Abstract
Background
Pancreatic carcinoma remains a treatment-refractory cancer with a poor prognosis. Here, we compared anti-EGF receptor and anti-HER2 monoclonal antibodies (2mAbs) injections with standard gemcitabine treatment on human pancreatic carcinoma xenografts.
Materials and Methods
Nude mice, bearing human pancreatic carcinoma, xenografts, were treated with either combined anti-EGFR (cetuximab) and anti-HER2 (trastuzumab), or gemcitabine and tumor growth were observed.
Results and Conclusion
In first-line therapy, mice survival was significantly longer in 2mAbs groups compared to gemcitabine (p<0.0001: BxPC-3; p=0.0679: MiaPaCa-2; p=0.0019: Capan-1) and to controls (p<0.0001). In second-line therapy tumor regressions were observed after replacing gemcitabine by 2mAbs treatment, resulting in significantly longer animal survival, compared with mice receiving continuous gemcitabine injections (p=0.008, BxPC-3; p=0.05; MiaPaCa-2; p<0.001, Capan-1). Therapeutic benefit of 2mAbs was observed, despite K-Ras mutation. Interestingly, concerning the mechanism of action, coinjection of F(ab)′2 fragments from 2mAbs induced significant tumor growth inhibition, compared to controls (p=0.001), indicating that the 2mAbs had an, Fc-independent, direct action on tumor cells.
This pre-clinical study demonstrated a significant improvement of survival and tumour regression in mice treated with anti-EGFR/anti-HER2 2mAbs in first and second-line treatments, compared to gemcitabine, independently of the K-Ras status.
Keywords: Animals; Antibodies, Monoclonal; administration & dosage; Antibodies, Monoclonal, Humanized; Antineoplastic Combined Chemotherapy Protocols; therapeutic use; Blotting, Western; Cell Line, Tumor; Deoxycytidine; analogs & derivatives; therapeutic use; Female; Humans; Immunohistochemistry; Mice; Mice, Nude; Pancreatic Neoplasms; drug therapy; Receptor, Epidermal Growth Factor; antagonists & inhibitors; immunology; Receptor, erbB-2; antagonists & inhibitors; immunology; Xenograft Model Antitumor Assays
Keywords: EGFR, gemcitabine, HER2, monoclonal antibodies, pancreatic carcinoma
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer death in both men and women. Currently, most pancreatic-cancer patients die within a year of diagnosis. When the tumor becomes symptomatic, 60 to 80% of the patients already have locally advanced or metastatic disease allowing essentially palliative therapy, with a 5-year survival rate of less than 5%.1
Recent evaluation of gemcitabine based combination chemotherapy trials in advanced pancreatic cancer have demonstrated significant, but low response rates and disappointing effects on survival.2 The strategies of earlier attack on cancer by perioperative adjuvant or neoadjuvant therapy were expected to be more promising. However, the recently reported retrospective and prospective analyses of adjuvant chemo-radiotherapy of pancreas carcinoma,3 as well as the results of preoperative chemo-radiation therapies,4 have again demonstrated significant, but very modest therapeutic gains. Similarly, the results from a recent phase III trial, evaluating the advantage of the association of erlotinib with gemcitabine, showed a significant, but low improvement, as compared to gemcitabine alone treatment, with a median survival of 6.24 vs 5.91 months, as well as a one year survival of 23% vs 17%, respectively.5
In the field of mAb therapies and following the demonstration of the expression of both EGFR and HER2 in pancreatic carcinomas6,7 and the known implication of those receptors in the malignant phenotype,8–11 we have recently demonstrated that the coinjection of anti-EGFR and anti-HER2 mAbs had a significant synergic effect in the treatment of human pancreatic carcinoma xenografts, as compared to the effect of each mAb alone.12 Our results were recently confirmed and extended by the demonstration that different pairs of anti-HER2 mAbs had a synergistic anti-tumor effect.13
Here, we are evaluating, if the combined anti-EGFR/anti-HER2 mAbs (2 mAbs) targeted therapy can result in better efficacy than the current standard chemotherapy, gemcitabine, in a first and second line therapy.
METHODS
Monoclonal antibodies and drugs
Cetuximab was purchased from Merck KGaA (Darmstadt, Germany), Trastuzumab from Roche Pharma AG (Grenzach-Wyhlen, Germany) and Gemcitabine from Lilly France (Pages, France). F(ab)′2 fragments of cetuximab and trastuzumab were prepared by pepsine digestion followed by filtration on a Superdex 200 column. Absence of intact mAb contamination was checked by lack of any cytotoxicity induction by NK cells on antigen positive target cells.14
Cell lines
BxPC-3 and MiaPaCa-2 pancreatic cell lines were from ATCC (Rockville, MD, USA). The Capan-1 was kindly provided by Pr L. Buscail (Toulouse, France).
Xenograft study and treatment procedure
All in vivo experiments were performed in compliance with the national regulations and ethical guidelines for experimental animal studies in an accredited establishment (Agreement No. B34-172-27). Six week-old female athymic mice, (Harlan, Le Malcourlet, France), were xenografted subcutaneously (s.c.) with BxPC-3 (3.5 × 106), MiaPaCa-2 (5 × 106), and Capan-1 (10 × 106) cells. Tumor-bearing mice were randomized when tumors reached a miminum of 50 mm3 and sacrificed when tumor reached a volume larger than 1000 mm3.
In first line, mice were treated twice a week either by intraperitoneal injections (i.p.) of combined trastuzumab/cetuximab (ratio 1:1; 2 mg/kg of each mAb) or gemcitabine (150 mg/kg) diluted in 0.15 ml saline.
In second line, 20 mice were treated twice a week with gemcitabine alone (150 mg/kg). For 10 mice presenting a tumour progression (volume increase at least twofold from initial measurement) the gemcitabine treatment was replaced by the combined trastuzumab/cetuximab i.p. injections twice a week (ratio 1:1; 2 mg/kg). Others 10 mice were continuously treated by gemcitabine.
To determine the implication of the Fc portion of antibodies, BxPC-3 xenografted mice were treated twice a week for four weeks with F(ab′)2 fragments from both trastuzumab and cetuximab (ratio 1:1; 1.35 mg/kg of each fragment) or cetuximab F(ab′)2 alone, at the same dose, or intact trastuzumab and cetuximab (ratio 1:1; 2mg/kg). The concentration of fragments was adjusted to be at the same molarity (2μM) as the intact antibodies.
Tumour dimensions were measured with a caliper and the volumes calculated by the formula: D1 × D2 × D3/2. In figure 3, tumor growth is expressed as the log of tumor progression: log [final volume(t)/initial volume(t0)].
Figure 3. Effect of 2mAbs treatment, versus gemcitabine, on the EGFR-expression, EGFR-phosphorylation, proliferation index-Ki67 and AKT-phosphorylation in BxPC-3 xenografts.

Tumor were resected 7 days after the beginning of therapy. Immunohistochemistry shows in: (A), EGFR expression (magnification × 10). (B), EGFR phosphorylation (magnification × 40). (C), Ki-67-index (magnification × 40). On the right: respective histograms in % positive cells. Western blot analysis shows in (D) the AKT-phosphorylation. GAPDH served as leading control. C+T: 2mAbs.
K-Ras mutation analysis
High-molecular-weight DNA was extracted using a Qiamp DNA mini kit (Qiagen, Courtaboeuf, France). Direct sequencing of the K-Ras gene codon 12 and 13 was done with a 3130 Genetic Analyzer (Applied Biosystems, Courtaboeuf, France), using the Bigdye terminators v1.1 cycle sequencing kit (Applied Biosystems).
Immunohistochemical analyses
Seven days post treatment, tumors were harvested and fixed 12h in buffered formalin, and embedded in paraffin.
Ki67 (MiB1, Dako corporation), EGFR (3C6, Ventana Medical Systems ) and pEGFR (tyr 1068, 1H12 cell signalling technology) immunostaining were performed on 3 mm sections with a BenchmarkXT immunostainer (Ventana Medical Systems, llkirch, France). Sections were scored under light microscopy by two independent pathologists, who analyzed five different fields per section. Error bars correspond to results obtained in the different fields.
For EGFR the two plus or more intensity staining of tumor cells membrane was scored positively, while for Ki67 index and pEGFR the percentage of tumor cells with one plus nuclear and/or cytoplasmic staining was recorded positively. The results are expressed on histograms.
Western blot analysis
Seven days post treatment, tumors were harvested and lysed as described12.
After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membranes (Millipore Co., Bedford, MA) which were saturated in PBS containing 0.1% Tween 20 and 5% nonfat dry milk and then incubated with the antibodies against the phosphorylated forms of AKT (Cell Signaling Technology, Beverly, MA). To ensure equal loading, immunoblots were also probed with anti-GAPDH (glyceraldehydes-3-phosphate deshydrogenase) antibody (Chemicon international, Australia).
Statistical Analyses
A linear mixed regression model (LMRM) was used to determine the relationship between tumor growth and the number of days after implantation. The fixed part of the model included variables corresponding to the number of days after implantation and to different groups. Interaction terms were built into the model. Random intercept and random slope were included to take into account time effect. The coefficients of the model were estimated by maximum likelihood and considered significant at the 0.05 level.
Survival rates were estimated from the date of the xenograft until the date when the tumor reach a volume of 1000 mm3 using the Kaplan-Meier method. Median survival was presented with 95% confidence intervals. Survival curves were compared using the Log-rank test. Statistical analysis was performed using STATA 10.0 software.
RESULTS
Pancreatic cell lines characteristics
Immunohistochemical analyses of the three human pancreatic carcinoma xenografted in nude mice showed a very low HER2 expression, but a high level of EGFR, classified as +++ for both BxPC-3 and Capan-1 and as ++ for MiaPaCa-2 (table 1). When tested by flow cytometry,15 the three pancreatic carcinoma cell lines showed a moderate expression of HER2 (table 1).
Table 1.
EGFR and HER2 expression and Kras status of the three human pancreatic cell lines
| Cell lines | EGFR | HER2 | Sequencing | ||
|---|---|---|---|---|---|
|
| |||||
| IHC | FACScan (MFI) | IHC | FACScan (MFI) | Kras status | |
| BxPC-3 | 3+ | 156 | ND | 37.0 | WT |
| MiaPaCa-2 | 2+ | 31 | ND | 10.7 | M |
| Capan-1 | 3+ | 135 | ND | 10.4 | M |
ND=non detectable; WT=wild-type; M=mutation; MFI=mean fluoresence intensity
The presence of G12C mutation was confirmed in the MiaPaCa-2 and Capan-1 carcinoma cell line, while it was not observed in BxPC-3 line (table 1).16,17
First line combined antibody therapy vs gemcitabine
Gemcitabine dose was fixed at 150 mg/kg/injection and antibody doses were selected on the basis of our previous experiments.12
In the three human pancreatic carcinoma xenografts, the survival of mice treated with the 2mAbs was significantly greater compared to gemcitabine (p = 0.0006: BxPC-3; p = 0.0679: MiaPaCa-2; p = 0.0018: Capan-1) and control groups (p = 0.0001: BxPC-3; p = 0.0006: MiaPaCa-2; p = 0.0025: Capan-1) (Figure 1). Median delays for each of the 3 tumors to reach a volume of 1000 mm3 under 2mAbs, gemcitabine or no treatment are reported in table 2. Only the groups treated with the 2mAbs showed complete responses with 20 to 33% of tumor-free mice after two months of follow-up (table 2).
Figure 1. Kaplan-Meyer survival curves of first line combined 2mAbs versus gemcitabine therapy.
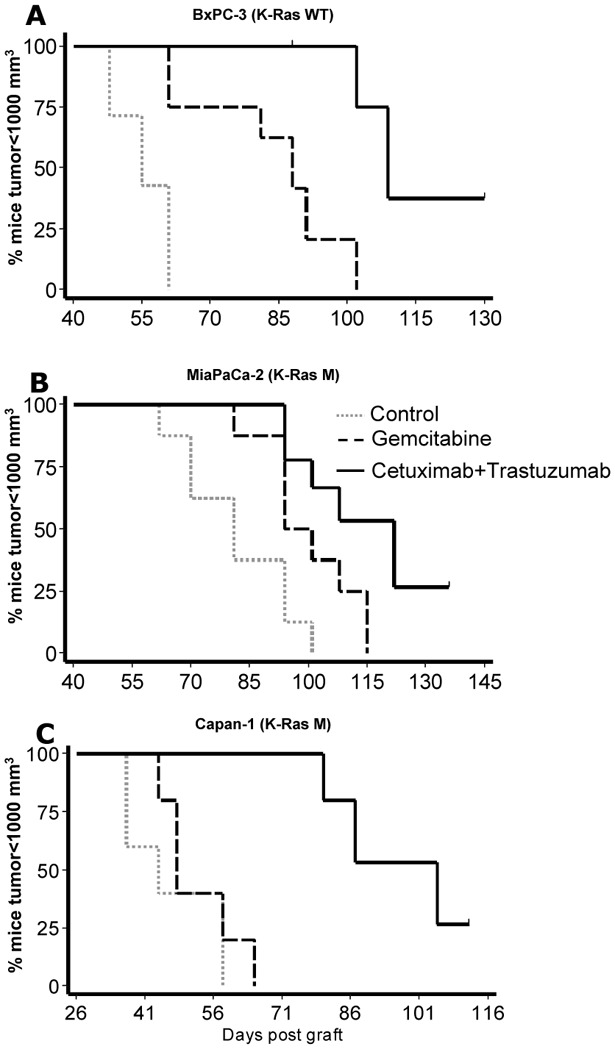
Percentage of mice with a tumor volume <1000 mm3 as a function of time post graft under either combined anti-EGFR and anti-HER2 mAbs or gemcitabine or no treatment controls. K-Ras WT, K-Ras wild-type; K-Ras M, K-Ras mutation.
Table 2.
Median survival and percent of cured mice after first line treatment
| Xenograft | Treatment | Mediana | Benefitb | Tumor-free mice % |
|---|---|---|---|---|
| BxPC-3 | Control | 55b | 0 | |
| Gemcitabine | 88 | +33 | 0 | |
| 2mAbs | 109 | +54 | 33 (3/9) | |
|
| ||||
| MiaPaCa-2 | Control | 81 | 0 | |
| Gemcitabine | 94 | +13 | 0 | |
| 2mAbs | 122 | +41 | 22 (2/9) | |
|
| ||||
| Capan-1 | Control | 44 | 0 | |
| Gemcitabine | 48 | +4 | 0 | |
| 2mAbs | 105 | +61 | 20 (2/10) | |
Median: days post-graft where 50% of the mice present a 1000 mm3 tumor volume
Benefit: gain in days of the treatment vs control group
Second line combined antibody therapy after progression under gemcitabine
The two mAbs were injected to mice with larger tumors, compared to first line therapy, since a minimum 2-fold tumor progression had to be recorded at the time of 2mAbs treatment initiation (Figure 2). In BxPC-3 and Capan-1 models significant tumour regressions were observed when the mice received 2mAbs, compared with continuous gemcitabine injection group (p<0.001), while in the MiaPaCa-2 model, 3 out of 10 mice showed a marked tumor regression and the others showed only a stabilisation of tumor growth (p=0.003). Despite moderate tumor regrowth, observed after tumor regressions or stabilisation, survival was significantly longer in mice from the 3 pancreatic carcinoma models, treated in second-line with 2mAbs compared to mice treated only with gemcitabine (p=0.008 for BxPC-3, p=0.052 for MiaPaCa-2, and p=0.0018 for Capan-1).
Figure 2. Tumor size evolution of second line combined 2mAbs therapy after gemcitabine progression.

Pancreatic carcinoma bearing mice were first treated with gemcitabine. At day 26, 37 or 40 for Capan-1, MiaPaCa-2 and BxPC-3, respectively, half of the mice in each tumor group were treated with the 2mAbs combination, while the other half received continuous gemcitabine treatment.
Immunohistochemistry (IHC) and Western Blot analysis
To assess the in vivo mechanisms underlying the anti-tumor activity of the two mAbs combination, we analyzed in BxPC-3 tumor xenografts, by IHC, the EGFR expression, the EGFR phosphorylation level, and the proliferative Ki67 index, after one week of 2mAbs or gemcitabine treatment. As shown in figure 3, the 2mAbs treatment induced a marked decrease of the EGFR expression, EGFR phosphorylation and Ki67 index compared to untreated controls and gemcitabine treatment. The AKT phosphorylation after the same two treatments was assessed by Western Blot analysis, which showed almost complete inhibition of P-AKT after 2mAbs treatment and only minor inhibition after gemcitabine compared to untreated tumor control.
Comparison of F(ab′)2 fragments from the 2 mAbs with intact antibodies
In order to separately analyze the in vivo direct effect of the mAbs binding sites on the EGF and HER2 receptors, as compared with the Fc-dependant effector functions of the antibodies, F(ab′)2 fragments from both cetuximab and trastuzumab were prepared and their therapeutic properties evaluated. As shown in figure 4, the F(ab′)2 fragments from the two mAbs had a significant anti-tumor effect compared with untreated controls (p<0.001), as well as with F(ab′)2 from anti-EGFR mAb alone, which had no anti-tumor effect (p=0.510). As expected, injection of the two intact mAbs had a more pronounced anti-tumor effect compared to the two fragments (p=0.002).
Figure 4. Anti-tumor effect of the combined treatment with two F(ab′)2 fragments mAbs, compared with two intact mAbs.
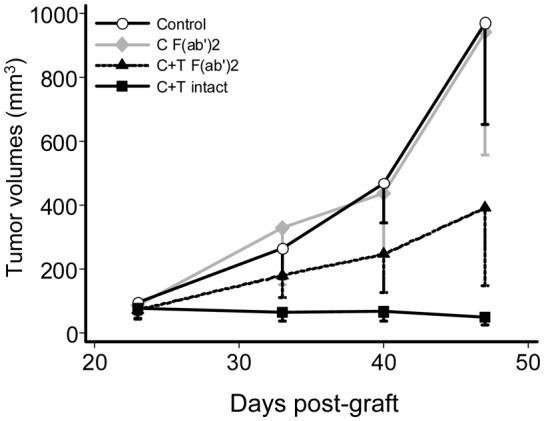
Mice bearing BxPC-3 pancreatic carcinoma were treated either by coinjection with F(ab′)2 fragments from both cetuximab and trastuzumab, or with the intact 2mAbs, or by injection with F(ab′)2 fragments from cetuximab alone, or were untreated controls.
DISCUSSION
This pre-clinical study demonstrates a significant improvement of survival and tumor regression in mice treated with combined anti-EGFR (cetuximab) and anti-HER2 (trastuzumab) mAbs in first and second line of treatment compared to gemcitabine, the gold standard in pancreatic cancers.2
Of particular interest was the positive impact of this treatment despite the low HER2 expression by all three target carcinomas and despite the presence of a K-ras mutation on two of them. It was recently shown that the efficacy of cetuximab in metastastic colorectal carcinoma was almost abolished in K-ras mutated tumors.18 In pancreatic cancer, where K-ras mutations are the norm (90%), trials evaluating cetuximab plus gemcitabine-cisplatin vs gemcitabine-cisplatine did not increase response or survival of patients.19 Here, a clear therapeutic benefit of cetuximab and trastuzumab combination was observed in two K-ras mutated pancreatic carcinomas, suggesting that the lack of clinical benefit of anti-EGFR mAb could be by-passed by combining this mAb with the anti-HER2 mAb.
All three tested pancreatic carcinoma lines responded better to 2mAbs whatever their respective sensitivity to gemcitabine. In case of high sensitivity to chemotherapy (BxPC-3), i.e. in the best condition of gemcitabine efficacy, 2mAbs significantly increased tumor responses and cured 3/9 mice, while no cure was obtained with gemcitabine.
The mechanism of action of the synergism of the two anti-EGFR and HER2 mAbs is not yet entirely understood, as is the case for several approved mAbs whose mode of action is not entirely unraveled. It was shown that an anti-EGFR mAb could increase the in vitro growth inhibition of an anti-HER2 mAb9 or that two anti-EGFR and HER2 mAbs had a synergistic effect in the down regulation of the receptor tyrosine-kinases activity 10. However, our in vivo results were the first to demonstrate unequivocally that the two anti-EGFR and HER2 mAbs can act synergistically in the treatment of human carcinoma xenografts.12 Thus, we concentrated our effort on some in vivo experiments that could shed some light on the mechanism of action of 2mAbs.
First, we showed that the 2mAbs induced a decrease of the proliferation index Ki-67 and a down regulation of the EGFR. These results were in agreement with those from other groups obtained after the injection of a single anti-EGF receptor or anti-HER2 mAbs9–11 or by treatment with two mAbs directed against different epitopes of either EGF receptor20 or HER210.
Second, our more original in vivo results were obtained by the combined injection of F(ab′)2 fragments from cetuximab and trastuzumab in order to determine if the tumor growth inhibition observed with the two intact mAbs, was due to a direct action of the two antibody binding sites on the two receptors, by inhibiting their dimerizations or triggering of a negative intracellular signal, as suggested by in vitro results8–11 or if it was due to an ADCC enhancement, as suggested by in vivo experiments21. Our results show for the first time in vivo that F(ab′)2 from two anti-EGFR and HER2 receptors mAbs significantly inhibited the growth of a pancreatic carcinoma xenograft, indicating that they can have a therapeutic efficiency independently from the Fc mediated effector functions and thus due to their direct binding activity to the HER1 and HER2 receptors. The therapeutic activity of the two intact mAbs was superior to that observed with the two F(ab′)2 fragments, suggesting that the recrutement of effector cells played also a role in the therapeutic efficiency of our 2mAbs treatment. Another explanation for the more efficient therapeutic activity of the two intact mAbs is that due to the property of their Fc to react with FcRn receptor,22 they have a much longer circulating half life than the F(ab′)2.23
Whatever the contribution of the two mentioned anti-tumor mechanisms, the present results show that the combined use of two anti-EGFR and anti-HER2 mAbs, broadly used in the clinic, can be efficient in the treatment of human pancreatic carcinoma xenografts with low expression of HER2 and in two of them with K-Ras mutations. Most impotantly, it demonstrates that the combined anti-EGFR and HER2 therapy is more efficient than the present standard chemotherapy, gemcitabine. In this context, a phase I-II clinical trial evaluating the combined cetuximab and tratuzumab in second line treatment of pancreatic carcinoma after gemcitabine first line progression appears justified and promising.
Acknowledgments
We thank G. Heintz, S. Bousquié, V. Garambois, R. Lavaill and A. Cayre for excellent technical assistance, M. Brissac and I. Aït-Arsa for help in performing the animal experiments.
References
- 1.Nelson NJ. Pancreatic cancer research matures. J Natl Cancer Inst. 2007;99:1432–1434. doi: 10.1093/jnci/djm180. [DOI] [PubMed] [Google Scholar]
- 2.Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 5.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 6.Tobita K, Kijima H, Dowaki S, et al. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int J Mol Med. 2003;11:305–309. [PubMed] [Google Scholar]
- 7.Dugan MC, Dergham ST, Kucway R, et al. HER-2/neu expression in pancreatic adenocarcinoma: relation to tumor differentiation and survival. Pancreas. 1997;14:229–236. doi: 10.1097/00006676-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ye D, Mendelsohn J, Fan Z. Augmentation of an anti-HER2 mAb 4D5 induced growth inhibition by a human -mouse chiméric anti-EGF receptor mAb C225. Oncogene. 1999;18:731–738. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]
- 9.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 10.Friedman LM, Rinon A, Schechter B, et al. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci U S A. 2005;102:1915–1920. doi: 10.1073/pnas.0409610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 12.Larbouret C, Robert B, Navarro-Teulon I, et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13:3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Kasus T, Schechter B, Lavi S. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: relevance of receptor endocytosis. Proc Natl Acad Sci USA. 2009;106(9):3294–3399. doi: 10.1073/pnas.0812059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germain C, Larbouret C, Cesson V, et al. MHC class I-related chain A conjugated to antitumor antibodies can sensitize tumor cells to specific lysis by natural killer cells. Clin Cancer Res. 2005;11:7516–7522. doi: 10.1158/1078-0432.CCR-05-0872. [DOI] [PubMed] [Google Scholar]
- 15.Mimura K, Kono K, Hanawa M, et al. Trastuzumab-mediated antibody-dependent cellular cytotoxicity against esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:4898–4904. doi: 10.1158/1078-0432.CCR-04-2476. [DOI] [PubMed] [Google Scholar]
- 16.Cogoi S, Codognotto A, Rapozzi V, et al. Transcription inhibition of oncogenic KRAS by a mutation-selective peptide nucleic acid conjugated to the PKKKRKV nuclear localization signal peptide. Biochemistry. 2005;44:10510–10519. doi: 10.1021/bi0505215. [DOI] [PubMed] [Google Scholar]
- 17.Butz J, Wickstrom E, Edwards J. Characterization of mutations and loss of heterozygosity of p53 and K-ras2 in pancreatic cancer cell lines by immobilized polymerase chain reaction. BMC Biotechnol. 2003;3:11. doi: 10.1186/1472-6750-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lievre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 19.Cascinu S, Berardi R, Labianca R, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. Lancet Oncol. 2008;9:39–44. doi: 10.1016/S1470-2045(07)70383-2. [DOI] [PubMed] [Google Scholar]
- 20.Perera RM, Narita Y, Furnari FB, et al. Treatment of human tumor xenografts with monoclonal antibody 806 in combination with a prototypical epidermal growth factor receptor-specific antibody generates enhanced antitumor activity. Clin Cancer Res. 2005;11:6390–6399. doi: 10.1158/1078-0432.CCR-04-2653. [DOI] [PubMed] [Google Scholar]
- 21.Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 22.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 23.Delaloye B, Bischof-Delaloye A, Buchegger F, et al. Detection of colorectal carcinoma by emission-computerized tomography after injection of 123I-labeled Fab or F(ab′)2 fragments from monoclonal anti-carcinoembryonic antigen antibodies. J Clin Invest. 1996;77:301–311. doi: 10.1172/JCI112291. [DOI] [PMC free article] [PubMed] [Google Scholar]


