Abstract
Cutaneous mechanoreceptors are localized in the various layers of the skin where they detect a wide range of mechanical stimuli, including light brush, stretch, vibration and noxious pressure. This variety of stimuli is matched by a diverse array of specialized mechanoreceptors that respond to cutaneous deformation in a specific way and relay these stimuli to higher brain structures. Studies across mechanoreceptors and genetically tractable sensory nerve endings are beginning to uncover touch sensation mechanisms. Work in this field has provided researchers with a more thorough understanding of the circuit organization underlying the perception of touch. Novel ion channels have emerged as candidates for transduction molecules and properties of mechanically gated currents improved our understanding of the mechanisms of adaptation to tactile stimuli. This review highlights the progress made in characterizing functional properties of mechanoreceptors in hairy and glabrous skin and ion channels that detect mechanical inputs and shape mechanoreceptor adaptation.
Keywords: mechanoreceptor, mechanosensitive channel, pain, skin, somatosensory system, touch
Introduction
Touch is the detection of mechanical stimulus impacting the skin, including innocuous and noxious mechanical stimuli. It is an essential sense for the survival and the development of mammals and human. Contact of solid objects and fluids with the skin gives necessary information to the central nervous system that allows exploration and recognition of the environment and initiates locomotion or planned hand movement. Touch is also very important for apprenticeship, social contacts and sexuality. Sense of touch is the least vulnerable sense, although it can be distorted (hyperesthesia, hypoesthesia) in many pathological conditions.1-3
Touch responses involve a very precise coding of mechanical information. Cutaneous mechanoreceptors are localized in the various layers of the skin where they detect a wide range of mechanical stimuli, including light brush, stretch, vibration, deflection of hair and noxious pressure. This variety of stimuli is matched by a diverse array of specialized mechanoreceptors that respond to cutaneous deformation in a specific way and relay these stimuli to higher brain structures. Somatosensory neurones of the skin fall into two groups: low-threshold mechanoreceptors (LTMRs) that react to benign pressure and high-threshold mechanoreceptors (HTMRs) that respond to harmful mechanical stimulation. LTMR and HTMR cell bodies reside within dorsal root ganglia (DRG) and cranial sensory ganglia (trigeminal ganglia). Nerve fibers associated with LTMRs and HTMRs are classified as Aβ-, Aδ- or C-fibers based on their action potential conduction velocities. C fibers are unmyelinated and have the slowest conduction velocities (~2 m/s), whereas Aδ and Aβ fibers are lightly and heavily myelinated, exhibiting intermediate (~12 m/s) and rapid (~20 m/s) conduction velocities, respectively. LTMRs are also classified as slowly, or rapidly adapting responses (SA- and RA-LTMRs) according to their rates of adaptation to sustained mechanical stimulus. They are further distinguished by the cutaneous end organs they innervate and their preferred stimuli.
Ability of mechanoreceptors to detect mechanical cues relies on the presence of mechanotransducer ion channels that rapidly transform mechanical forces into electrical signals and depolarise the receptive field. This local depolarisation, called receptor potential, can generate action potentials that propagate toward the central nervous system. However, properties of molecules that mediate mechanotransduction and adaptation to mechanical forces remain unclear.
In this review, we provide an overview of mammalian mechanoreceptor properties in innocuous and noxious touch in the hairy and glabrous skin. We also consider the recent knowledge about the properties of mechanically-gated currents in an attempt to explain the mechanism of mechanoreceptor’s adaptation. Finally, we review recent progress made in identifying ion channels and associated proteins responsible for the generation of mechano-gated currents.
Innocuous Touch
Hair follicle-associated LTMRs
The hair follicles represent hair shaft-producing mini-organs that detect light touch. Fibers associated with hair follicles respond to hair motion and its direction by firing trains of action potentials at the onset and removal of the stimulus. They are rapidly adapting receptors.
Cat and rabbit
In cat and rabbit coat, hair follicles can be divided in three hair follicle types, the Down hair, the Guard hair and the Tylotrichs. The Down hairs (underhair, wool, vellus)4 are the most numerous, the shortest and finest hairs of the coat. They are wavy, colorless and emerged in groups of two to four hairs from a common orifice in the skin. The Guard hairs (monotrichs, overhears, tophair)4 are slightly curved, either pigmented or unpigmented, and emerged singly from the mouths of their follicles. The tylotrichs are the least numerous, the longest and thickest hairs.5,6 They are pigmented or unpigmented, sometimes both and emerged singly from a follicle which is surrounded by a loop of capillary blood vessels. The sensory fibers supply to a hair follicle is located below the sebaceous gland and are attributed to Aβ or Aδ-LTMR fibers.7
In close apposition to the down hair shaft, just below the level of the sebaceous gland is the ring of lanceolate pilo-Ruffini endings. These sensory nerve endings are positioned in a spiral course around the hair shaft within the connective tissue forming the hair follicle. Within the hair follicle, there are also free nerve endings, some of them forming mechanoreceptors. Frequently, touch corpuscles (see glabrous skin) are surrounding the neck region of tylotrich follicle.
Properties of myelinated nerve endings in cat and rabbit hairy skin have been explored intensively in the 1930–1970 period (review in Hamann, 1995).8 Remarkably, Brown and Iggo, studying 772 units with myelinated afferent nerve fibers in the saphenous nerves from cat and rabbit, have classified responses in three receptor types corresponding to the movements of Down hairs (type D receptors), Guard hair (type G receptors) and Tylotrich hair (type T receptor).9 All the afferent nerve fiber responses have been brought together in the Rapidly Adapting receptor of type I (RA I) by opposition to the Pacinian receptor named RA II. RA I mechanoreceptors detect velocity of mechanical stimulus and have sharp border. They do not detect thermal variations. Burgess et al. also described a rapidly adapting field receptor that responds optimally to stroking of the skin or movement of several hairs, which was attributed to stimulation of pilo-Ruffini endings. None of the hair follicle response was attributed to C fiber activity.10
Mice
In the dorsal hairy skin of mice, three major types of hair follicles have been described: zigzag (around 72%), awl/auchene (around 23%) and guard or tylotrich (around 5%).11-14 Zigzag and Awl/auchenne hair follicles produce the thinner and shorter hair shafts and are associated with one sebaceous gland. Guard or tylotrich hairs are the longest of the hair follicle types. They are characterized by a large hair bulb associated with two sebaceous glands. Guard and awl/auchene hairs are arranged in an iterative, regularly spaced pattern whereas zigzag hairs densely populate skin areas surrounding the two larger hair follicle types [Fig. 1 (A1, A2 and A3)].
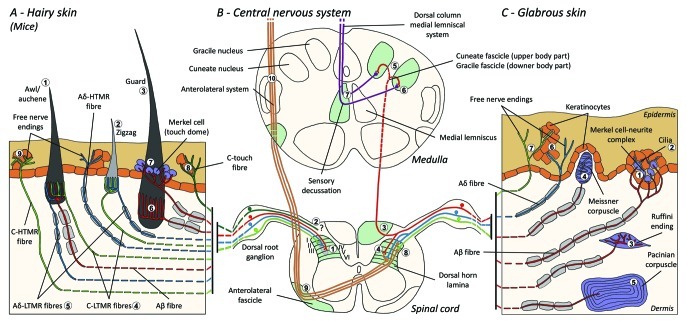
Figure 1. Organization and projections of cutaneous mechanoreceptors. In hairy skin, light brush and touch are mainly detected by the innervation around the hair follicles: awl/auchenne (A1), zigzag (A2) and guard (A3). Awl/auchene hairs are triply innervated by C-LTMR lanceolate endings (A4), Aδ-LTMR and Aβ rapidly adapting-LTMR (A6). Zigzag hair follicles are the shorter hair shafts and are innervated by both C-LTMR (A4) and Aδ -LTMR lanceolate endings (A5). The longest guard hair follicles are innervated by Aβ rapidly adapting-LTMR longitudinal lanceolate endings (A6) and are associated with Aβ slowly adapting-LTMR of touch dome endings (A7). The central projections of all these fibers terminate in distinct, but partially overlapping laminae of the spinal cord dorsal horn (C-LTMR in lamina II, Aδ-LTMR in lamina III and Aβ-LTMR in lamina IV and V). The projections of LTMR that innervate the same or adjacent hair follicles are aligned to form a narrow column in the spinal cord dorsal horn (B1 in gray). Only in hairy skin, a subpopulation of C-fibers free ending innervates the epidermis and responds to pleasant touch (A8). These C-touch fibers don’t respond to noxious touch and their pathway travel is not yet known (B2). In glabrous skin, innocuous touch is mediated by four types of LTMRs. The Merkel cell-neurite complex is in the basal layer of the epidermis (C1). This mechanoreceptor consists of an arrangement between many Merkel cells and an enlarged nerve terminal from a single Aβ fiber. Merkel cells exhibits finger like processes contacting keratinocytes (C2). The Ruffini ending is localized in the dermis. It is a thin cigar-shaped encapsulated sensory endings connected to Aβ fiber (C3). The Meissner corpuscle connected to Aβ nerve ending and is located in the dermal papillae. This encapsulated mechanoreceptor consists of packed down supportive cells arranged as horizontal lamellae surrounded by connective tissue (C4). Pacinian corpuscle is the deeper mechanoreceptor. One single Aβ unmyelinated nerve ending terminates in the center of this large ovoid corpuscle made of concentric lamellae. Projections of these Aβ-LTMR fibers in the spinal cord are divided in two branches. The principal central branch (B3) ascends in the spinal cord in the ipsilateral dorsal forming cuneate or gracile fascicles (B5) upon medulla level where the primary afferents make their first synapse (B6). The secondary neurons make a sensory decussation (B7) to form a tract on the medial lemniscus which ascends through the brainstem to the midbrain, specifically in the thalamus. Secondary branche of LTMR terminates in the dorsal horn in the lamina II, IV, V and interfere with the pain transmission (B4). Noxious touch is detected by the free nerve ending in the epidermis of both hairy (A9) and glabrous skin (C7). These mechanoreceptors are the ending of Aδ-HTMR and C-HTMR in close contact with neighboring keratinocytes (C6). Aδ-hTMR terminate in the lamina I and V; C-HTMR terminate in the lamina I and II (B8). At spinal cord dorsal horn level, primary afferents HTMRs make synapses with secondary neurons which cross the midline and climb to the higher brain structure in the anterolateral fascicle (B9, B10). LTMR, low threshold mechanoreceptor; HTMR, high threshold mechanoreceptor
Recently, Ginty and collaborators used a combination of molecular-genetic labeling and somatotopic retrograde tracing approaches to visualize the organization of peripheral and central axonal endings of the LTMRs in mice.15 Their findings support a model in which individual features of a complex tactile stimulus are extracted by the three hair follicle types and conveyed via the activities of unique combinations of Aβ-, Aδ- and C- fibers to dorsal horn.
They showed that the genetic labeling of tyrosine hydroxylase positive (TH+) DRG neurones characterize a population of nonpeptidergic, small-diameter sensory neurones and allow for visualization of C-LTMR peripheral endings in the skin. Surprisingly, the axoneal branches of individual C-LTMRs were found to arborise and form longitudinal lanceolate endings that are intimately associated with zigzag (80% of endings) and awl/auchene (20% of endings), but not tylotrich hair follicles [Fig. 1 (A4)]. Longitudinal lanceolate endings have been long thought to belong exclusively to Aβ-LTMRs and therefore it was unexpected that the endings of C-LTMRs would form longitudinal lanceolate endings.15 These C-LTMRs have an intermediate adaptation in comparison with the slowly and rapidly adapting myelinated mechanoreceptors [Fig. 2 (C1)].
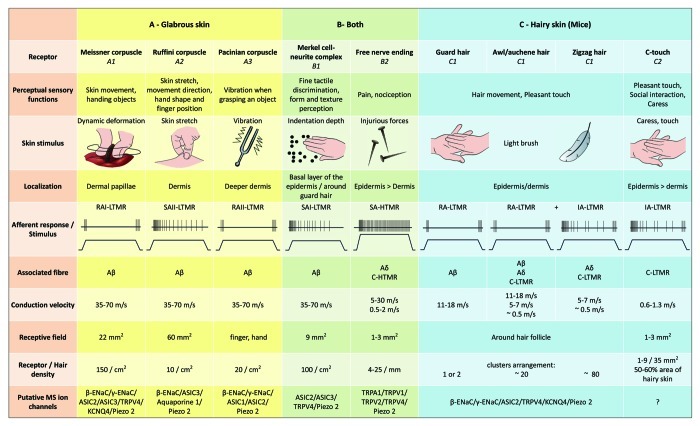
Figure 2. Tactile receptors in mammals: Cutaneous tactile receptors differentiate into innocuous touch supported by multiple receptors with low mechanical threshold (LTMRs) in glabrous and hairy skin and noxious touch supported by high mechanical threshold receptor (HTMRs). They make up nerve free endings that terminate mainly in epidermis. (A) Glabrous skin. A1: Meissner corpuscles detect skin motion and slipping of object in the hand. They are important for handing object and dexterity. Receptors rapidly adapt to stimulus, are connected to Aβ fibers and sparsely to C fibers and have large receptor field. A2: Ruffini corpuscles detect skin stretch and are important to detect finger position and handing object. Receptor slowly adapt to stimulus and maintained activity as long as the stimulus was applied. Receptors are connected to Aβ fibers and have large receptive field. A3: Pacinian corpuscles are deeper in the dermis and detect vibration. Receptors are connected to Aβ fibers; they rapidly adapt to stimulus and have the largest receptive field. (B) Whole skin. B1: Merkel-cell complexes are present in both glabrous skin and around hair. They are densely expressed in the hand and are important for texture perception and finest discrimination between two points. They are responsible for finger precision. Receptors are connected to Aβ fibers; they slowly adapt to stimulus and have short receptive field. B2: Noxious touch HTMRs with very slow adaptation to the stimulus, i.e., active as long as the nociceptive stimulus is applied. They are formed by the free nerve ending of Aδ and C-fibers associated to keratinocytes. (C) Hairy skin. C1: Hair follicles are associated with the different hair types. In mice Guard hairs are the longer and sparsely expressed one, awl/auchenne are of medium size and zigzag are the smallest and the most densely expressed hair. They are connected to Aβ fibers but also to Aδ and C-LTMRs fibers for awl/auchenne and zizag hair. They detect hair movement including pleasant touch during caress. They adapt rapidly or with intermediate kinetic to stimulus. C2: C-touch nerve endings correspond to a subtype of C fibers terminus with free ending characterized by a low mechanical threshold. They are supposed to encode for pleasant sensation induced by caress. They moderately adapt to stimulus and have short receptive field. Putative mechanosensitive (MS) ion channels expressed in the different tactile receptors are indicated accordingly to preliminary data and summarize present hypothesis under evaluation.
A second major population identified concerns the Aδ-LTMR endings in Awl/Auchenne and zigzag follicles to be compared with the Down hair follicle extensively studied in cat and rabbit. Ginty and collaborators showed that TrkB is expressed at high levels in a subset of medium-diametre DRG neurones. Intracellular recordings using the ex vivo skin-nerve preparation of labeled fibers revealed that they exhibit the physiological properties of fibers previously studied in cat and rabbit: exquisite mechanical sensitivity (Von Frey threshold < 0.07 mN), rapidly adapting responses to suprathreshold stimuli, intermediate conduction velocities (5.8 ± 0.9 m/s) and narrow uninflected soma spikes.15 These Aδ-LTMRs form longitudinal lanceolate endings associated with virtually every zigzag and awl/auchene hair follicle of the trunk [Fig. 1 (A5)].
Finally, they showed that the peripheral endings of rapidly adapting Aβ LTMRs form longitudinal lanceolate endings associated with guard (or tylotrich) and awl/auchene hair follicles [Fig. 1 (A6)].15 In addition, Guard hairs are also associated with a Merkel cell complex forming a touch dome connected to Aβ slowly adapting LTMR [Fig. 1 (A7)].
In summary, virtually all zigzag hair follicles are innervated by both C-LTMR and Aδ-LTMR lanceolate endings; awl/auchene hairs are triply innervated by Aβ rapidly adapting-LTMR, Aδ-LTMR and C-LTMR lanceolate endings; Guard hair follicles are innervated by Aβ rapidly adapting-LTMR longitudinal lanceolate endings and interact with Aβ slowly adapting-LTMR of touch dome endings. Thus, each mouse hair follicle receives unique and invariant combinations of LTMR endings corresponding to neurophysiologically distinct mechanosensory end organs. Considering the iterative arrangement of these three hair types, Ginty and collaborators propose that hairy skin consists of iterative repeat of peripheral unit containing, (1) one or two centrally located guard hairs, (2) ~20 surrounding awl/auchenne hairs and (3) ~80 interspersed zigzag hairs [Fig. 2 (C1)].
Spinal cord projection
The central projections of Aβ rapidly adapting-LTMRs, Aδ-LTMRs and C-LTMRs terminate in distinct, but partially overlapping laminae (II, III, IV) of the spinal cord dorsal horn. In addition, the central terminals of LTMRs that innervate the same or adjacent hair follicles within a peripheral LTMR unit are aligned to form a narrow LTMR column in the spinal cord dorsal horn [Fig. 1 (B1)]. Thus, it appears likely that a wedge, or column of somatotopically organized primary sensory afferent endings in the dorsal horn represents the alignment of the central projections of Aβ-, Aδ- and C-LTMRs that innervate the same peripheral unit and detect mechanical stimuli acting upon the same small group of hairs follicles. Based on the numbers of guard, awl/ auchene and zigzag hairs of the trunk and limbs and the numbers of each LTMR subtype, Ginty and collaborators estimate that the mouse dorsal horn contains 2,000–4,000 LTMR columns, which corresponds to the approximate number of peripheral LTMR units.15
Furthermore, axones of LTMR subtypes are closely associated with one another, having entwined projections and interdigitated lanceolate endings that innervate the same hair follicle. In addition, because the three hair follicle types exhibit different shapes, sizes and cellular compositions, they are likely to have distinct deflectional or vibrational tuning properties. These findings are consistent with classic neurophysiological measurements in the cat and rabbit indicating that Aβ RA-LTMRs and Aδ-LTMRs can be differentially activated by deflection of distinct hair follicle types.16,17
In conclusion, touch in hairy skin is the combination of: (1) the relative numbers, unique spatial distributions and distinct morphological and deflectional properties of the three types of hair follicles; (2) the unique combinations of LTMR subtype endings associated with each of the three hair follicle types; and (3) distinct sensitivities, conduction velocities, spike train patterns and adaptation properties of the four main classes of hair-follicle-associated LTMRs that enable the hairy skin mechanosensory system to extract and convey to the CNS the complex combinations of qualities that define a touch.
Free-nerve endings LTMRs
Generally, C-fibers free endings in the skin are HTMRs, but a subpopulation of C-fibers doesn’t respond to noxious touch. This subset of tactile C-fiber (CT) afferents represents a distinct type of unmyelinated, low-threshold mechanoreceptive units existing in the hairy but not glabrous skin of humans and mammals [Fig. 1 (A8)].18,19 CTs are generally associated with the perception of pleasant tactile stimulation in body contact.20,21
CT afferents respond to indentation forces in the range 0.3–2.5 mN and are thus as sensitive to skin deformation as many of the Aβ afferents.19 The adaptation characteristics of CT afferents are thus intermediate in comparison with the slowly and rapidly adapting myelinated mechanoreceptors. The receptive fields of human CT afferents are roughly round or oval in shape. The field consists of one to nine small responsive spots distributed over an area up to 35 mm2.22 The mouse homolog receptors are organized in a pattern of discontinuous patches covering about 50–60% of the area in the hairy skin [Fig. 2 (C2)].23
Evidence from patients lacking myelinated tactile afferents indicates that signaling in CT fibers activate the insular cortex. Since this system is poor in encoding discriminative aspects of touch, but well-suited to encoding slow, gentle touch, CT fibers in hairy skin may be part of a system for processing pleasant and socially relevant aspects of touch.24 CT fiber activation may also have a role in pain inhibition and it has recently been proposed that inflammation or trauma may change the sensation conveyed by C-fiber LTMRs from pleasant touch to pain.25,26
Which pathway CT-afferents travel is not yet known [Fig. 1 (B2)], but low-threshold tactile inputs to spinothalamic projection cells have been documented,27 lending credence to reports of subtle, contralateral deficits of touch detection in human patients following destruction of these pathways after chordotomy procedures.28
LTMRs in Glabrous skin
Merkel cell-neurite complexes and touch dome
Merkel (1875) was the first to give a histological description of clusters of epidermal cells with large lobulated nuclei, making contact with presumed afferent nerve fibers. He assumed that they subserved sense of touch by calling them Tastzellen (tactile cells). In humans, Merkel cell–neurite complexes are enriched in touch sensitive areas of the skin, they are found in the basal layer of the epidermis in fingers, lips and genitals. They also exist in hairy skin at lower density. The Merkel cell–neurite complex consists of a Merkel cell in close apposition to an enlarged nerve terminal from a single myelinated Aβ fiber [Fig. 1 (C1)] (review in Halata and collaborators).29 At the epidermal side Merkel cell exhibits finger-like processes extending between neighboring keratinocytes [Fig. 1 (C2)]. Merkel cells are keratinocyte-derived epidermal cells.30,31 The term of touch dome was introduced to name the large concentration of Merkel cell complexes in the hairy skin of cat forepaw. A touch dome could have up to 150 Merkel cells innervated by a single Aβ-fiber and in humans besides Aβ-fibers, Aδ and C-fibers were also regularly present.32-34
Stimulation of Merkel cell–neurite complexes results in slowly-adapting Type I (SA I) responses, which originate from punctuate receptive fields with sharp borders. There is no spontaneous discharge. These complexes respond to indentation depth of the skin and have the highest spatial resolution (0.5 mm) of the cutaneous mechanoreceptors. They transmit a precise spatial image of tactile stimuli and are proposed to be responsible for shape and texture discrimination [Fig. 2 (B1)]. Mice devoid of Merkel cells cannot detect textured surfaces with their feet while they do so using their whiskers.35
Whether the Merkel cell, the sensory neuron or both are sites of mechanotransduction is still a matter of debate. In rats, phototoxic destruction of Merkel cells abolishes SA I response.36 In mice with genetically suppressed-Merkel cells, the SA I response recorded in ex vivo skin/nerve preparation completely disappeared, demonstrating that Merkel cells are required for the proper encoding of Merkel receptor responses.37 However, the mechanical stimulation of isolated Merkel cells in culture by motor driven pressure does not generate mechanically-gated currents.38,39 Keratinocytes may play an important role in the normal functioning of the Merkel cell–neurite complex. The Merkel cell finger-like processes can move with skin deformation and epidermis cell movement, and this may be the first step of mechanical transduction. Clearly, the conditions required to study mechano-sensitivity of Merkel cells have yet to be established.
Ruffini endings
Ruffini endings are thin cigar-shaped encapsulated sensory endings connected to Aβ nerve endings. Ruffini endings are small connective tissue cylinders arranged along dermal collagen strands which are supplied by one to three myelinated nerve fibers of 4–6 µm diametre. Up to three cylinders of different orientation in the dermis may merge to form one receptor [Fig. 1 (C3)]. Structurally, Ruffini endings are similar to Golgi tendon organs. They are broadly expressed in the dermis and have been identified as the slowly adapting type II (SA II) cutaneous mechanoreceptors. Against the background of spontaneous nervous activity, a slowly-adapting regular discharge is elicited by perpendicular low force maintained mechanical stimulation or more effectively by dermal stretch. SA II response originates from large receptive fields with obscure borders. Ruffini receptors contribute to the perception of the direction of object motion through the pattern of skin stretch [Fig. 2 (A2)].
In mice, SA I and SA II responses can be separated electrophysiologically in ex-vivo nerve-skin preparation.40 Nandasena and collaborators reported the immunolocalization of aquaporin 1 (AQP1) in the periodontal Ruffini endings of the rat incisors suggesting that AQP1 is involved in the maintenance of the dental osmotic balance necessary for the mechanotransduction.41 The periodontal Ruffini endings also expressed the putative mechanosensitive ion channel ASIC3.42
Meissner corpuscles
Meissner corpuscles are localized in the dermal papillae of the glabrous skin, mainly in hand palms and foot soles but also in lips, in tongue, in face, in nipples and in genitals. Anatomically, they consist of an encapsulated nerve ending, the capsule being made of flattened supportive cells arranged as horizontal lamellae embedded in connective tissue. There is one single nerve fiber Aβ afferents connected per corpuscle [Fig. 1 (C4)]. Any physical deformation of the corpuscle triggers a volley of action potentials that quickly ceases, i.e., they are rapidly adapting receptors. When the stimulus is removed, the corpuscle regains its shape and while doing so produces another volley of action potentials. Due to their superficial location in the dermis, these corpuscles selectively respond to skin motion, tactile detection of slip and vibrations (20–40 Hz). They are sensitive to dynamic skin - for example, between the skin and an object that is being handled [Fig. 2 (A1)].
Pacinian corpuscles
Pacinian corpuscles are the deeper mechanoreceptors of the skin and are the most sensitive encapsulated cutaneous mechanoreceptor of skin motion. These large ovoid corpuscles (1 mm in length) made of concentric lamellae of fibrous connective tissue and fibroblasts lined by flat modified Schwann cells are expressed in the deep dermis.43 In the center of the corpuscle, in a fluid-filled cavity called inner bulb, terminates one single Aβ afferent unmyelinated nerve ending [Fig. 1 (C5)]. They have a large receptive field on the skin’s surface with a particularly sensitive center. The development and function of several rapidly adapting mechanoreceptor types are disrupted in c-Maf mutant mice. In particular, Pacinian corpuscles are severely atrophied.44
Pacinian corpuscles display very rapid adaptation in response to the indentation of the skin, the rapidly-adapting II (RA II) nervous discharge that are capable of following high frequency of vibratory stimuli, and allow perception of distant events through transmitted vibrations.45 Pacinian corpuscle afferents respond to sustained indentation with transient activity at the onset and offset of the stimulus. They are also called acceleration detectors because they can detect changes in the strength of the stimulus and, if the rate of change in the stimulus is altered (as happens in vibrations), their response becomes proportional to this change. Pacinian corpuscles sense gross pressure changes and most of all vibrations (150–300 Hz), which they can detect even centimeters away [Fig. 2 (A3)].
Tonic response was observed in decapsulated Pacinian corpuscle.46 In addition, intact Pacinian corpuscles respond with sustained activity during constant indentation stimuli, without altering mechanical thresholds or response frequency when GABA-mediated signaling is blocked between lamellate glia and a nerve ending.47 Thus, the non-neuronal components of the Pacinian corpuscle may have dual roles in filtering the mechanical stimulus as well as in modulating the response properties of the sensory neurone.
Spinal cord projections
Projections of the Aβ-LTMRs in the spinal cord are divided in two branches. The principal central branch ascends in the spinal cord in the ipsilateral dorsal columns to the cervical level [Fig. 1 (B3)]. Secondary branches terminate in the dorsal horn in the laminae IV and interfere with the pain transmission, for example. This may attenuate pain as a part of the gate control [Fig. 1 (B4)].48
At cervical levels, axones of the principal branch separate in two tracts: the midline tract comprises the gracile fascicle conveying information from the lower half of the body (legs and trunk), and the outer tract comprises the cuneate fascicle conveying information from the upper half of the body (arms and trunk) [Fig. 1 (B5)].
Primary tactile afferents make their first synapse with second order neurones at the medulla where fibers from each tract synapse in a nucleus of the same name: the gracile fasciculus axones synapse in the gracile nucleus and the cuneate axones synapse in the cuneate nucleus [Fig. 1 (B6)]. Neurones receiving the synapse provide the secondary afferents and cross the midline immediately to form a tract on the contralateral side of the brainstem—the medial lemniscus—which ascends through the brainstem to the next relay station in the midbrain, specifically, in the thalamus [Fig. 1 (B7)].
Molecular specification of LTMRs
Molecular mechanisms controlling the early diversification of LTMRs have been recently partly elucidated. Bourane and collaborators have shown that the neuronal populations expressing the Ret tyrosine kinase receptor (Ret) and its co-receptor GFRα2 in E11–13 embryonic mice DRG selectively coexpress the transcription factor Mafa.49,50 These authors demonstrate that the Mafa/Ret/GFRα2 neurones destined to become three specific types of LTRMs at birth: the SA1 neurones innervating Merkel-cell complexes, the rapidly adapting neurones innervating Meissner corpuscles and the rapidly adapting afferents (RA I) forming lanceolate endings around hair follicles. Ginty and collaborators also report that DRG neurones expressing early-Ret are rapidly adapting mechanoreceptors from Meissner corpuscles, Pacinian corpuscles and lanceolate endings around hair follicles.51 They innervate discrete target zones within the gracile and cuneate nuclei, revealing a modality-specific pattern of mechanosensory neurone axonal projections within the brainstem.
Exploration of human skin mechanoreceptors
The technique of “microneurography” described by Hagbarth and Vallbo in 1968 has been applied to study the discharge behavior of single human mechanosensitive endings supplying muscle, joint and skin (see for review Macefield, 2005).52,53 The majority of human skin microneurography studies have characterized the physiology of tactile afferents in the glabrous skin of the hand. Microelectrode recordings from the median and ulnar nerves in human subjects have revealed touch sensation generated by the four classes of LTMRs: Meissner afferents are particularly sensitive to light stroking across the skin, responding to local shear forces and incipient or overt slips within the receptive field. Pacinian afferents are exquisitively sensitive to brisk mechanical transients. Afferents respond vigorously to blowing over the receptive field. A Pacinian corpuscle located in a digit will usually respond to tapping the table supporting the arm. Merkel afferents characteristically have a high dynamic sensitivity to indentation stimuli applied to a discrete area and often respond with an off-discharge during release. Although the Ruffini afferents do respond to forces applied normally to the skin, a unique feature of SA II afferents is their capacity to respond also to lateral skin stretch. Finally, hair units in the forearm have large ovoid or irregular receptive fields composed of multiple sensitive spots that corresponded to individual hairs (each afferent supply ~20 hairs).
Mechanical sensitivity of keratinocytes
Any mechanical stimulus on the skin must be transmitted through keratinocytes that form the epidermis. These ubiquitous cells may perform signaling functions in addition to their supportive or protective roles. For example, keratinocytes secrete ATP, an important sensory signaling molecule, in response to mechanical and osmotic stimuli.54,55 The release of ATP induces intracellular calcium increase by autocrine stimulation of purinergic receptors.55 Furthermore, there is evidence that hypotonicity activates the Rho-kinase signaling pathway and the subsequent F-actin stress fiber formation suggesting that the mechanical deformation of the keratinocytes may mechanically interfere with the neighbor cells such as Merkel cells for innocuous touch and C-fiber free endings for noxious touch [Fig. 1 (C6)].56,57
Noxious Touch
High threshold mechanoreceptors (HTMRs) are epidermal C- and Aδ free nerve-endings. They are not associated with specialized structures and are observed in both hairy skin [Fig. 1 (A9)] and glabrous skin [Fig. 1(C7)]. However, the term of free nerve-ending has to be considered prudently since nerve endings are always in close apposition with keratinocyte or Langherans’ cell or melanocytes. Ultrastructural analysis of nerve endings reveals the presence of rough endoplasmic reticulum, abundant mitochondria and dense-core vesicle. Adjacent membranes of epidermal cells are thickened and resembling post-synaptic membrane in nervous tissues. Note that the interactions between nerve endings and epidermal cells may be bidirectional since epidermal cells may release mediators as ATP, interleukine (IL6, IL10) and bradykinin and conversely peptidergic nerve endings may release peptides such as CGRP or substance P acting on epidermal cells. HTMRs comprise mechano-nociceptors excited only by noxious mechanical stimuli and polymodal nociceptors that also respond to noxious heat and exogenous chemical [Fig. 2 (B2)].58
HTMR afferent fibers terminate on projection neurones in the dorsal horn of the spinal cord. Aδ-HTMRs contact second order neurones predominantly in the lamina I and V, whereas C-HTMRs terminate in the lamina II [Fig. 1 (B8)]. Second order nociceptive neurones project to the controlateral side of the spinal cord and ascend in the white matter, forming the anterolateral system. These neurones terminate mainly in the thalamus [Fig. 1 (B9 and B10)].
Mechano-Currents in Somatosensory Neurones
The mechanisms of slow or rapid adaptation of mechanoreceptors are not yet elucidated. It is not clear to what extent mechanoreceptor adaptation is provided by the cellular environment of the sensory nerve ending, the intrinsic properties of the mechanically-gated channels and the properties of the axonal voltage-gated ion channels in sensory neurones (Fig. 2). However, recent progress in the characterization of mechanically-gated currents has demonstrated that different classes of mechanosensitive channels exist in DRG neurones and may explain some aspects of the adaptation of mechanoreceptors.
In vitro recording in rodents has shown that the soma of DRG neurons is intrinsically mechanosensitive and express cationic mechano-gated currents.59-64 Gadolinium and ruthenium red fully block mechanosensitive currents, whereas external calcium and magnesium, at physiological concentrations, as well as amiloride and benzamil, cause partial block.60,62,63 FM1-43 acts as a lasting blocker, and the injection of FM1-43 into the hind paw of mice decreases pain sensitivity in the Randall–Selitto test and increases the paw withdrawal threshold assessed with von Frey hairs.65
In response to sustained mechanical stimulation, mechanosensitive currents decline through closure. Based on the time constants of current decay, four distinct types of mechanosensitive currents have been distinguished: rapidly adapting currents (~3–6 ms), intermediately adapting currents (~15–30 ms), slowly adapting currents (~200–300 ms) and ultra-slowly adapting currents (~1000 ms).64 All these currents are present with variable incidence in rat DRG neurones innervating the glabrous skin of the hindpaw.64
The mechanical sensitivity of mechanosensitive currents can be determined by applying a series of incremental mechanical stimuli, allowing for relatively detailed stimulus-current analysis.66 The stimulus–current relationship is typically sigmoidal, and the maximum amplitude of the current is determined by the number of channels that are simultaneously open.64,67 Interestingly, the rapidly adapting mechanosensitive current has been reported to display low mechanical threshold and half-activation midpoint compared with the ultra-slowly adapting mechanosensitive current.63,65
Sensory neurones with non-nociceptive phenotypes preferentially express rapidly adapting mechanosensitive currents with lower mechanical threshold.60,61,63,64,68 Conversely, slowly and ultra-slowly adapting mechanosensitive currents are occasionally reported in putative non-nociceptive cells.64,68 This prompted suggestion that these currents might contribute to the different mechanical thresholds seen in LTMRs and HTMRs in vivo. Although these in vitro experiments should be taken with caution, support for the presence in the soma of the DRG neurones of low- and high-threshold mechanotransducers was also provided by radial stretch-based stimulation of cultured mouse sensory neurones.69 This paradigm revealed two main populations of stretch-sensitive neurones, one that responds to low stimulus amplitude and another one that selectively responds to high stimulus amplitude.
These results have important, yet speculative, mechanistic implications: the mechanical threshold of sensory neurones might have little to do with the cellular organization of the mechanoreceptor but may lie in the properties of the mechanically-gated ion channels.
The mechanisms that underlie desensitization of mechanosensitive cation currents in rat DRG neurones have been recently unraveled.64,67 It results from two concurrent mechanisms that affect channel properties: adaptation and inactivation. Adaptation was first reported in auditory hair cell studies. It can be described operationally as a simple translation of the transducer channel’s activation curve along the mechanical stimulus axis.70-72 Adaptation allows sensory receptors to maintain their sensitivity to new stimuli in the presence of an existing stimulus. However, a substantial fraction of mechanosensitive currents in DRG neurones cannot be reactivated following conditioning mechanical stimulation, indicating inactivation of some transducer channels.64,67 Therefore, both inactivation and adaptation act in tandem to regulate mechanosensitive currents. These two mechanisms are common to all mechanosensitive currents identified in rat DRG neurones, suggesting that related physicochemical elements determine the kinetics of these channels.64
In conclusion, determining the properties of endogenous mechanosensitive currents in vitro is crucial in the quest to identify transduction mechanisms at the molecular level. The variability observed in the mechanical threshold and the adapting kinetics of the different mechanically-gated currents in DRG neurones suggest that intrinsic properties of ion channels may explain, at least in part, mechanical threshold and adaptation kinetics of the mechanoreceptors described in the decades 1960–80 using ex vivo preparations.
Putative Mechanosensitive Proteins
Mechanosensitive ion currents in somatosensory neurones are well characterized, by contrast, little is known about the identity of molecules that mediate mechanotransduction in mammals. Genetic screens in Drosophila and C. elegans have identified candidate mechanotransduction molecules, including the TRP and degenerin/epithelial Na+ channel (Deg/ENaC) families.73 Recent attempts to elucidate the molecular basis of mechanotransduction in mammals have largely focused on homologs of these candidates. Additionally, many of these candidates are present in cutaneous mechanoreceptors and somatosensory neurones (Fig. 2).
Acid-sensing ion channels
ASICs belong to a proton-gated subgroup of the degenerin–epithelial Na+ channel family.74 Three members of the ASIC family (ASIC1, ASIC2 and ASIC3) are expressed in mechanoreceptors and nociceptors. The role of ASIC channels has been investigated in behavioral studies using mice with targeted deletion of ASIC channel genes. Deletion of ASIC1 does not alter the function of cutaneous mechanoreceptors but increases mechanical sensitivity of afferents innervating the gut.75 ASIC2 knockout mice exhibit a decreased sensitivity of rapidly adapting cutaneous LTMRs.76 However, subsequent studies reported a lack of effects of knocking out ASIC2 on both visceral mechano-nociception and cutaneous mechanosensation.77 ASIC3 disruption decreases mechano sensitivity of visceral afferents and reduces responses of cutaneous HTMRs to noxious stimuli.76
The transient receptor channel
THE TRP superfamily is subdivided into six subfamilies in mammals.78 Nearly all TRP subfamilies have members linked to mechanosensation in a variety of cell systems.79 In mammalian sensory neurones, however, TRP channels are best known for sensing thermal information and mediating neurogenic inflammation, and only two TRP channels, TRPV4 and TRPA1, have been implicated in touch responsiveness. Disrupting TRPV4 expression in mice has only modest effects on acute mechanosensory thresholds, but strongly reduces sensitivity to noxious mechanical stimuli.80,81 TRPV4 is a crucial determinant in shaping the response of nociceptive neurones to osmotic stress and to mechanical hyperalgesia during inflammation.82,83 TRPA1 seems to have a role in mechanical hyperalgesia. TRPA1-deficient mice exhibit pain hypersensitivity. TRPA1 contributes to the transduction of mechanical, cold and chemical stimuli in nociceptor sensory neurones but it appears that is not essential for hair-cell transduction.84,85
There is no clear evidence indicating that TRP channels and ASICs channels expressed in mammals are mechanically gated. None of these channels expressed heterologously recapitulates the electrical signature of mechanosensitive currents observed in their native environment. This does not rule out the possibility that ASICs and TRPs channels are mechanotransducers, given the uncertainty of whether a mechanotransduction channel may function outside of its cellular context (see section on SLP3).
Piezo proteins
Piezo protiens have been recently identified like as promising candidates for mechanosensing proteins by Coste and collaborators.86,87 Vertebrates have two Piezo members, Piezo 1 and Piezo 2, previously known as FAM38A and FAM38B, respectively, which are well conserved throughout multi cellular eukaryotes. Piezo 2 is abundant in DRGs, whereas Piezo 1 is barely detectable. Piezo-induced mechanosensitive currents are prevented inhibited by gadolinium, ruthenium red and GsMTx4 (a toxin from the tarantula Grammostola spatulata).88 Expression of Piezo 1 or Piezo 2 in heterologous systems produces mechanosensitive currents, the kinetics of inactivation of Piezo 2 current being faster than Piezo 1. Similar to endogenous mechanosensitive currents, Piezo-dependent currents have reversal potentials around 0 mV and are cation no selective, with Na+, K+, Ca2+ and Mg2+ all permeating the underlying channel. Likewise, piezo-dependent currents are regulated by membrane potential, with a marked slowing of current kinetics at depolarized potentials.86
Piezo proteins are undoubtedly mechanosensing proteins and share many properties of rapidly adapting mechanosensitive currents in sensory neurones. Treatment of cultured DRG neurones with Piezo 2 short interfering RNA decreased the proportion of neurones with rapidly adapting current and decreased the percentage of mechanosensitive neurones.86 Transmembrane domains are located throughout the piezo proteins but no obvious pore-containing motifs or ion channel signatures have been identified. However, mouse Piezo 1 protein purified and reconstituted into asymmetric lipid bilayers and liposome forms ion channels sensitive to ruthenium red.87 An essential step in validating mechanotransduction through Piezo channels is to use in vivo approaches to determine the functional importance in touch signaling. Information was given in Drosophila where deletion of the single Piezo member reduced mechanical response to noxious stimuli, without affecting normal touch.89 Although their structure remains to be determined, this novel family of mechanosensitive proteins is a promising subject for future research, beyond the border of touch sensation. For exemple, a recent study on patients with anemia (hereditary xerocytosis) shows the role of Piezo 1 in maintaining erythrocyte volume homeostasis.90
Transmembrane channel-like (TMC)
A recent study indicates that two proteins, TMC1 and TMC2, are necessary for hair cell mechanotransduction.91 Hereditary deafness due to TMC1 gene mutation was reported in human and mice.92,93 Presence of these channels had not yet been shown in the somatosensory system, but it seems to be a good lead to investigate.
Stomatin-like protein 3 (SLP3)
Additionally to the transduction channels, some accessory proteins linked to the channel have been shown to play a role in touch sensivity. SLP3 is expressed in mammalian DRG neurones. Studies using mutant mice lacking SLP3 had shown change in mechanosensation and mechanosentive currents.94,95 SLP3 precise function remains unknown. It may be a linker between the mechanosensitive channel and the underlying microtubules, as proposed for its C. elegans homolog MEC2.96 Recently GR. Lewin lab has suggested that a tether is synthesized by DRG sensory neurones and links mechanosensitive ion channel to the extracellular matrix.97 Disrupting the link abolishes the RA-mechanosensitive current suggesting that some ion channels are mechanosensitive only when tethered. RA-mechanosensitive currents are also inhibited by laminin-332, a matrix protein produced by keratinocytes, reinforcing the hypothesis of a modulation of the mechanosensitive current by extracellular proteins.98
K+ channel subfamily
In parallel to cationic depolarizing mechanosensitive currents, the presence of repolarizing mechanosensitive K+ currents is under investigation. K+ channels in mechanosensitive cells can step in the current balance and contribute to define the mechanical threshold and the time course of adaptation of mechanoreceptors.
KCNK members belong to the two-pore domain K+ channel (K2P) family.99,100 The K2P display a remarkable range of regulation by cellular, physical and pharmacological agents, including pH changes, heat, stretch and membrane deformation. These K2P are active at resting membrane potential. Several KCNK subunits are expressed in somatosensory neurones.101 KCNK2 (TREK-1), KCNK4 (TRAAK) and TREK-2 channels are among the few channels for which a direct mechanical gating by membrane stretch has been shown.102,103
Mice with a disrupted KCNK2 gene displayed an enhanced sensitivity to heat and mild mechanical stimuli but a normal withdrawal threshold to noxious mechanical pressure applied to the hindpaw using the Randall–Selitto test.104 KCNK2-deficient mice also displays increased thermal and mechanical hyperalgesia in inflammatory conditions. KCNK4 knockout mice were hypersensitive to mild mechanical stimulation, and this hypersensitivity was increased by additional inactivation of KCNK2.105 Increased mechanosensitivity of these knockout mice could mean that stretch normally activates both depolarizing and repolarizing mechanosensitive currents in a coordinated way, similarly to the unbalance of depolarizing and repolarizing voltage-gated currents.
KCNK18 (TRESK) is a major contributor to the background K+ conductance that regulates the resting membrane potential of somatosensory neurones.106 Although it is not known if KCNK18 is directly sensitive to mechanical stimulation, it may play a role in mediating responses to light touch, as well as painful mechanical stimuli. KCNK18 and to a lesser extent KCNK3, are proposed to be the molecular target of hydroxy-α-sanshool, a compound found in Schezuan peppercorns that activates touch receptors and induces a tingling sensation in humans.107,108
The voltage dependent K+ channel KCNQ4 (Kv7.4) is crucial for setting the velocity and frequency preference of a subpopulation of rapidly adapting mechanoreceptors in both mice and humans. Mutation of KCNQ4 has been initially associated with a form of hereditary deafness. Interestingly a recent study localizes KCNQ4 in the peripheral nerve endings of cutaneous rapidly adapting hair follicle and Meissner corpuscle. Accordingly, loss of KCNQ4 function leads to a selective enhancement of mechanoreceptor sensitivity to low-frequency vibration. Notably, people with late-onset hearing loss due to dominant mutations of the KCNQ4 gene show enhanced performance in detecting small-amplitude, low-frequency vibration.109
Conclusion
Touch is a complex sense because it represents different tactile qualities, namely, vibration, shape, texture, pleasure and pain, with different discriminative performances. Up to now, the correspondence between a touch-organ and the psychophysical sense was correlative and class-specific molecular markers are just emerging. The development of rodent tests matching the diversity of touch behavior is now required to facilitate future genomics identification. The use of mice that lack specific subsets of sensory afferent types will greatly facilitate identification of mechanoreceptors and sensory afferent fibers associated with a particular touch modality. Interestingly, a recent paper opens the important question of the genetic basis of mechanosensory traits in human and suggests that single gene mutation could negatively influence touch sensitivity.110 This underlines that the pathophysiology of the human touch deficit is in a large part unknown and would certainly progress by identifying precisely the subset of sensory neurones linked to a touch modality or a touch deficit.
In return, progress has been made to define the biophysical properties of the mechano-gated currents.64 The development of new techniques in recent years, allowing monitoring of membrane tension changes, while recording mechano-gated current, has proved valuable experimental method to describe mechanosensitive currents with rapid, intermediate and slow adaptation (reviewed in Delmas and collaborators).66,111 The future will be to determine the role of the current properties in the mechanisms of adaptation of functionally diverse mechanoreceptors and the contribution of mechanosensitive K+ currents to the excitability of LTMRs and HTMRs.
The molecular nature of mechano-gated currents in mammals is also a future promising research topic. Future research will progress in two perspectives, first to determine the role of accessory molecule that tether channels to the cytoskeleton and would be required to confer or regulate mechanosensitivity of ion channels of the like of TRP and ASIC/EnaC families. Second, to investigate the large and promising area of the contribution of the Piezo channels by answering key questions, relative to the permeation and gating mechanisms, the subset of sensory neurones and touch modalities involving Piezo and the role of Piezo in non neuronal cells associated with mechanosensation.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22213
References
- 1.Moriwaki K, Yuge O. Topographical features of cutaneous tactile hypoesthetic and hyperesthetic abnormalities in chronic pain. Pain. 1999;81:1–6. doi: 10.1016/S0304-3959(98)00257-7. [DOI] [PubMed] [Google Scholar]
- 2.Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005;132:193–201. doi: 10.1016/j.neuroscience.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 3.Kleggetveit IP, Jørum E. Large and small fiber dysfunction in peripheral nerve injuries with or without spontaneous pain. J Pain. 2010;11:1305–10. doi: 10.1016/j.jpain.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Noback CR. Morphology and phylogeny of hair. Ann N Y Acad Sci. 1951;53:476–92. doi: 10.1111/j.1749-6632.1951.tb31950.x. [DOI] [PubMed] [Google Scholar]
- 5.Straile WE. Atypical guard-hair follicles in the skin of the rabbit. Nature. 1958;181:1604–5. doi: 10.1038/1811604a0. [DOI] [PubMed] [Google Scholar]
- 6.Straile WE. The morphology of tylotrich follicles in the skin of the rabbit. Am J Anat. 1961;109:1–13. doi: 10.1002/aja.1001090102. [DOI] [PubMed] [Google Scholar]
- 7.Millard CL, Woolf CJ. Sensory innervation of the hairs of the rat hindlimb: a light microscopic analysis. J Comp Neurol. 1988;277:183–94. doi: 10.1002/cne.902770203. [DOI] [PubMed] [Google Scholar]
- 8.Hamann W. Mammalian cutaneous mechanoreceptors. Prog Biophys Mol Biol. 1995;64:81–104. doi: 10.1016/0079-6107(95)00011-9. [Review] [DOI] [PubMed] [Google Scholar]
- 9.Brown AG, Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967;193:707–33. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess PR, Petit D, Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968;31:833–48. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- 11.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–23. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussein MA. The overall pattern of hair follicle arrangement in the rat and mouse. J Anat. 1971;109:307–16. [PMC free article] [PubMed] [Google Scholar]
- 13.Vielkind U, Hardy MH. Changing patterns of cell adhesion molecules during mouse pelage hair follicle development. 2. Follicle morphogenesis in the hair mutants, Tabby and downy. Acta Anat (Basel) 1996;157:183–94. doi: 10.1159/000147880. [DOI] [PubMed] [Google Scholar]
- 14.Hardy MH, Vielkind U. Changing patterns of cell adhesion molecules during mouse pelage hair follicle development. 1. Follicle morphogenesis in wild-type mice. Acta Anat (Basel) 1996;157:169–82. doi: 10.1159/000147879. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–27. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown AG, Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967;193:707–33. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess PR, Petit D, Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968;31:833–48. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- 18.Vallbo A, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993;628:301–4. doi: 10.1016/0006-8993(93)90968-S. [DOI] [PubMed] [Google Scholar]
- 19.Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81:2753–63. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- 20.Hertenstein MJ, Keltner D, App B, Bulleit BA, Jaskolka AR. Touch communicates distinct emotions. Emotion. 2006;6:528–33. doi: 10.1037/1528-3542.6.3.528. [DOI] [PubMed] [Google Scholar]
- 21.McGlone F, Vallbo AB, Olausson H, Loken L, Wessberg J. Discriminative touch and emotional touch. Can J Exp Psychol. 2007;61:173–83. doi: 10.1037/cjep2007019. [DOI] [PubMed] [Google Scholar]
- 22.Wessberg J, Olausson H, Fernström KW, Vallbo AB. Receptive field properties of unmyelinated tactile afferents in the human skin. J Neurophysiol. 2003;89:1567–75. doi: 10.1152/jn.00256.2002. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10:946–8. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- 24.Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–4. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- 25.Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev. 2010;34:185–91. doi: 10.1016/j.neubiorev.2008.09.011. [Review] [DOI] [PubMed] [Google Scholar]
- 26.Krämer HH, Lundblad L, Birklein F, Linde M, Karlsson T, Elam M, et al. Activation of the cortical pain network by soft tactile stimulation after injection of sumatriptan. Pain. 2007;133:72–8. doi: 10.1016/j.pain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Applebaum AE, Beall JE, Foreman RD, Willis WD. Organization and receptive fields of primate spinothalamic tract neurons. J Neurophysiol. 1975;38:572–86. doi: 10.1152/jn.1975.38.3.572. [DOI] [PubMed] [Google Scholar]
- 28.White JC, Sweet WH. Effectiveness of chordotomy in phantom pain after amputation. AMA Arch Neurol Psychiatry. 1952;67:315–22. doi: 10.1001/archneurpsyc.1952.02320150048004. [DOI] [PubMed] [Google Scholar]
- 29.Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:225–39. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- 30.Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol. 2009;336:76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebara S, Kumamoto K, Baumann KI, Halata Z. Three-dimensional analyses of touch domes in the hairy skin of the cat paw reveal morphological substrates for complex sensory processing. Neurosci Res. 2008;61:159–71. doi: 10.1016/j.neures.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Guinard D, Usson Y, Guillermet C, Saxod R. Merkel complexes of human digital skin: three-dimensional imaging with confocal laser microscopy and double immunofluorescence. J Comp Neurol. 1998;398:98–104. doi: 10.1002/(SICI)1096-9861(19980817)398:1<98::AID-CNE6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Reinisch CM, Tschachler E. The touch dome in human skin is supplied by different types of nerve fibers. Ann Neurol. 2005;58:88–95. doi: 10.1002/ana.20527. [DOI] [PubMed] [Google Scholar]
- 35.Maricich SM, Morrison KM, Mathes EL, Brewer BM. Rodents rely on Merkel cells for texture discrimination tasks. J Neurosci. 2012;32:3296–300. doi: 10.1523/JNEUROSCI.5307-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeda I, Yamashita Y, Ono T, Ogawa H. Selective phototoxic destruction of rat Merkel cells abolishes responses of slowly adapting type I mechanoreceptor units. J Physiol. 1994;479:247–56. doi: 10.1113/jphysiol.1994.sp020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, et al. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–2. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diamond J, Holmes M, Nurse CA. Are Merkel cell-neurite reciprocal synapses involved in the initiation of tactile responses in salamander skin? J Physiol. 1986;376:101–20. doi: 10.1113/jphysiol.1986.sp016144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita Y, Akaike N, Wakamori M, Ikeda I, Ogawa H. Voltage-dependent currents in isolated single Merkel cells of rats. J Physiol. 1992;450:143–62. doi: 10.1113/jphysiol.1992.sp019120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wellnitz SA, Lesniak DR, Gerling GJ, Lumpkin EA. The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. J Neurophysiol. 2010;103:3378–88. doi: 10.1152/jn.00810.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nandasena BG, Suzuki A, Aita M, Kawano Y, Nozawa-Inoue K, Maeda T. Immunolocalization of aquaporin-1 in the mechanoreceptive Ruffini endings in the periodontal ligament. Brain Res. 2007;1157:32–40. doi: 10.1016/j.brainres.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Rahman F, Harada F, Saito I, Suzuki A, Kawano Y, Izumi K, et al. Detection of acid-sensing ion channel 3 (ASIC3) in periodontal Ruffini endings of mouse incisors. Neurosci Lett. 2011;488:173–7. doi: 10.1016/j.neulet.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11:455–61. doi: 10.1016/S0959-4388(00)00234-8. [Review] [DOI] [PubMed] [Google Scholar]
- 44.Wende H, Lechner SG, Cheret C, Bourane S, Kolanczyk ME, Pattyn A, et al. The transcription factor c-Maf controls touch receptor development and function. Science. 2012;335:1373–6. doi: 10.1126/science.1214314. [DOI] [PubMed] [Google Scholar]
- 45.Mendelson M, Lowenstein WR. Mechanisms of receptor adaptation. Science. 1964;144:554–5. doi: 10.1126/science.144.3618.554. [DOI] [PubMed] [Google Scholar]
- 46.Loewenstein WR, Mendelson M. Components of receptor adaptation in a pacinian corpuscle. J Physiol. 1965;177:377–97. doi: 10.1113/jphysiol.1965.sp007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawson L, Prestia LT, Mahoney GK, Güçlü B, Cox PJ, Pack AK. GABAergic/glutamatergic-glial/neuronal interaction contributes to rapid adaptation in pacinian corpuscles. J Neurosci. 2009;29:2695–705. doi: 10.1523/JNEUROSCI.5974-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basbaum AI, Jessell TM. The perception of pain. In: Kandel ER, Schwartz JH, Jessell TM, eds. Principles of neural science. Fourth edition. The McGraw-Hill compagies, 2000: 472-490. [Google Scholar]
- 49.Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, et al. Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron. 2009;64:857–70. doi: 10.1016/j.neuron.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–93. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–56. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallbo AB, Hagbarth KE. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968;21:270–89. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- 53.Macefield VG. Physiological characteristics of low-threshold mechanoreceptors in joints, muscle and skin in human subjects. Clin Exp Pharmacol Physiol. 2005;32:135–44. doi: 10.1111/j.1440-1681.2005.04143.x. [Review] [DOI] [PubMed] [Google Scholar]
- 54.Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M, Inoue K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380:329–38. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azorin N, Raoux M, Rodat-Despoix L, Merrot T, Delmas P, Crest M. ATP signalling is crucial for the response of human keratinocytes to mechanical stimulation by hypo-osmotic shock. Exp Dermatol. 2011;20:401–7. doi: 10.1111/j.1600-0625.2010.01219.x. [DOI] [PubMed] [Google Scholar]
- 56.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [Review] [DOI] [PubMed] [Google Scholar]
- 57.Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol. 2001;532:759–69. doi: 10.1111/j.1469-7793.2001.0759e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perl ER. Cutaneous polymodal receptors: characteristics and plasticity. Prog Brain Res. 1996;113:21–37. doi: 10.1016/S0079-6123(08)61079-1. [Review] [DOI] [PubMed] [Google Scholar]
- 59.McCarter GC, Reichling DB, Levine JD. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci Lett. 1999;273:179–82. doi: 10.1016/S0304-3940(99)00665-5. [DOI] [PubMed] [Google Scholar]
- 60.Drew LJ, Wood JN, Cesare P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J Neurosci. 2002;22:RC228. doi: 10.1523/JNEUROSCI.22-12-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, et al. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarter GC, Levine JD. Ionic basis of a mechanotransduction current in adult rat dorsal root ganglion neurons. Mol Pain. 2006;2:28. doi: 10.1186/1744-8069-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coste B, Crest M, Delmas P. Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J Gen Physiol. 2007;129:57–77. doi: 10.1085/jgp.200609665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hao J, Delmas P. Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. J Neurosci. 2010;30:13384–95. doi: 10.1523/JNEUROSCI.2926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drew LJ, Wood JN. FM1-43 is a permeant blocker of mechanosensitive ion channels in sensory neurons and inhibits behavioural responses to mechanical stimuli. Mol Pain. 2007;3:1. doi: 10.1186/1744-8069-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hao J, Delmas P. Recording of mechanosensitive currents using piezoelectrically driven mechanostimulator. Nat Protoc. 2011;6:979–90. doi: 10.1038/nprot.2011.343. [DOI] [PubMed] [Google Scholar]
- 67.Rugiero F, Drew LJ, Wood JN. Kinetic properties of mechanically activated currents in spinal sensory neurons. J Physiol. 2010;588:301–14. doi: 10.1113/jphysiol.2009.182360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol. 2006;577:815–28. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhattacharya MR, Bautista DM, Wu K, Haeberle H, Lumpkin EA, Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc Natl Acad Sci U S A. 2008;105:20015–20. doi: 10.1073/pnas.0810801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crawford AC, Evans MG, Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J Physiol. 1989;419:405–34. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci. 1998;18:8261–77. doi: 10.1523/JNEUROSCI.18-20-08261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci. 2007;30:339–65. doi: 10.1146/annurev.neuro.29.051605.112917. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodman MB, Schwarz EM. Transducing touch in Caenorhabditis elegans. Annu Rev Physiol. 2003;65:429–52. doi: 10.1146/annurev.physiol.65.092101.142659. [Review] [DOI] [PubMed] [Google Scholar]
- 74.Waldmann R, Lazdunski MH. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–24. doi: 10.1016/S0959-4388(98)80070-6. [Review] [DOI] [PubMed] [Google Scholar]
- 75.Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–47. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 76.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–83. doi: 10.1016/S0896-6273(01)00547-5. [Erratum in: Neuron 2002 Jul 18;35] [2] [DOI] [PubMed] [Google Scholar]
- 77.Roza C, Puel JL, Kress M, Baron A, Diochot S, Lazdunski M, et al. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol. 2004;558:659–69. doi: 10.1113/jphysiol.2004.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol. 2008;18:R880–9. doi: 10.1016/j.cub.2008.07.063. [Review] [DOI] [PubMed] [Google Scholar]
- 79.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–21. doi: 10.1038/nrn2149. [Review] [DOI] [PubMed] [Google Scholar]
- 80.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14531–6. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–8. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 82.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–35. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–52. doi: 10.1523/JNEUROSCI.0242-04.2004. [Erratum in: J Neurosci. 2004 Jun;24] [23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–82. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 85.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–89. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 86.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–81. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295–300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–12. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120:1908–15. doi: 10.1182/blood-2012-04-422253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawashima Y, Géléoc GS, Kurima K, Labay V, Lelli A, Asai Y, et al. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest. 2011;121:4796–809. doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tlili A, Rebeh IB, Aifa-Hmani M, Dhouib H, Moalla J, Tlili-Chouchène J, et al. TMC1 but not TMC2 is responsible for autosomal recessive nonsyndromic hearing impairment in Tunisian families. Audiol Neurootol. 2008;13:213–8. doi: 10.1159/000115430. [DOI] [PubMed] [Google Scholar]
- 93.Manji SS, Miller KA, Williams LH, Dahl HH. Identification of three novel hearing loss mouse strains with mutations in the Tmc1 gene. Am J Pathol. 2012;180:1560–9. doi: 10.1016/j.ajpath.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 94.Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–9. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- 95.Martinez-Salgado C, Benckendorff AG, Chiang LY, Wang R, Milenkovic N, Wetzel C, et al. Stomatin and sensory neuron mechanotransduction. J Neurophysiol. 2007;98:3802–8. doi: 10.1152/jn.00860.2007. [DOI] [PubMed] [Google Scholar]
- 96.Huang M, Gu G, Ferguson EL, Chalfie M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature. 1995;378:292–5. doi: 10.1038/378292a0. [DOI] [PubMed] [Google Scholar]
- 97.Hu J, Chiang LY, Koch M, Lewin GR. Evidence for a protein tether involved in somatic touch. EMBO J. 2010;29:855–67. doi: 10.1038/emboj.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiang LY, Poole K, Oliveira BE, Duarte N, Sierra YA, Bruckner-Tuderman L, et al. Laminin-332 coordinates mechanotransduction and growth cone bifurcation in sensory neurons. Nat Neurosci. 2011;14:993–1000. doi: 10.1038/nn.2873. [DOI] [PubMed] [Google Scholar]
- 99.Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, et al. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996;15:1004–11. [PMC free article] [PubMed] [Google Scholar]
- 100.Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/S0028-3908(02)00339-8. [Review] [DOI] [PubMed] [Google Scholar]
- 101.Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, et al. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res. 2001;86:101–14. doi: 10.1016/S0169-328X(00)00263-1. [DOI] [PubMed] [Google Scholar]
- 102.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–6. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 103.Maingret F, Fosset M, Lesage F, Lazdunski M, Honoré E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274:1381–7. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- 104.Alloui A, Zimmermann K, Mamet J, Duprat F, Noël J, Chemin J, et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–76. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–18. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dobler T, Springauf A, Tovornik S, Weber M, Schmitt A, Sedlmeier R, et al. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J Physiol. 2007;585:867–79. doi: 10.1113/jphysiol.2007.145649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, et al. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci. 2008;11:772–9. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lennertz RC, Tsunozaki M, Bautista DM, Stucky CL. Physiological basis of tingling paresthesia evoked by hydroxy-alpha-sanshool. J Neurosci. 2010;30:4353–61. doi: 10.1523/JNEUROSCI.4666-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heidenreich M, Lechner SG, Vardanyan V, Wetzel C, Cremers CW, De Leenheer EM, et al. KCNQ4 K(+) channels tune mechanoreceptors for normal touch sensation in mouse and man. Nat Neurosci. 2012;15:138–45. doi: 10.1038/nn.2985. [DOI] [PubMed] [Google Scholar]
- 110.Frenzel H, Bohlender J, Pinsker K, Wohlleben B, Tank J, Lechner SG, et al. A genetic basis for mechanosensory traits in humans. PLoS Biol. 2012;10:e1001318. doi: 10.1371/journal.pbio.1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011;12:139–53. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]