) and its structure factor (Si = 0.78), indicating an inverted cone-like shape39,40 (Fig. 1B). In parallel to their shape, lysolipids also affect thermotropic behavior of phospholipids through their miscibility. It has been proven that LPC mixes homogeneously both below and above the Tm, but at concentrations beyond 40 mol% induces micellization, disrupting lipid bilayers. At LPC concentrations under this value, LPC liquefies synchronously with DPPC showing a high degree of miscibility.41,42
As will be discussed below, it is clear that LPC elicits an extremely subtle effect on bacterial MS channel activation at micromolar concentrations.43,44 LPC (at concentrations < 30 mol%) can induce some reduction in thickness of the lipid bilayers by forming an interdigitated gel phase (LβI). Indeed, X-ray diffraction experiments show that the thickness of DPPC lipid bilayers diminishes from 7.30 nm to 6.75 and 5.52 nm when LPC is mixed at 14.1 or 27.0 mol%, respectively.42 However, micromolar incorporation of LPC has no detectable effect on the thermotropic behavior of liposomes formed with DPPC. Figure 1 shows the thermotropic behavior of multilamellar vesicles (MLVs) formed with DPPC and mixed with different molar concentrations of LPC. At micromolar doses, thermograms are practically the same as the control without lysolipid, and negligible changes in Tm (+ 41.5°C) were observed (not shown). In contrast, LPC at > 10 mol% induces a broadening in the pretransition peak as well as a slight displacement in the main transition, reducing the Tm to 38–39°C at 25 mol% of LPC. The pretransition peak (Tp) shifts to lower temperature values and completely disappears at 20 mol%, suggesting a new interdigitated phase. Moreover, if the LPC concentration reaches 50 mol%, samples became translucid, indicating disruption of the bilayer and formation of small aggregates (not shown). Thus, at high concentrations, LPC prevents the rotation of the polar headgroups of lipids, a kinetic interpretation of the pretransition in DPPC bilayers.29 In conclusion, at high concentrations, lysolipids are highly miscible, change the thermotropic profile of DPPC and can even induce micellization of lipid bilayers. Since the observed behavior on pretransition in Figure 1 can also be attributed to an increase in surface tension,28 it becomes interesting to determine whether the effects observed in MS channels are, in some extent, related to a lipid interdigitation induced by LPC incorporation and/or to a change in the rotational kinetics of the headgroups. These questions deserve to be explored.
Geometrical properties of phospholipids and their effect on MscL and MscS activities
Inverted cone-shape LPC incorporation into lamellar phases generates tension/stress, known as curvature “frustration”.45 This reduces the positive pressure generated by the thermal motion of acyl chains and increases the tension in the inserted leaflet, which favors changes in protein conformation.32,45,46 In this way, asymmetrical incorporation of lysolipids would perturb the energetic profile of the lipid packing surrounding membrane proteins. Additionally, the fluidizing effect on bilayers induced by LPC or polyunsaturated fatty acids (PUFA) on membrane proteins can also be understood in terms of a relaxation in compressibility between lipid molecules by a reduction of the attractions resulting from the formation of intermolecular H-bonds at the polar headgroups.47 Likewise, PUFA induce great fluidity/elasticity in membranes because of their high degree of conformational flexibility.48 Importantly, surface flow properties of lipids, including their visco-elasticity, are directly related to the high lateral fluidity and structural rigidity of membranes, which is key for their biological function.49
Asymmetric micromolar inclusion of lysolipids into the outer leaflet of the lipid bilayer strongly contributes to activating MscL channels,43 while their incorporation into the cytoplasmic (inner) monolayer produces a similar effect on MscS homologs.44,50 Nevertheless, liposome co-reconstitution of MscL/MscS channels has proven that lipid tail length and LPC have differential effects on these channels, MscS being less sensitive to bilayer thinning than MscL, whereas relatively more sensitive to LPC.51 Such subtle differences surely depend on the distinct topologies of both proteins. Indeed, the asymmetric incorporation of lysolipids induces a high degree of compression in the leaflet where it is added and a concomitant dilation in the opposite monolayer. Therefore, a local stress is created leading to the redistribution of pressure profiles in the membrane, which can affect protein conformation.46,52 This rationale is also in accordance with the experimental observation that asymmetrical incorporation of LPC stabilizes intermediate conformational states and full opening in MscL channels, whereas symmetrical addition of LPC equilibrates the pressure profiles, maintaining the channel in the closed state.43,53
Organization of lipids in domains and its implication in MS channel segregation
Membrane tension, spontaneous curvature and intrinsic bending, all can significantly contribute to the lipid domain energetics.54 Lipid phase-transition may also induce protein redistribution and clustering, at least for the KcsA channel.55 In this case, in the context of the transition from an Ld phase (T > Tm), in which the channels are homogeneously distributed, to a temperature at which a coexistence of Ld and Lo/Lc phases takes place, KcsA channels get preferentially distributed into Ld domains, tending to protein clustering. In addition to a marked preference for Ld (fluid) phases, clustering of membrane proteins and peptides is also dependent on the hydrophobic mismatch (HM), as shown for synthetic, Trp-flanked, α-helical model WALP peptides.56 In bacterial MS channels, a cooperative MscL gating induced by spatial clustering of proteins has been recently described.57 This behavior is explained in terms of the bilayer elasticity and the communication between proteins, where the conformational status of an embedded protein can be transmitted to another protein through membrane deformations.58 In the case of the MscL channel, the substantial rearrangement of the TM helices upon gating induces changes in the protein solvation by the surrounding lipids (the annular layers) that can be “sensed” by a neighboring protein. Considering the non-uniform distribution observed in the case of KcsA channels using liposomes with lipid domains forming mixtures,55 and since MscL and MscS undergo important structural rearrangements upon an iris-like expansion,59-61 it remains important to be determined: (1) if MS channels change their location under a phase transition in model lipids and (2) if such phase transitions can affect in some way the gating of those channels. In addition, domains resulting from segregation of fluid-fluid phases are much more difficult to be established, but physiologically they are perhaps the most significant domains in biological membranes.49 Thus, the possible segregation of MS channels in mixtures of lipids with different degrees of fluidity also remains an open question. Moreover, since line tension is a key parameter determining the distribution and properties of Lo domains,62 it becomes interesting to ask whether MS channels are capable of segregating in mixtures of lipids with a low degree(s) of fluidity (i.e., in two different solid phases).
To study the phase behavior of lipid mixtures, a widespread method is to use well-defined model membrane systems, such as large- and giant-unilamellar vesicles (LUVs and GUVs, respectively), which can be obtained from controlled lipid compositions. GUVs are an important tool to study lipid-lipid and lipid-protein interactions, lipid asymmetry, control on contents and the attachment of His-tagged proteins to solid phases.63-65 One of the most important advantages of using GUV methodology lies in the reconstitution of membrane proteins (proteo-GUVs) as suitable systems for patch-clamp recordings and experiments of liberation of solutes. This has been demonstrated for several transport proteins, including VDAC channels, porins, and the MscL channel itself.66-68 Indeed, since the MscS channel shows a slow inactivation in azolectin liposomes,69 the use of proteo-GUVs becomes an interesting experimental alternative to study controlled lipid compositions, with or without the presence of solid lipid domains, in addition to the successful use of Xenopus oocytes.70 Importantly, the use of liposomes for co-reconstitution of MscL/MscS is an interesting approach to finely discern the effects of HM and material properties of lipid bilayers on these channels.51
Lipid-Protein Interactions in the MS Channels MscL and MscS
In contrast to water-soluble proteins, membrane embedded proteins interact simultaneously with two drastically different environments. The structural requirements as well as the functional consequences of this circumstance, necessarily derive from the interactions at the edge where the lipids surround the protein71,72 and in the lipid-water interface.73
Protein adaptations to hydrophobic mismatch in MscL channels
As could be anticipated, membrane proteins and lipids are entities with an intrinsic capability for adaptation to each other since neither are rigid entities. Unlike lipids, in general, membrane proteins have a higher compressibility modulus. As a consequence of this situation, any HM between the bilayer and the protein causes lipid adaptation to the surface of the protein.33,34 Many studies with model lipids, well-characterized model peptides and transmembrane segments of proteins, have shown that lipids influence protein structure/organization and vice versa. Positive HM (when the TM segment of a peptide or a protein is longer than the membrane thickness) can be alleviated often by peptide tilting, in order to achieve a better adjustment to the thinner membrane with a low energy cost. Other adaptations to this circumstance are bilayer distortion and peptide aggregation but, in addition, the α-helix can reduce its apolar length by becoming a less compact π-helix.74 On the contrary, if the mismatch is negative (TM segments shorter than the bilayer) the observed consequences are a combination of local bilayer bending and snorkeling of flanking Lys/Arg residues.74 In general, the Trp functional group (an aromatic indole with a flat rigid shape) and Tyr (a 4-hydroxyphenylalanine) reside at the level of the electronegative lipid carbonyls, at the membrane interface. They both form aromatic belts in TM segments of membrane proteins,75 which result in a strong electrostatic interaction that confers some rigidity to the whole protein. In opposition, Lys, which also flanks TM segments of embedded proteins, has a long and flexible side chain, which facilitates its interaction with a broader interfacial zone. This feature enables changes in tilting/conformation of α-helices and allows Lys residues to perform the role of a flexible anchor.73,76 In this way, the “snorkeling” behavior of Lys side chains, where the aliphatic part of the residue can get immersed into the hydrophobic core of lipid bilayer, while the charged -NH3+ group lies at the surface of the membrane, makes possible a fine adaptation to membrane thickness because the whole residue does not move in the interface.76
The relevance of this behavior could be revealed by mutagenesis of the MscL channel. The P-loop (Figs. 2 and 3) is poorly conserved between homologs and it has been predicted to form a short amphipathic α-helix (so-called S2 segment) attached to a distorted β-hairpin.77 Upon opening, hydrophobic residues from periplasmic S2 can interact with lipid chains at the external monolayer, whereas its hydrophilic residues can form salt bridges inside the pore. In the closed state, the S2 and the β-hairpin interact through their hydrophobic residues forming a compact core. Remarkably, Gln65 in EcoMscL forms part of S2 and its hydrophilicity is important for the proper function of the channel. When this residue is changed to a hydrophobic Leu (Q65L mutant) a loss of function (LOF) phenotype is acquired and the channel requires more negative pressure to open, so the tension threshold rises. However, if Q65 is mutated to a snorkeling Arg, a gain of function (GOF) phenotype is obtained and the channel opens more easily, indicating that the threshold was significantly reduced.16 Like Lys, Arg residues also favorably interact with the oxygen atoms of lipid headgroups and the snorkeling property of their side chains allows a better coupling to relieve HMs.74,76 In parallel, if Q65 is mutated to a Tyr (Q65Y), channels showing GOF phenotypes with an increased mechanosensitivity are suppressed, and normal function is recovered.78 Thus, it is conceivable that Tyr at position 65 in EcoMscL contributes to the stability of TM2 by strong hydrophobic interactions at the external monolayer, which makes difficult the tension-induced tilting when the channel is activated. However, if a positively charged Arg occupies such a position in the S2 within the P-loop, the effect is the contrary and tilting is favored by the snorkeling effect.
Figure 2. Multiple alignments between homologs of MscL channel from Bacteria, Archaea and Eukaryotes. Bold letters represent residues forming the lipid-exposed TM2, which is important for mechanosensitivity. Thin letters represent poor conserved residues in the periplasmic loop (P-loop) and into the first part of the C-terminal domain. Red letters show hydrophobic residues whereas black letters show hydrophilic ones. Brown bold letters are flanking Tyr or Trp residues at the periplasmic face of the membrane. Blue bold letters represent flanking Lys or Arg residues facing to the cytosol. Snorkeling side chains attached to the TM2 are shown also in blue with a positive charge. Residues showing a high degree of conservation are highlighted in yellow. Trp (W) is highlighted in orange and represents a poor conservation of this residue at this place (~10%).
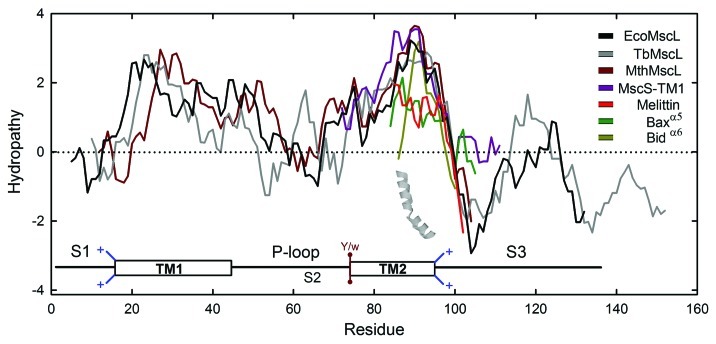
Figure 3. Hydrophobicity plot for MscL channel homologs from E. coli (EcoMscL), Mycobacterium tuberculosis (TbMscL), the archaeon Methanosarcina barkeri (MthMscL), MscS encompassing TM1 (residues 22–61 from EcoMscS), as well as the α-PFPs: melittin, fragment α6 from Bid and α5 from Bax proteins (see text). The hydrophobicity plots were developed by the algorithm of Kyte and Doolittle (ref. 82) using a window of 9 and aligning residues according to the lipid-exposed EcoMscL-TM2 profile. The topology of EcoMscL protein is included indicating only TM segments and the P-loop. As in Figure 2, snorkeling side chains attached to the TM1 and TM2 are shown in blue with a positive charge. Position 75 (E. coli numbering) is occupied by a Tyr or, less frequently, a Trp and are shown as a rigid brown lateral chain. The inserted 3D helix shown corresponds to a monomer of melittin (PDB: 2MLT).
In MscL homologs, a persistent consensus is observed in flanking lipid-facing residues of TM2: Tyr75 (E. coli numbering) is conserved in > 70% and Lys97 is present in 55% of 230 putative MscL homologs.8 However, in ~10% of them, a rigid Trp residue takes the corresponding place of Tyr75 (Fig. 2). If these channels are functional, the significance of this sequence peculiarity could be a tempting issue to be explored. It has been shown that capping the TM2 on both sides with Trp or Tyr at position 93 (a highly conserved Phe immersed into the hydrophobic core of the bilayer), slows the kinetics of gating with an increment in the activation threshold and a decrease in cell viability in osmotic downshock assays.79 Interestingly, as the distance between aromatic belts increases, channel function is partially restored, presumably by facilitation of TM2 tilting.
In order to understand better the functioning of MS channels and contribute to the discovery of new insights regarding their tension-induced gating, the roles of different domains in MscL have been studied. The cooperativity between two or more MscL channels, has been integrated into a more simplistic “loopless” model of MscL, which has proved to promote a wider open pore.80 Importantly, this model lacking the P-loop connecting TM1 and TM2 shows a channel with a high elasticity and flexibility, confirming the restrictive role of such a domain in the MscL gating.81 According to this model, under equilateral tension, the ring formed by the TM2 bundle in direct contact with the lipids, opens and expands simultaneously, reducing the tilt angle in order to adapt the TM segments to the membrane compression that the lipid bilayer suffers (i.e., thinning). This compression is critical at the edge of the lipid pore formed (surrounded by the lipid-exposed TM2 domains), whereas the TM1 bundle is partially immersed in the center of the TM2 bundle and their contacts with the lipids are expected to be less numerous. In agreement with this model, hydropathy analyses show that although TM1 is hydrophobic and larger than TM2 by at least five residues, TM2 has a stronger hydrophobic character. In an attempt to compare their properties and interactions with lipids, Figure 3 shows a hydrophobicity plot of some MscL homologs aligned with selected α-PFPs discussed below (based in ref. 82). The slightly attenuated hydrophobicity seen for TM1 is in accordance with its function during gating, which forms a large water-filled pore lined almost exclusively by these segments; although residues from TM2 are also implicated.60 The N-terminus of the protein (so-called S1 domain) is amphipathic and has the potential to form a short α-helix attached to the inner side of the membrane.77,83 Thus, under tension TM1 also tilts and rotates but the angle of tilting for such a segment is more pronounced in comparison with their TM2 counterpart.80,81 Indeed, the tilt angles observed for TM segments from EcoMscL embedded in two membranes differing by their hydrophobic tail lengths are also different. In a simulated bilayer under tension, formed by 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE, C14:0), the tilt angle increases from 35° to 50° for TM1, whereas for TM2 it changes from 35° to 45°, compared with a bilayer of 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE, C16:0–C18:1) which simulates a relaxed membrane.84 The large tilting change experienced by TM1 also reveals the helix adaptation to achieve optimal lipid interactions. This effect probably is because TM1 is longer and has no rigid structure anchors, like Trp or Tyr residues, but rather it recurrently has snorkeling residues, such as Arg (53%) or Lys (46%), facing to the cytoplasmic side at position 13 (E. coli numbering).8 Indeed, the change of Arg13 to Cys has been described as a GOF mutant85 whereas the R13L mutant confers a LOF phenotype.86 This is consistent with the important snorkeling role proposed for Arg at that position. Furthermore, considering subhelix S1, TM1 is potentially extensible, acting as a single gate that stabilizes the open structure.83,87 Thus, hydrophobic matching has been constantly evoked as the theoretical explanation to understand the nature of this class of interactions.72-75,88
Beyond interactions to adapt to HMs, the lipid-water interface provides electrostatically another potential source for interaction with embedded proteins. One consistent observation is the effect that the presence of anionic lipids like phosphatidylserine (PS) has on membrane channels. Even at acidic pH, the Po of the KcsA channel is very low but in the presence of at least 50% anionic lipids it increases significantly in planar bilayer experiments.89 Studies in MscL have also shown this effect; in this channel, a conserved cluster of positive charges (EcoMscL: Arg104, Lys105, Lys106) on the cytoplasmic side of the protein is predicted to strongly interact with negatively charged lipid headgroups at the inner leaflet. This interplay increases the rate of solute flux through the open channel.90 Such requirement is only for the internal leaflet of the bilayer because there is no selectivity for anionic lipids on the periplasmic side of the channel. Indeed, a characterizing preference for dioleoyl-PS (DOPS) and dioleoyl-phosphatidic acid (DOPA) has been established for the inner part of the protein.91 These effects have been explained in terms of the ability to form H-bonds between the phospholipid headgroups and the lateral chains of the residues on the protein.90,91 However, it has been demonstrated that the tension threshold is not affected in DOPS or dioleoyl-phosphatidylglycerol (DOPG) bilayers, whereas the tension required to gate the channel is ~50% larger in DOPE:DOPC (1:1) than in DOPC bilayers.92 These apparently contradictory results have been explained in terms of a major contribution of hydrophobic interactions in the surface cavities of the protein with fatty acyl chains for tension transmission in comparison with the electrostatic interactions for channel function.10,93 Rather, the electropositive cluster of the protein binds to negatively charged lipids once the channel opens by tension. In consequence, tension is transmitted mainly by hydrophobic interactions and then the cluster transiently acts as an anchor during gating, modulating TM tilting as if it were a pivot.94 Therefore, important contacts with membrane proteins can also be achieved by the flexible lipid tails in external adjacent layers, which are not in the first shell of annular lipids.71
Protein adaptations to hydrophobic mismatch in MscS channels
The general principles described for MscL can also be applied to MscS, although compared with MscL, HM seems to have less influential on MscS gating.51 It has been shown that MscL reconstituted into liposomes mimicking the native composition of E. coli membranes, DOPE:DOPG:CL (14:5:1), channel activity is three times lower than in liposomes formed with DOPC:DOPG:CL (14:5:1).95 Similarly, MscS expressed in Xenopus oocytes requires at least three times less negative pressure to be activated in comparison with channels reconstituted in giant bacterial spheroplasts.70 However, although pipette tip geometry contributes to MscS adaptation (i.e., the complex behavior shown by MscS including inactivation or desensitization in response to a sustained mechanical stimulus),96 a contribution of lipid composition in both systems should not be ruled out. Indeed, X. laevis membranes have 65% PC, 19% PE and 10% of polyunsaturated phosphatidylinositol (PI), whereas E. coli has 70–80% PE and 15–20% phosphatidyglycerol (PG).97 PE is a reverse hexagonal (HII)-forming lipid, which has been shown to be densely packed with the highly ordered acyl chains. Moreover, the presence of PG in mixed membranes increases acyl chain ordering and makes them more compact (a high KA) and less fluid.47,98 This is opposite to the decreasing effect in lipid order and the rise in fluidity induced by tension in DPPC membranes.30,31 As in MscL, important residues implicated in sensitivity to mechanical changes in the membrane have been mainly located at the periplasmic interface of MscS.99 Thus, the tension sensor for both channels is predicted to be located at this place, involving residues interacting with lipids through both electrostatic as well as hydrophobic interactions.
Nevertheless, the situation is somewhat different in the heptameric MscS superfamily because each protein monomer spans the membrane three times and the large cytoplasmic domain (CDMscS) surrounds a water-filled chamber with a molecular sieve function.9,100 Even so, both MscL and MscS channels show a similar basic behavior in their responses to the force transmission from lipids through their lipid-exposed TMs. A great number of MscS-like homologs have additional N-terminal TM segments.17,26 Such diversity implies a more complex mechanism for tension sensing and transmission. In order to circumscribe our discussion to simple models, in the next, final section, a close re-examination of the interactions between lipids and TM segments of MS channels will be expose from the perspective of membrane active peptides. Likewise, their responses to membrane tension by deformation, rotation, tilting and the formation of stable pores will be treated. The information contained there could contribute to the discussion about the evolution of these proteins, as well as stimulate the design of synthetic MS channels.
α-Helical Pore-Forming Peptides (α-PFPs) as Experimental Tools to Understand MS Channel Evolution
Antimicrobial peptides are α-PFPs that have been extensively used to study peptide-lipid interactions and they represent simplified models for studies on peptide-induced pore formation in lipid bilayers.101 In general, they bind to microbial membranes, forming lipid pores and can elicit cell lysis at high concentrations.102-104 Alamethicin, melittin, MG-H2 (a magainin analog) and piscidin bind strongly and stabilize preformed lipidic pores.105 Thus, it is reasonable to assume that inclusion of these kind of peptides alleviates tension by adsorbing to the high-curved edges of lipidic pores. This has been described, for example, for protregrin-1(PG-1), which preferentially adsorbs to the rim of preformed lipid pores, and significantly reduces the line energy and tension of the system.106 This observation is not exclusive for PG-1. By different methods, two of the best-studied peptides are alamethicin (Alm) from Trichoderma viridae and melittin, the main toxic peptide (26-residues) from honeybee venom. Several studies on these α-PFPs have provided valuable information regarding the relationship between tension-induced lipid pores, their close coupling with peptide-induced pores and their stabilization.21,22,107-109 Importantly, the pore formation coupled to internal tension observed in both Alm and melittin, has also been reported in synthetic lytic peptides such as the class L amphipathic α-helix 18L (a model peptide with the sequence: GIKKFLGSIWKFIKAFVG). Once inserted in the membrane, 18L derivative peptides disturb the bilayer and create a potential site to form a pore by nucleation, which strongly responds to osmotic swelling.110 This modulates permeabilization in liposomes, facilitating the release of osmolytes, a similar attribute that is well known for MS channels.1,4 Thus, α-PFPs create perturbations at the surface of the monolayer where they are added, which is equivalent to the membrane tension required to open lipidic pores.101 The tension is then relieved through expansion and incorporation of more peptides that stabilize the formed pores, although with a precarious control on membrane tension.22,110-112
Studies with Alamethicin and Melittin and their responses to membrane tension
Alm has long been considered as a paradigm for channel formation and, along with melittin, is one of the two α-PFPs where adaptation to HMs by tilting has been studied both theoretically and experimentally.113-115 Alm is a hydrophobic peptaibol (20-residues) that adopts predominantly helical conformations, whose degree of helicity and conformational flexibility depends on several biophysical factors such as temperature, lipid composition, physical state of lipids, membrane hydration, salt content, pH, and the peptide-to-lipid (P/L) molar ratio.103,114-116 Channel formation by Alm has been explained through parallel “TM helical bundles” or “barrel staves” composed of several membrane spanning peptides that aggregate to line an aqueous pore,103,104 although it has been proposed that Alm inserts into bilayers using also a “toroidal” mechanism when it is exposed to ether-linked phospholipids.116 Nevertheless, folding and oligomerization of Alm are seriously affected if ether lipids are used instead of ester-linked lipids.117
Alm can form dimers, trimers, tetramers and large aggregates stabilizated by interhelical H-bonding which determines a multiplicity of conductances. Indeed, hexameric Alm channels are more stable than other assemblages.103 This can be done by the integration of the internal tension that is associated with the membrane thinning derived from the creation of a lipidic pore. Depending on lipid composition, at this specific value, peptides start to change their orientation with respect to the membrane plane from a parallel-to-perpendicular arrangement.21 In contrast, melittin has a stretch of positively charged amino acids at the C-terminus and only forms toroidal pores (wormholes).104 These structures are characterized by a bending of the lipid bilayer at the edge of the pore, with intercalation of lipid headgroups forming an integral part of it118 (see Fig. 4D) .Thus, Alm and analogs on the one hand and melittin and their derivatives (v gr magainins) on the other hand are different regarding pore size, being toroidal pores significantly larger than barrel-staves induced by Alm.119 Depending on lipid composition, melittin also increases membrane permeability at determined P/L* molar ratios, internal membrane tension, temperature and pH.21,22 Likewise, their channel-like activity in planar bilayers, in addition to micellization and induction of membrane fusion, has been well documented.120
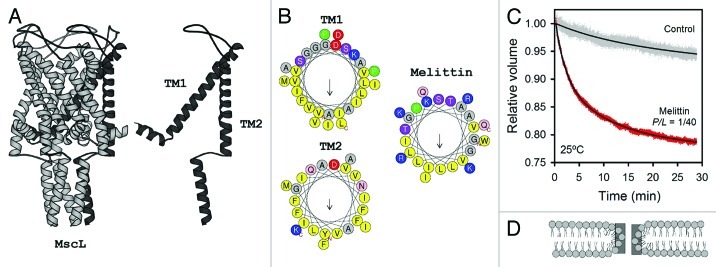
Figure 4. (A) Structure of the mechanosensitive channel MscL (PDB 2OAR, taken from ref. 9). (B) Helical wheel representation for the TM fragments of MscL and a monomer of melittin. Melittin and TM2 were modeled in α-helical wheel representations; since TM1 is larger, it was modeled as a π-helix, which promotes a better lipid exposing face. Hydrophobicity factors (H) obtained are: 0.817 (TM1); 0.959 (TM2) and melittin (0.511). Net charge (z): –1 (TM1); 0 (TM2); + 5 (melittin). Residues with hydrophobic side chains are shown in yellow. Small residues Gly and Ala are represented in gray and positively charged snorkeling residues, in blue. N- and C-ends are shown. Arrows show the hydrophobic moment. (C) Osmotically induced inflow of NH4+ through melittin pores in MLVs of DOPC:DPPC (2:1). MLVs were concentrated at 30 mM in choline chloride. Melittin was added to a P/L ratio of 1/40 in the presence and absence of a hypoosmotic downshock. For the osmotic shock, the sample was diluted 1:70 into an ammonium solution (100 mM). Permeability was determined by the swelling rate for a period of time, measuring the change in turbidity at Abs = 450nm. All flux measurements were performed at 25°C. (D) Schematic drawing of a toroidal pore in a lipid bilayer, taken from ref. 118.
Studies with Alm and melittin provide important information about their structural properties and energetics, the mechanisms and kinetics of induced pores as well as their stabilization in lipid bilayers under tension. In Alm channels, membrane tension directly couples changes associated with membrane expansion and the conductance state of the formed channel,107 whereas melittin pores couple permeability changes with the osmotically induced tension when transmembrane gradients are established.111 Thus, Alm and melittin both create local stresses at the surface of the bilayer, which cause membrane perturbations and, under specific conditions, they can stabilize pores at constant membrane tensions.21,22,109 Indeed, in molecular dynamics studies, Alm helices become more stable and compact under surface tension than in tension-free stages, because H-bonding reinforces Alm bundles by their anchorage to lipids.109 Interestingly, the MscL channel has also been modeled as being stable in closed state at low tension and highly stable under tension (i.e., open).121 In comparison, membrane tension controls electrostatic interactions between several melittin peptides and their contacts with the lipid interface determine their insertion rate and pore size.108 Moreover, the change in tilting observed in the TM segments of MS channels as a response to an increase in the lateral tension has also been described and simulated for Alm and melittin as a function of the equivalent bilayer hydrophobic thickness.113,122-124 As was anticipated, the main trend observed is that the tilt angles of these peptides increase with a reduction in lipid tail length in order to compensate positive HMs between the peptide length and bilayer thickness.73,74,88
Studies with TM segments of proteins showing channel activity
Notably, the responses observed to membrane tension by aggregates of Alm or melittin are not exclusive for antimicrobial peptides. For example, cell-death inducers of the Bcl-2 family, like Bax/Bid proteins facilitate the release of apoptotic factors through pore formation in the outer membranes of the mitochondria.125 Indeed, the chemically synthesized peptides encompassing the colicin-like hydrophobic hairpin (helix-5 or Bax-α5) or the helix-6 (Bax-α6) from the hydrosoluble Bax protein, emulate the permeation activity of the parental protein by forming toroidal pores.126,127 In both cases, the amphipatic fragments are positively charged by the presence of snorkeling residues Lys and/or Arg, as in magainin and melittin. Bax-α5 also induces a decrease of the line tension between two coexisting lipidic phases by releasing curvature stress and it has been shown to affect the energetics at the edge of the pore through its stabilization.128 Furthermore, Bid-α6 (23-residues), a peptide containing only the fragment α6 of Bid, another proapoptotic protein, forms pores presumably by a bundle of helices such as in Alm, which is consistent with the barrel-stave model.126 Colicin E1, a cytotoxic bacteriocin (522-residues) produced by some strains of E. coli but lethal for related strains of this species, has also a channel-forming activity, with a strong dependence on membrane thickness and curvature, also showing activation by LPC.129,130 These proteins induce toroidal pores in membranes but, unfortunately, their pore-forming TM α-helices have not been tested separately, and their activity has not yet been compared with the complete protein.
α-PFPs as models of TM segments of MS channels and the design of an osmotic nanovalve
The gating of the MscL and MscS channels have been persistently explained in terms of “barrel-staves.”59,77,131 However, although this gating model does not fit well for the MscL channel, according to solvent accessibility experiments with a Ni2+ chelate complex (NiEdda),60 toroidal pores are favored in thick membranes,119,130 in contrast to the thinning effect that tension exerts on lipid bilayers.30,31,80,84 Ion channels formed by a “barrel-stave” mode of aggregation show reproducible multiple discrete states.107,132-134 In addition, such as the TM segments of MscL and MscS channels, α-PFPs are amphipathic, exposing both hydrophilic as well as hydrophobic residues in opposite faces of the α-helix77 (Fig. 4B). The tendency to expose hydrophobic residues to the lipid bilayer on one side, and hydrophilic residues facing an open pore on the other side, is crucial to sense tension in both types of channels. In consequence, hydrophobic interactions with lipid tails and H-bonding with headgroups are key.90,92,135 Likewise, as in MS channels in general, natural α-PFPs lack Trp, which are flanking residues that restrict the tilting and rotational angles of TM α-helices in membrane proteins.75
Therefore, some relevant parallelisms appear between α-PFPs and bacterial MS channels (particularly in MscL homologs) if one considers their TM segments separately. Besides, studies in extramembranous domains of the protein are also important. Indeed, mutations in the P-loop facilitate channel opening by means of a reduction in its constraining effect, which contributes to channel’s gating,16,136 and studies on the cytoplasmic domain (CD or S3) of MscL indicate that it is quite stable upon gating and it remains associated as a bundle during channel opening, regulating the pore size.5,137 This is in accordance with the observation of the cytoplasmic bundle as a dispensable element of the protein, since an S3 deletion of EcoMscL (Δ110–136) or SaMscL (Δ95–119) does not abolish gating, albeit it induces a great variability of conductances.137,138 Together, these data suggest that TM-bundles in both MscL and MscS are essential elements to obtain the basic function of an MS channel. Thus, the concept of a “minimal channel” with dispensable regulatory components and still showing mechanosensitivity, opens interesting new questions in relation to the evolution of these proteins. How TM segments of these proteins interact with the lipids in a stretched membrane is one of the first questions to address in order to get some new insights.
Considering all this evidence, the similarities between α-PFPs and the TM segments of MS channels should be taken, however, with caution. α-PFPs only adopt helical conformations in membranes and the great majority form toroidal pores, disrupting membranes at high concentrations. The use of simulated “loopless” MscL models,80,81 as well as the studies on the role of the HM on mobile TM helices,73-75,88 make it possible to contemplate the use of attractive new molecular tools. Thus, it is reasonable to anticipate that the study of mechanisms of pore formation induced by natural or designed α-PFPs can provide key details for the design of specific nanovalves. From this perspective, the evolution of MS channels can also be experimentally addressed. Nevertheless, the design of an alternative functional channel, formed by a barrel-stave aggregate of α-PFPs, should necessarily consider new levels of complexity. For example, the role of regulating elements such as the linking loops between TM segments or the inclusion of extramembranous domains can also be approached.
As an example, in Figure 4C, ammonium permeation through melittin pores into liposomes was induced under a sudden osmotic downshock. Melittin was added at a peptide/lipid ratio of 1/40 to preformed MLVs of DOPC:DPPC (2:1). Close to these conditions, it has been demonstrated that melittin forms stable transmembrane pores.139 Here, at this P/L ratio and a specific lipid concentration of 500 μM, the presence of an imposed osmotic pressure across membranes results in enhanced pore-formation by melittin, which facilitates ammonium permeation. This seems to indicate that melittin forms pores coupled to the membrane expansion associated to downshock. Through these pores, [NH4+] ions are internalized in the vesicles, while this cation per se has a significantly low intrinsic permeability under the same conditions. Similar results were also found for glycine betaine, a common osmolyte in bacteria (not shown). Thus, it becomes imaginable to achieve improvements in functional peptide design with minimal sequence requirements, as models of protobiologic MS channels. Further advances in studying the molecular events that lead to establishing stable proteinaceous pores in vesicles, with the concomitant property to sense osmotic swelling, will surely contribute to a better understanding of how these proteins have been selected through evolution.
Concluding Remarks and Outlook
The interesting perspective to use model peptides corresponding to TM segments to mimic the complex functional properties of ion channels has been proposed elsewhere.140,141 Indeed, the design of a Gly repeat peptide (24-residues) has revealed the formation of multimeric porin-like channels, which exhibit a similar complex conductance, poor selectivity and voltage dependence in lipid bilayers.142 Synthetic peptides mimicking the M2 channel-lining segment from the Gly receptor and the nicotinic acetylcholine receptor (AChR) have also been achieved.143 Of equal importance is the use of ion-channel engineering to produce, for instance, pH-sensitive channels, reversibly light-activated MscL channels, osmotic tension-modulated channels, as well as the de novo design of molecular nanovalves to allow the controlled release of molecules with biotechnological ends.110,144,145
As a final point, the ultimate goal will be the design of “prebiotic peptides” with a desirable osmo-valve activity in a pre-cell context. Considering that oligomerization was found to be inherent to the primary sequence of MscL for its in vitro synthesis,18 and since chemically synthesized MscL proteins can be directly reconstituted in liposomes with high efficiency,19,20 it becomes tempting to study alternative “prebiotic” sequences146 in the context of model protocells. Indeed, the role of ancient peptides responding to membrane tension has been claimed as a determining factor allowing protocells to gain volume-control and osmoregulation.147 In a plausible prebiotic scenario on the early Earth, a precursor osmo-valve embedded into a phospholipid vesicle would be able to sense sudden severe environmental changes with the consequent mechanical stress generation on the membrane. After that shock, they will become active to counterbalance this potential harm. The envisaged result will be an autocatalytically closed system capable of sustaining a protometabolism.148,149 To this end, it becomes promising, for instance, to use fluorescent burst tools in order to monitor the release of specific molecules through MscL or α-PFPs reconstituted in liposomes, which has been achieved for melittin with a comparable performance.67 Alternatively, the use of molecular simulation has reinforced the principle that MS channels act as safety valves in prokaryotic cells, opening a large pore to release pressure during hypo-osmotic challenges.150 By using these approaches, one can imagine the design/modeling of a prebiotic osmo-nanovalve with such specific functions.
In summary:
(1) The mechanical properties of lipid bilayers, such as hydrophobic thickness, intrinsic curvature, and compressibility are key factors in the activity of the MS channels MscL and MscS. They also influence the spatial segregation of these proteins.
(2) Hydrophobic mismatch is a critical factor which determines how these proteins interact with their contiguous lipid environment. In MS channels, important adaptations to alleviate such situations are TM tilting, membrane snorkeling of specific residues (Lys/Arg) and electrostatic interactions at the lipid-water interface.
(3) The responses to tension and the adaptation to hydrophobic mismatch shown by α-PFPs indicate that they perform similar, simpler behaviors, like prokaryotic MS channels do. This raises interesting perspectives for the design of specific osmotic nanovalves.
Acknowledgments
The author wishes to thank G. Basañez and K. Ruiz-Mirazo for useful discussions and careful reading of the manuscript and J. Sot for technical assistance. This work was supported in part by a postdoctoral Fellow JAEDoc-07_00502 from the Spanish Consejo Superior de la Investigación Científica (CSIC).
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/21085
References
- 1.Martinac B. 3.5 Billion years of mechanosensory transduction: structure and function of mechanosensitive channels in prokaryotes. Current Topics Membr. 2007;58:25–57. doi: 10.1016/S1063-5823(06)58002-0. [DOI] [Google Scholar]
- 2.Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys J. 2002;83:290–8. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–29. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 4.Levina N, Tötemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–7. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang LM, Wray R, Parker J, Wilson D, Duran RS, Blount P. Three routes to modulate the pore size of the MscL channel/nanovalve. ACS Nano. 2012;6:1134–41. doi: 10.1021/nn203703j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Gandhi CS, Rees DC. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461:120–4. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi CS, Walton TA, Rees DC. OCAM: a new tool for studying the oligomeric diversity of MscL channels. Protein Sci. 2011;20:313–26. doi: 10.1002/pro.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balleza D, Gómez-Lagunas F. Conserved motifs in mechanosensitive channels MscL and MscS. Eur Biophys J. 2009;38:1013–27. doi: 10.1007/s00249-009-0460-y. [DOI] [PubMed] [Google Scholar]
- 9.Steinbacher S, Bass R, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Current Topics Membr. 2007;58:1–24. doi: 10.1016/S1063-5823(06)58001-9. [DOI] [Google Scholar]
- 10.Carney J, East JM, Lee AG. Penetration of lipid chains into transmembrane surfaces of membrane proteins: studies with MscL. Biophys J. 2007;92:3556–63. doi: 10.1529/biophysj.106.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moe PC, Blount P, Kung C. Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol Microbiol. 1998;28:583–92. doi: 10.1046/j.1365-2958.1998.00821.x. [DOI] [PubMed] [Google Scholar]
- 12.Moe PC, Levin G, Blount P. Correlating a protein structure with function of a bacterial mechanosensitive channel. J Biol Chem. 2000;275:31121–7. doi: 10.1074/jbc.M002971200. [DOI] [PubMed] [Google Scholar]
- 13.Gokhale RS, Saxena P, Chopra T, Mohanty D. Versatile polyketide enzymatic machinery for the biosynthesis of complex mycobacterial lipids. Nat Prod Rep. 2007;24:267–77. doi: 10.1039/b616817p. [DOI] [PubMed] [Google Scholar]
- 14.Ajouz B, Berrier C, Besnard M, Martinac B, Ghazi A. Contributions of the different extramembranous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J Biol Chem. 2000;275:1015–22. doi: 10.1074/jbc.275.2.1015. [DOI] [PubMed] [Google Scholar]
- 15.Park KH, Berrier C, Martinac B, Ghazi A. Purification and functional reconstitution of N- and C-halves of the MscL channel. Biophys J. 2004;86:2129–36. doi: 10.1016/S0006-3495(04)74272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai IJ, Liu ZW, Rayment J, Norman C, McKinley A, Martinac B. The role of the periplasmic loop residue glutamine 65 for MscL mechanosensitivity. Eur Biophys J. 2005;34:403–12. doi: 10.1007/s00249-005-0476-x. [DOI] [PubMed] [Google Scholar]
- 17.Malcolm HR, Elmore DE, Maurer JA. Mechanosensitive behavior of bacterial cyclic nucleotide gated (bCNG) ion channels: Insights into the mechanism of channel gating in the mechanosensitive channel of small conductance superfamily. Biochem Biophys Res Commun. 2012;417:972–6. doi: 10.1016/j.bbrc.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 18.Clayton D, Shapovalov G, Maurer JA, Dougherty DA, Lester HA, Kochendoerfer GG. Total chemical synthesis and electrophysiological characterization of mechanosensitive channels from Escherichia coli and Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2004;101:4764–9. doi: 10.1073/pnas.0305693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berrier C, Guilvout I, Bayan N, Park KH, Mesneau A, Chami M, et al. Coupled cell-free synthesis and lipid vesicle insertion of a functional oligomeric channel MscL MscL does not need the insertase YidC for insertion in vitro. Biochim Biophys Acta. 2011;1808:41–6. doi: 10.1016/j.bbamem.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Price CE, Kocer A, Kol S, van der Berg JP, Driessen AJM. In vitro synthesis and oligomerization of the mechanosensitive channel of large conductance, MscL, into a functional ion channel. FEBS Lett. 2011;585:249–54. doi: 10.1016/j.febslet.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 21.Lee MT, Chen FY, Huang HW. Energetics of pore formation induced by membrane active peptides. Biochemistry. 2004;43:3590–9. doi: 10.1021/bi036153r. [DOI] [PubMed] [Google Scholar]
- 22.Lee MT, Hung WC, Chen FY, Huang HW. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci U S A. 2008;105:5087–92. doi: 10.1073/pnas.0710625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimura K, Sokabe M. Mechanosensitivity of ion channels based on protein-lipid interactions. J R Soc Interface. 2010;7(Suppl 3):S307–20. doi: 10.1098/rsif.2010.0095.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–69. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinac B. Bacterial mechanosensitive channels as a paradigm for mechanosensory transduction. Cell Physiol Biochem. 2011;28:1051–60. doi: 10.1159/000335842. [DOI] [PubMed] [Google Scholar]
- 26.Naismith JH, Booth IR. Bacterial mechanosensitive channels-MscS: Evolution’s solution to creating sensitivity in function. Annu Rev Biophys. 2012;41:157–77. doi: 10.1146/annurev-biophys-101211-113227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tristram-Nagle S, Nagle JF. Lipid bilayers: thermodynamics, structure, fluctuations, and interactions. Chem Phys Lipids. 2004;127:3–14. doi: 10.1016/j.chemphyslip.2003.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uline MJ, Schick M, Szleifer I. Phase behavior of lipid bilayers under tension. Biophys J. 2012;102:517–22. doi: 10.1016/j.bpj.2011.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riske KA, Barroso RP, Vequi-Suplicy CC, Germano R, Henriques VB, Lamy MT. Lipid bilayer pre-transition as the beginning of the melting process. Biochim Biophys Acta. 2009;1788:954–63. doi: 10.1016/j.bbamem.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Neder J, West B, Nielaba P, Schmid F. Coarse-grained simulations of membranes under tension. J Chem Phys. 2010;132:115101. doi: 10.1063/1.3352583. [DOI] [PubMed] [Google Scholar]
- 31.Muddana HS, Gullapalli RR, Manias E, Butler PJ. Atomistic simulation of lipid and DiI dynamics in membrane bilayers under tension. Phys Chem Chem Phys. 2011;13:1368–78. doi: 10.1039/c0cp00430h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantor RS. Size distribution of barrel-stave aggregates of membrane peptides: influence of the bilayer lateral pressure profile. Biophys J. 2002;82:2520–5. doi: 10.1016/S0006-3495(02)75595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIntosh TJ, Simon SA. Roles of bilayer material properties in function and distribution of membrane proteins. Annu Rev Biophys Biomol Struct. 2006;35:177–98. doi: 10.1146/annurev.biophys.35.040405.102022. [DOI] [PubMed] [Google Scholar]
- 34.Andersen OS, Koeppe RE., 2nd Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–30. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 35.Seeger HM, Aldrovandi L, Alessandrini A, Facci P. Changes in single K(+) channel behavior induced by a lipid phase transition. Biophys J. 2010;99:3675–83. doi: 10.1016/j.bpj.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt D, MacKinnon R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci U S A. 2008;105:19276–81. doi: 10.1073/pnas.0810187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ermakov YA, Kamaraju K, Sengupta K, Sukharev S. Gadolinium ions block mechanosensitive channels by altering the packing and lateral pressure of anionic lipids. Biophys J. 2010;98:1018–27. doi: 10.1016/j.bpj.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrov E, Martinac B. Modulation of channel activity and gadolinium block of MscL by static magnetic fields. Eur Biophys J. 2007;36:95–105. doi: 10.1007/s00249-006-0109-z. [DOI] [PubMed] [Google Scholar]
- 39.Fuller N, Rand RP. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys J. 2001;81:243–54. doi: 10.1016/S0006-3495(01)75695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanghellini J, Wodlei F, von Grünberg HH. Phospholipid demixing and the birth of a lipid droplet. J Theor Biol. 2010;264:952–61. doi: 10.1016/j.jtbi.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Van Echteld CJ, de Kruijff B, de Gier J. Differential miscibility properties of various phosphatidylcholine/lysophosphatidylcholine mixtures. Biochim Biophys Acta. 1980;595:71–81. doi: 10.1016/0005-2736(80)90249-7. [DOI] [PubMed] [Google Scholar]
- 42.Lu J, Xu Y, Chen J, Huang F. Effect of lysophosphatidylcholine on behavior and structure of phosphatidylcholine liposomes. Sci China C Life Sci. 1997;40:622–9. doi: 10.1007/BF02882692. [DOI] [PubMed] [Google Scholar]
- 43.Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 44.Vásquez V, Sotomayor M, Cordero-Morales J, Schulten K, Perozo E. A structural mechanism for MscS gating in lipid bilayers. Science. 2008;321:1210–4. doi: 10.1126/science.1159674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsh D. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys J. 2007;93:3884–99. doi: 10.1529/biophysj.107.107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo J, Cui Q. Curvature generation and pressure profile modulation in membrane by lysolipids: insights from coarse-grained simulations. Biophys J. 2009;97:2267–76. doi: 10.1016/j.bpj.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wydro P, Witkowska K. The interactions between phosphatidylglycerol and phosphatidylethanolamines in model bacterial membranes: the effect of the acyl chain length and saturation. Colloids Surf B Biointerfaces. 2009;72:32–9. doi: 10.1016/j.colsurfb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Feller SE, Gawrisch K, MacKerell AD., Jr. Polyunsaturated fatty acids in lipid bilayers: intrinsic and environmental contributions to their unique physical properties. J Am Chem Soc. 2002;124:318–26. doi: 10.1021/ja0118340. [DOI] [PubMed] [Google Scholar]
- 49.Espinosa G, López-Montero I, Monroy F, Langevin D. Shear rheology of lipid monolayers and insights on membrane fluidity. Proc Natl Acad Sci U S A. 2011;108:6008–13. doi: 10.1073/pnas.1018572108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machiyama H, Tatsumi H, Sokabe M. Structural changes in the cytoplasmic domain of the mechanosensitive channel MscS during opening. Biophys J. 2009;97:1048–57. doi: 10.1016/j.bpj.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nomura T, Cranfield CG, Deplazes E, Owen DM, Macmillan A, Battle AR, et al. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc Natl Acad Sci U S A. 2012;109:8770–5. doi: 10.1073/pnas.1200051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–85. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charalambous K, Booth PJ, Woscholski R, Seddon JM, Templer RH, Law RV, et al. Engineering de novo membrane-mediated protein-protein communication networks. J Am Chem Soc. 2012;134:5746–9. doi: 10.1021/ja300523q. [DOI] [PubMed] [Google Scholar]
- 54.Ursell TS, Klug WS, Phillips R. Morphology and interaction between lipid domains. Proc Natl Acad Sci U S A. 2009;106:13301–6. doi: 10.1073/pnas.0903825106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seeger HM, Bortolotti CA, Alessandrini A, Facci P. Phase-transition-induced protein redistribution in lipid bilayers. J Phys Chem B. 2009;113:16654–9. doi: 10.1021/jp907505m. [DOI] [PubMed] [Google Scholar]
- 56.Schäfer LV, de Jong DH, Holt A, Rzepiela AJ, de Vries AH, Poolman B, et al. Lipid packing drives the segregation of transmembrane helices into disordered lipid domains in model membranes. Proc Natl Acad Sci U S A. 2011;108:1343–8. doi: 10.1073/pnas.1009362108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ursell TS, Huang KC, Peterson E, Phillips R. Cooperative gating and spatial organization of membrane proteins through elastic interactions. PLoS Comput Biol. 2007;3:e81. doi: 10.1371/journal.pcbi.0030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grage SL, Keleshian AM, Turdzeladze T, Battle AR, Tay WC, May RP, et al. Bilayer-mediated clustering and functional interaction of MscL channels. Biophys J. 2011;100:1252–60. doi: 10.1016/j.bpj.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Betanzos M, Chiang CS, Guy HR, Sukharev S. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nat Struct Biol. 2002;9:704–10. doi: 10.1038/nsb828. [DOI] [PubMed] [Google Scholar]
- 60.Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418:942–8. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 61.Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, et al. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008;321:1179–83. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.García-Sáez AJ, Chiantia S, Schwille P. Effect of line tension on the lateral organization of lipid membranes. J Biol Chem. 2007;282:33537–44. doi: 10.1074/jbc.M706162200. [DOI] [PubMed] [Google Scholar]
- 63.Kahya N. Protein-protein and protein-lipid interactions in domain-assembly: lessons from giant unilamellar vesicles. Biochim Biophys Acta. 2010;1798:1392–8. doi: 10.1016/j.bbamem.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 64.Richmond DL, Schmid EM, Martens S, Stachowiak JC, Liska N, Fletcher DA. Forming giant vesicles with controlled membrane composition, asymmetry, and contents. Proc Natl Acad Sci U S A. 2011;108:9431–6. doi: 10.1073/pnas.1016410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stachowiak JC, Hayden CC, Sanchez MAA, Wang J, Bunker BC, Voigt JA, et al. Targeting proteins to liquid-ordered domains in lipid membranes. Langmuir. 2011;27:1457–62. doi: 10.1021/la1041458. [DOI] [PubMed] [Google Scholar]
- 66.Doeven MK, Folgering JH, Krasnikov V, Geertsma ER, van den Bogaart G, Poolman B. Distribution, lateral mobility and function of membrane proteins incorporated into giant unilamellar vesicles. Biophys J. 2005;88:1134–42. doi: 10.1529/biophysj.104.053413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Bogaart G, Kusters I, Velásquez J, Mika JT, Krasnikov V, Driessen AJ, et al. Dual-color fluorescence-burst analysis to study pore formation and protein-protein interactions. Methods. 2008;46:123–30. doi: 10.1016/j.ymeth.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Varnier A, Kermarrec F, Blesneac I, Moreau C, Liguori L, Lenormand JL, et al. A simple method for the reconstitution of membrane proteins into giant unilamellar vesicles. J Membr Biol. 2010;233:85–92. doi: 10.1007/s00232-010-9227-8. [DOI] [PubMed] [Google Scholar]
- 69.Battle AR, Petrov E, Pal P, Martinac B. Rapid and improved reconstitution of bacterial mechanosensitive ion channel proteins MscS and MscL into liposomes using a modified sucrose method. FEBS Lett. 2009;583:407–12. doi: 10.1016/j.febslet.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 70.Maksaev G, Haswell ES. Expression and characterization of the bacterial mechanosensitive channel MscS in Xenopus laevis oocytes. J Gen Physiol. 2011;138:641–9. doi: 10.1085/jgp.201110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marsh D, Páli T. The protein-lipid interface: perspectives from magnetic resonance and crystal structures. Biochim Biophys Acta. 2004;1666:118–41. doi: 10.1016/j.bbamem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 72.Lee AG. Lipid-protein interactions. Biochem Soc Trans. 2011;39:761–6. doi: 10.1042/BST0390761. [DOI] [PubMed] [Google Scholar]
- 73.Killian JA, Nyholm TK. Peptides in lipid bilayers: the power of simple models. Curr Opin Struct Biol. 2006;16:473–9. doi: 10.1016/j.sbi.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 74.Kandasamy SK, Larson RG. Molecular dynamics simulations of model trans-membrane peptides in lipid bilayers: a systematic investigation of hydrophobic mismatch. Biophys J. 2006;90:2326–43. doi: 10.1529/biophysj.105.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nyholm TK, Ozdirekcan S, Killian JA. How protein transmembrane segments sense the lipid environment. Biochemistry. 2007;46:1457–65. doi: 10.1021/bi061941c. [DOI] [PubMed] [Google Scholar]
- 76.Strandberg E, Killian JA. Snorkeling of lysine side chains in transmembrane helices: how easy can it get? FEBS Lett. 2003;544:69–73. doi: 10.1016/S0014-5793(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 77.Sukharev S, Durell SR, Guy HR. Structural models of the MscL gating mechanism. Biophys J. 2001;81:917–36. doi: 10.1016/S0006-3495(01)75751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Wray R, Blount P. Intragenic suppression of gain-of-function mutations in the Escherichia coli mechanosensitive channel, MscL. Mol Microbiol. 2004;53:485–95. doi: 10.1111/j.1365-2958.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 79.Chiang CS, Shirinian L, Sukharev S. Capping transmembrane helices of MscL with aromatic residues changes channel response to membrane stretch. Biochemistry. 2005;44:12589–97. doi: 10.1021/bi050750r. [DOI] [PubMed] [Google Scholar]
- 80.Tang Y, Cao G, Chen X, Yoo J, Yethiraj A, Cui Q. A finite element framework for studying the mechanical response of macromolecules: application to the gating of the mechanosensitive channel MscL. Biophys J. 2006;91:1248–63. doi: 10.1529/biophysj.106.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang Y, Yoo J, Yethiraj A, Cui Q, Chen X. Gating mechanisms of mechanosensitive channels of large conductance, II: systematic study of conformational transitions. Biophys J. 2008;95:581–96. doi: 10.1529/biophysj.107.128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–32. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 83.Islca I, Wray R, Blount P. On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface. Biophys J. 2008;95:2283–2291. doi: 10.1073/pnas.092051099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Debret G, Valadié H, Stadler AM, Etchebest C. New insights of membrane environment effects on MscL channel mechanics from theoretical approaches. Proteins. 2008;71:1183–96. doi: 10.1002/prot.21810. [DOI] [PubMed] [Google Scholar]
- 85.Levin G, Blount P. Cysteine scanning of MscL transmembrane domains reveals residues critical for mechanosensitive channel gating. Biophys J. 2004;86:2862–70. doi: 10.1016/S0006-3495(04)74338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maurer JA, Dougherty DA. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL: implications for channel gating and evolutionary design. J Biol Chem. 2003;278:21076–82. doi: 10.1074/jbc.M302892200. [DOI] [PubMed] [Google Scholar]
- 87.Jeon J, Voth GA. Gating of the mechanosensitive channel protein MscL: the interplay of membrane and protein. Biophys J. 2008;94:3497–511. doi: 10.1529/biophysj.107.109850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strandberg E, Esteban-Martín S, Ulrich AS, Salgado J. Hydrophobic mismatch of mobile transmembrane helices: Merging theory and experiments. Biochim Biophys Acta. 2012;1818:1242–9. doi: 10.1016/j.bbamem.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 89.Marius P, Zagnoni M, Sandison ME, East JM, Morgan H, Lee AG. Binding of anionic lipids to at least three nonannular sites on the potassium channel KcsA is required for channel opening. Biophys J. 2008;94:1689–98. doi: 10.1529/biophysj.107.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Powl AM, East JM, Lee AG. Importance of direct interactions with lipids for the function of the mechanosensitive channel MscL. Biochemistry. 2008;47:12175–84. doi: 10.1021/bi801352a. [DOI] [PubMed] [Google Scholar]
- 91.Powl AM, Carney J, Marius P, East JM, Lee AG. Lipid interactions with bacterial channels: fluorescence studies. Biochem Soc Trans. 2005;33:905–9. doi: 10.1042/BST20050905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moe PC, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–44. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 93.Powl AM, East JM, Lee AG. Different effects of lipid chain length on the two sides of a membrane and the lipid annulus of MscL. Biophys J. 2007;93:113–22. doi: 10.1529/biophysj.107.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iscla I, Wray R, Blount P. An in vivo screen reveals protein-lipid interactions crucial for gating a mechanosensitive channel. FASEB J. 2011;25:694–702. doi: 10.1096/fj.10-170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powl AM, East JM, Lee AG. Anionic phospholipids affect the rate and extent of flux through the mechanosensitive channel of large conductance MscL. Biochemistry. 2008;47:4317–28. doi: 10.1021/bi702409t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belyy V, Kamaraju K, Akitake B, Anishkin A, Sukharev S. Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics. J Gen Physiol. 2010;135:641–52. doi: 10.1085/jgp.200910371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Opekarová M, Tanner W. Specific lipid requirements of membrane proteins - a putative bottleneck in heterologous expression. Biochim Biophys Acta. 2003;1610:11–22. doi: 10.1016/S0005-2736(02)00708-3. [DOI] [PubMed] [Google Scholar]
- 98.Zhao W, Róg T, Gurtovenko AA, Vattulainen I, Karttunen M. Role of phosphatidylglycerols in the stability of bacterial membranes. Biochimie. 2008;90:930–8. doi: 10.1016/j.biochi.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 99.Edwards MD, Li Y, Kim S, Miller S, Bartlett W, Black S, et al. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat Struct Mol Biol. 2005;12:113–9. doi: 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- 100.Gamini R, Sotomayor M, Chipot C, Schulten K. Cytoplasmic domain filter function in the mechanosensitive channel of small conductance. Biophys J. 2011;101:80–9. doi: 10.1016/j.bpj.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fuertes G, Giménez D, Esteban-Martín S, Sánchez-Muñoz OL, Salgado J. A lipocentric view of peptide-induced pores. Eur Biophys J. 2011;40:399–415. doi: 10.1007/s00249-011-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sato H, Feix JB. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1245–56. doi: 10.1016/j.bbamem.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 103.Leitgeb B, Szekeres A, Manczinger L, Vágvölgyi C, Kredics L. The history of alamethicin: a review of the most extensively studied peptaibol. Chem Biodivers. 2007;4:1027–51. doi: 10.1002/cbdv.200790095. [DOI] [PubMed] [Google Scholar]
- 104.Mihajlovic M, Lazaridis T. Antimicrobial peptides in toroidal and cylindrical pores. Biochim Biophys Acta. 2010;1798:1485–93. doi: 10.1016/j.bbamem.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mihajlovic M, Lazaridis T. Antimicrobial peptides bind more strongly to membrane pores. Biochim Biophys Acta. 2010;1798:1494–502. doi: 10.1016/j.bbamem.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lam KL, Wang H, Siaw TA, Chapman MR, Waring AJ, Kindt JT, et al. Mechanism of structural transformations induced by antimicrobial peptides in lipid membranes. Biochim Biophys Acta. 2012;1818:194–204. doi: 10.1016/j.bbamem.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 107.Opsahl LR, Webb WW. Transduction of membrane tension by the ion channel alamethicin. Biophys J. 1994;66:71–4. doi: 10.1016/S0006-3495(94)80751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin JH, Baumgaertner A. Stability of a melittin pore in a lipid bilayer: a molecular dynamics study. Biophys J. 2000;78:1714–24. doi: 10.1016/S0006-3495(00)76723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu Q, Vaughn MW. Surface tension effect on transmembrane channel stability in a model membrane. J Phys Chem B. 2005;109:19474–83. doi: 10.1021/jp051419k. [DOI] [PubMed] [Google Scholar]
- 110.Polozov IV, Anantharamaiah GM, Segrest JP, Epand RM. Osmotically induced membrane tension modulates membrane permeabilization by class L amphipathic helical peptides: nucleation model of defect formation. Biophys J. 2001;81:949–59. doi: 10.1016/S0006-3495(01)75753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Benachir T, Lafleur M. Osmotic and pH transmembrane gradients control the lytic power of melittin. Biophys J. 1996;70:831–40. doi: 10.1016/S0006-3495(96)79622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang HW, Chen FY, Lee MT. Molecular mechanism of Peptide-induced pores in membranes. Phys Rev Lett. 2004;92:198304. doi: 10.1103/PhysRevLett.92.198304. [DOI] [PubMed] [Google Scholar]
- 113.Marsh D. Orientation and peptide-lipid interactions of alamethicin incorporated in phospholipid membranes: polarized infrared and spin-label EPR spectroscopy. Biochemistry. 2009;48:729–37. doi: 10.1021/bi801279n. [DOI] [PubMed] [Google Scholar]
- 114.Duclohier H, Wróblewski H. Voltage-dependent pore formation and antimicrobial activity by alamethicin and analogues. J Membr Biol. 2001;184:1–12. doi: 10.1007/s00232-001-0077-2. [DOI] [PubMed] [Google Scholar]
- 115.Ye S, Nguyen KT, Chen Z. Interactions of alamethicin with model cell membranes investigated using sum frequency generation vibrational spectroscopy in real time in situ. J Phys Chem B. 2010;114:3334–40. doi: 10.1021/jp911174d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dave PC, Billington E, Pan YL, Straus SK. Interaction of alamethicin with ether-linked phospholipid bilayers: oriented circular dichroism, 31P solid-state NMR, and differential scanning calorimetry studies. Biophys J. 2005;89:2434–42. doi: 10.1529/biophysj.105.067678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bertelsen K, Vad B, Nielsen EH, Hansen SK, Skrydstrup T, Otzen DE, et al. Long-term-stable ether-lipid vs conventional ester-lipid bicelles in oriented solid-state NMR: altered structural information in studies of antimicrobial peptides. J Phys Chem B. 2011;115:1767–74. doi: 10.1021/jp110866g. [DOI] [PubMed] [Google Scholar]
- 118.Allende D, Simon SA, McIntosh TJ. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophys J. 2005;88:1828–37. doi: 10.1529/biophysj.104.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J. 2001;81:1475–85. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Biosci Rep. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 121.Wiggins P, Phillips R. Analytic models for mechanotransduction: gating a mechanosensitive channel. Proc Natl Acad Sci U S A. 2004;101:4071–6. doi: 10.1073/pnas.0307804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toraya S, Nishimura K, Naito A. Dynamic structure of vesicle-bound melittin in a variety of lipid chain lengths by solid-state NMR. Biophys J. 2004;87:3323–35. doi: 10.1529/biophysj.104.046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sengupta D, Meinhold L, Langosch D, Ullmann GM, Smith JC. Understanding the energetics of helical peptide orientation in membranes. Proteins. 2005;58:913–22. doi: 10.1002/prot.20383. [DOI] [PubMed] [Google Scholar]
- 124.Salnikov ES, Friedrich H, Li X, Bertani P, Reissmann S, Hertweck C, et al. Structure and alignment of the membrane-associated peptaibols ampullosporin A and alamethicin by oriented 15N and 31P solid-state NMR spectroscopy. Biophys J. 2009;96:86–100. doi: 10.1529/biophysj.108.136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Basañez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem. 2002;277:49360–5. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- 126.García-Sáez AJ, Coraiola M, Dalla Serra M, Mingarro I, Menestrina G, Salgado J. Peptides derived from apoptotic Bax and Bid reproduce the poration activity of the parent full-length proteins. Biophys J. 2005;88:3976–90. doi: 10.1529/biophysj.104.058008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.García-Sáez AJ, Coraiola M, Serra MD, Mingarro I, Müller P, Salgado J. Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J. 2006;273:971–81. doi: 10.1111/j.1742-4658.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- 128.García-Sáez AJ, Chiantia S, Salgado J, Schwille P. Pore formation by a Bax-derived peptide: effect on the line tension of the membrane probed by AFM. Biophys J. 2007;93:103–12. doi: 10.1529/biophysj.106.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sobko AA, Kotova EA, Antonenko YN, Zakharov SD, Cramer WA. Effect of lipids with different spontaneous curvature on the channel activity of colicin E1: evidence in favor of a toroidal pore. FEBS Lett. 2004;576:205–10. doi: 10.1016/j.febslet.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 130.Sobko AA, Kotova EA, Antonenko YN, Zakharov SD, Cramer WA. Lipid dependence of the channel properties of a colicin E1-lipid toroidal pore. J Biol Chem. 2006;281:14408–16. doi: 10.1074/jbc.M513634200. [DOI] [PubMed] [Google Scholar]
- 131.Anishkin A, Kamaraju K, Sukharev S. Mechanosensitive channel MscS in the open state: modeling of the transition, explicit simulations, and experimental measurements of conductance. J Gen Physiol. 2008;132:67–83. doi: 10.1085/jgp.200810000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keller SL, Bezrukov SM, Gruner SM, Tate MW, Vodyanoy I, Parsegian VA. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys J. 1993;65:23–7. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bezrukov SM, Rand RP, Vodyanoy I, Parsegian VA. Lipid packing stress and polypeptide aggregation: alamethicin channel probed by proton titration of lipid charge. Faraday Discuss. 1998;111:173–83, discussion 225-46. doi: 10.1039/a806579i. [DOI] [PubMed] [Google Scholar]
- 134.Mak DO, Webb WW. Two classes of alamethicin transmembrane channels: molecular models from single-channel properties. Biophys J. 1995;69:2323–36. doi: 10.1016/S0006-3495(95)80102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Malcolm HR, Heo YY, Elmore DE, Maurer JA. Defining the role of the tension sensor in the mechanosensitive channel of small conductance. Biophys J. 2011;101:345–52. doi: 10.1016/j.bpj.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maurer JA, Elmore DE, Lester HA, Dougherty DA. Comparing and contrasting Escherichia coli and Mycobacterium tuberculosis mechanosensitive channels (MscL). New gain of function mutations in the loop region. J Biol Chem. 2000;275:22238–44. doi: 10.1074/jbc.M003056200. [DOI] [PubMed] [Google Scholar]
- 137.Anishkin A, Gendel V, Sharifi NA, Chiang CS, Shirinian L, Guy HR, et al. On the conformation of the COOH-terminal domain of the large mechanosensitive channel MscL. J Gen Physiol. 2003;121:227–44. doi: 10.1085/jgp.20028768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iscla I, Wray R, Blount P. The oligomeric state of the truncated mechanosensitive channel of large conductance shows no variance in vivo. Protein Sci. 2011;20:1638–42. doi: 10.1002/pro.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van den Bogaart G, Guzmán JV, Mika JT, Poolman B. On the mechanism of pore formation by melittin. J Biol Chem. 2008;283:33854–7. doi: 10.1074/jbc.M805171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iwamoto T, Grove A, Montal MO, Montal M, Tomich JM. Chemical synthesis and characterization of peptides and oligomeric proteins designed to form transmembrane ion channels. Int J Pept Protein Res. 1994;43:597–607. doi: 10.1111/j.1399-3011.1994.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 141.Marsh D. Peptide models for membrane channels. Biochem J. 1996;315:345–61. doi: 10.1042/bj3150345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thundimadathil J, Roeske RW, Guo L. A synthetic peptide forms voltage-gated porin-like ion channels in lipid bilayer membranes. Biochem Biophys Res Commun. 2005;330:585–90. doi: 10.1016/j.bbrc.2005.02.184. [DOI] [PubMed] [Google Scholar]
- 143.Shank LP, Broughman JR, Takeguchi W, Cook G, Robbins AS, Hahn L, et al. Redesigning channel-forming peptides: amino acid substitutions that enhance rates of supramolecular self-assembly and raise ion transport activity. Biophys J. 2006;90:2138–50. doi: 10.1529/biophysj.105.070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grosse W, Essen LO, Koert U. Strategies and perspectives in ion-channel engineering. Chembiochem. 2011;12:830–9. doi: 10.1002/cbic.201000793. [DOI] [PubMed] [Google Scholar]
- 145.Bonardi F, Nouwen N, Feringa BL, Driessen AJ. Protein conducting channels-mechanisms, structures and applications. Mol Biosyst. 2012;8:709–19. doi: 10.1039/c2mb05433g. [DOI] [PubMed] [Google Scholar]
- 146.Pohorille A, Wilson MA, Chipot C. Membrane peptides and their role in protobiological evolution. Orig Life Evol Biosph. 2003;33:173–97. doi: 10.1023/A:1024627726231. [DOI] [PubMed] [Google Scholar]
- 147.Morris CE. How did cells get their size? Anat Rec. 2002;268:239–51. doi: 10.1002/ar.10158. [DOI] [PubMed] [Google Scholar]
- 148.Monnard PA, Deamer DW. Membrane self-assembly processes: steps toward the first cellular life. Anat Rec. 2002;268:196–207. doi: 10.1002/ar.10154. [DOI] [PubMed] [Google Scholar]
- 149.Mulkidjanian AY, Galperin MY, Koonin EV. Co-evolution of primordial membranes and membrane proteins. Trends Biochem Sci. 2009;34:206–15. doi: 10.1016/j.tibs.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Louhivuori M, Risselada HJ, van der Giessen E, Marrink SJ. Release of content through mechano-sensitive gates in pressurized liposomes. Proc Natl Acad Sci U S A. 2010;107:19856–60. doi: 10.1073/pnas.1001316107. [DOI] [PMC free article] [PubMed] [Google Scholar]