Abstract
A cDNA encoding a novel, inwardly rectifying K+ (K+in) channel protein, SKT1, was cloned from potato (Solanum tuberosum L.). SKT1 is related to members of the AKT family of K+in channels previously identified in Arabidopsis thaliana and potato. Skt1 mRNA is most strongly expressed in leaf epidermal fragments and in roots. In electrophysiological, whole-cell, patch-clamp measurements performed on baculovirus-infected insect (Spodoptera frugiperda) cells, SKT1 was identified as a K+in channel that activates with slow kinetics by hyperpolarizing voltage pulses to more negative potentials than −60 mV. The pharmacological inhibitor Cs+, when applied externally, inhibited SKT1-mediated K+in currents half-maximally with an inhibitor concentration (IC50) of 105 μm. An almost identical high Cs+ sensitivity (IC50 = 90 μm) was found for the potato guard-cell K+in channel KST1 after expression in insect cells. SKT1 currents were reversibly activated by a shift in external pH from 6.6 to 5.5, which indicates a physiological role for pH-dependent regulation of AKT-type K+in channels. Comparative studies revealed generally higher current amplitudes for KST1-expressing cells than for SKT1-expressing insect cells, which correlated with a higher targeting efficiency of the KST1 protein to the insect cell's plasma membrane, as demonstrated by fusions to green fluorescence protein.
K+ channels and transport systems play multiple roles in higher-plant processes, including opening and closing of stomatal pores, leaf movements, and ion uptake in roots (Hedrich and Becker, 1994; Schroeder et al., 1994; Coté, 1995). Different approaches led to the molecular cloning of plant K+in channel cDNAs from Arabidopsis thaliana (kat1: Anderson et al., 1992; kat2: Butt et al., 1997; akt1: Sentenac et al., 1992; akt2 and akt3: Cao et al., 1995; Ketchum and Slayman, 1996), potato (Solanum tuberosum L.) (kst1: Müller-Röber et al., 1995; skt2 and skt3: Ehrhardt et al., 1997), and other plant species (Hoth et al., 1997). Considering structural domains, these plant K+in channels can be divided into the KAT and the AKT subfamilies (Schroeder et al., 1994). Recently, the first plant member of a novel class of “two-pore” K+ channels has been identified in Arabidopsis thaliana (Czempinski et al., 1997).
Functional characterization of newly cloned channel proteins requires their expression in heterologous expression systems. Xenopus oocytes are widely used to characterize channel proteins and other transporters from animals (Sigel, 1990) and, more recently, also from plants. In addition to metabolite transport proteins (e.g. Boorer et al., 1994) and water channels (e.g. Maurel et al., 1993; Yamada et al., 1995), functional expression of Arabidopsis KAT1 (Schachtman et al., 1992; Véry et al., 1994, 1995; Hedrich et al., 1995; Hoshi, 1995) and potato KST1 (Müller-Röber et al., 1995; Hoth et al., 1997) in oocytes has been reported. However, in some cases expression of plant channel proteins in oocytes can be difficult (e.g. AKT1) or may be dependent on the injected cRNA. Although AKT2 and AKT3 are obviously encoded by the same gene, only Ketchum and Slayman (1996) could demonstrate K+in currents in Xenopus oocytes after injection of the akt3 cRNA. On the other hand, Cao et al. (1995) were not able to show any activity of AKT2 in either Xenopus oocytes, Saccharomyces cerevisiae, or baculovirus-infected insect (Spodoptera frugiperda) cells. Although the reason for this discrepancy is not known at present, slight differences in the 5′ end of the constructs used for the experiments could be responsible. Furthermore, the expression strength and biophysical properties of KAT1 were dependent on polyadenylation of the injected cRNA (Cao et al., 1995). Arabidopsis AKT1 was never reported to be functionally expressed in Xenopus oocytes, although it was active in S. cerevisiae cells (Bertl et al., 1997).
Baculovirus-infected insect cells represent an alternative system for the expression and characterization of channel proteins. Insect cells have been used for the expression of a wide variety of proteins (King and Possee, 1992), including several proteins from higher plants (see Bustos et al., 1988; Vernet et al., 1990; Nagai et al., 1992; Macdonald et al., 1994). More recently, K+in channels from Arabidopsis (AKT1: Gaymard et al., 1996; KAT1: Marten et al., 1996) and potato (KST1: Ehrhardt et al., 1997) were functionally expressed and electrophysiologically studied using this heterologous expression system.
We report here the molecular cloning, functional expression, and electrophysiological characterization of SKT1, another K+in channel from potato. Initial experiments in our laboratory indicated that SKT1 was not functionally expressed in oocytes. The use of baculovirus-infected insect cells enabled us to analyze and compare the potato K+in channels SKT1 and KST1 in the same expression system, such as pH dependence and inhibition of K+ currents by Cs+. Furthermore, the targeting of the potato channels within the insect cells was studied by protein fusion to GFP, allowing us to visualize differences of K+in channel distribution.
MATERIALS AND METHODS
Enzymes and Chemicals
Enzymes for restriction and modification of DNA were obtained from New England Biolabs and Boehringer Mannheim. Reagents for SDS-PAGE were purchased from Bio-Rad, Roth (Karlsruhe, Germany), and Sigma. Other chemicals were from Boehringer Mannheim, Sigma, or Merck (Darmstadt, Germany).
Bacteria and Plants
Escherichia coli was cultivated at 37°C in standard yeast tryptone medium supplemented with appropriate antibiotics using standard methods (Sambrook et al., 1989). E. coli strain XL1-Blue (Stratagene) was used for DNA cloning procedures. E. coli strain DH10αBac (GIBCO-BRL) was used for in vivo transposition to obtain recombinant virus DNA for transfection of insect (Spodoptera frugiperda) cells (see below).
Potato (Solanum tuberosum L. cv Désirée) was obtained through Saatzucht Fritz Lange (GmbH, Bad Schwartau, Germany). Plants for epidermal fragment preparation were grown in soil in a greenhouse as described by Landschütze et al. (1995).
Isolation of the skt1 cDNA
Standard DNA manipulation procedures were as described by Sambrook et al. (1989). A λ ZAP II cDNA library established from potato epidermal fragments (Müller-Röber et al., 1995) was screened under low stringency in PEG buffer (Amasino, 1986), using a PCR-generated DNA fragment encoding the N-terminal part (including the membrane-spanning regions S1–S6) of the AKT1 protein (Anderson et al., 1992) as radioactively labeled hybridization probe. Plaque-purified phage clones were converted to pBluescript SK derivatives by in vivo excision according to the manufacturer's protocols (Stratagene). The skt1 cDNA, present in plasmid pSKT42, was sequenced on both strands using [α-35S]dATP (Amersham) and the T7 Sequencing Kit (Pharmacia). Sequence analysis was performed with the help of the programs of the Genetics Computer Group (GCG Package, version 8.1, Madison, WI; Devereux et al., 1984).
RNA Extraction and Northern-Blot Analysis
RNA from leaves and roots was extracted according to the method of Logemann et al. (1987). Extraction of RNA from epidermal fragments (highly enriched for guard cells) was performed as described (Kopka et al., 1997). Poly(A+) RNA was isolated using Oligotex Poly-dT (Qiagen, Darmstadt, Germany). Two micrograms of poly(A+) RNA was used for northern-blot analysis as described (Landschütze et al., 1995), using the radioactively labeled Asp718/BamHI cDNA insert of plasmid clone pSKT42 as the hybridization probe. Autoradiography using intensifying screens was performed overnight at −70°C.
Insect Cells and Virus Infection
Insect Sf9 cells (Invitrogen, Leek, The Netherlands) and Sf21 cells (kindly provided by Dr. Hiroshi Nyunoya, Tokyo University of Agriculture and Technology, Japan) were maintained as described (Luckow and Summers, 1988) as monolayer cultures at 27°C in TNM-FH medium (Sigma) supplemented with 10% fetal calf serum. Recombinant viruses obtained after transfection of bacmid DNA into insect cells were amplified twice to yield high-titer virus stocks (bacmids are E. coli-based baculovirus shuttle vectors). For expression of SKT1, KST1, GFP-SKT1, GFP-KST1, and GFP, 0.5 mL of virus stocks was used to infect cells (60–80% confluence) in 25-cm2 culture flasks.
Constructs Used for Expression in Insect Cells
Expression of SKT1, KST1, GFP, GFP-SKT1, and GFP-KST1 in insect cells was performed with the help of the Bac-to-Bac Expression System from GIBCO-BRL. Transfer plasmids pSKT1, pKST1, pGFP, pGFP-SKT1, and pGFP-KST1 were constructed as described below and used for in vivo recombination with bacmid DNA according to the manufacturer's protocol. pSKT1: pSKT42 was cut with Asp718 and ends were filled in with T4 DNA polymerase followed by a second digest with SpeI. The resulting fragment was ligated to pFastBac1 (GIBCO-BRL) cut with StuI/SpeI. pKST1: The NotI/PstI fragment of plasmid pKST#8–1 (Müller-Röber et al., 1995) was ligated into NotI/PstI-digested vector pFastBac1. pGFP: pEGFP-C1 (Clontech, Palo Alto, CA) was cut with Eco47III/BclI, blunted, and ligated to BamHI/HindIII-digested and blunted vector pFastBac1. pGFP-SKT1: 5′ to the skt1 start ATG of SKT42 an Asp718 restriction site was created by PCR, resulting in pSKT1B, of which an Asp718/BamHI fragment was transferred to pGFP, which was cut with the same enzymes. pGFP-KST1: The first two codons of the kst1 coding region were modified by PCR, generating a BamHI restriction site. A BamHI/PstI fragment of the resulting plasmid was ligated into vector pBlueBacHisB (Invitrogen). From this construct a BamHI/HindIII fragment was transferred to pGFP-C3 (Clontech) cut with BglII/HindIII. Finally, the GFP-KST1 coding region was cloned as a NheI/PstI fragment into pFastBac1 cut with XbaI/PstI.
SDS-PAGE
Membrane proteins were isolated from baculovirus-infected insect cells as follows. Cells were harvested from two 25-cm2 culture flasks, washed once with PBS, resuspended in 2 mL of buffer A (10 mm sodium phosphate buffer, pH 8.0, 1 mm EDTA, 1 mm PMSF), and kept on ice for 5 min. After addition of 130 μL of 5 m NaCl, cells were sonicated on ice for 30 s with a sonicator (HD200, Bandelin, Berlin, Germany). Eleven milliliters of buffer A was added and membranes were collected at 4°C by centrifugation for 30 min at 120,000g. Pellets were resuspended in 100 μL of SDS gel-loading buffer by brief sonication. Proteins were separated on 8% SDS-polyacrylamide gels and visualized using the Silver Stain Plus Kit (Bio-Rad).
Electrophysiology
Insect cells that had been infected with the recombinant viruses vir-SKT1 or vir-KST1 for 1 to 3 d, respectively (with strong signs of infections), were transferred to patch-clamp chambers. Uninfected cells or cells expressing the Arabidopsis two-pore K+ channel KCO1 (Czempinski et al., 1997) were used as controls. The whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) was obtained by gentle suction, leading easily to high-ohmic seals, followed by a short suction pulse to break the membrane. Pipettes (resistances around 5 MΩ) were fabricated from Kimax-51 glass capillaries (Kimble Glass, Inc., Owens, IL). All recordings were made at room temperature (20–22°C) with standard bath solution containing 30 mm K-gluconate, 1 mm CaCl2, 1 mm MgCl2, 225 mm sorbitol, 10 mm Mes-Tris, pH 6.2, and standard pipette solution containing 150 mm K-gluconate, 2 mm MgCl2, 10 mm MgATP, 10 mm EGTA, 10 mm Hepes-Tris, pH 7.4. Osmolarities were adjusted with sorbitol to 300 to 320 mosmol/kg using a vapor-pressure osmometer (model 5500, Wescor, Logan, UT). In tail-current experiments, [K+ext] were varied while osmolarities were maintained. To study selectivities, external K-gluconate was replaced by K-, NH4-, Na-, and Li-chloride salts. CsCl was applied from Cs+-bath stock solutions. Whole-cell current recordings and application of voltage programs were performed using the patch-clamp amplifier Axopatch 200B together with a Digidata 1200 interface (Axon Instruments, Foster City, CA). The patch-clamp software pClamp 6.0.3 with Clampex and Clampfit (Axon Instruments) was used to apply voltage stimulation and analyze the data. The filter frequency was set to 1 kHz; the acquisition time of data points was in the range of 2 to 10 ms depending on the voltage protocols (usually 5 ms). The voltage protocols used are described in the figure legends. Tail currents were measured at the beginning of the second voltage pulse. The Erev of these tail currents was determined from relationships of current transients versus potential. Ionic activities were calculated according to the work of Robinson and Stokes (1965). Liquid junction potentials were determined and corrected according to the method of Neher (1992). With the standard bath solution (30 mm K-gluconate), the junction potential was −10 mV, and with the other K-gluconate concentrations (150, 100, 50, 20, and 10 mm), the potential values used for correction were 0, −2, −8, −15, and −17 mV, respectively. In 30 mm KCl, NaCl, NH4Cl, and LiCl, junction potentials of −10, −17, −11, and −19 mV, respectively, were determined. To determine the current-voltage relations of the whole-cell current, leak subtractions were applied and voltage decreases were corrected by series resistance (Rs) compensations calculated according to current amplitudes and access resistances. Rs values were in the range of 4 to 8 MΩ.
Unless otherwise indicated, figures are shown for one representative cell, and statistics are given as means ± sd. For comparisons statistical Student's t tests were performed using SigmaPlot (Jandel Scientific, Carle Madera, CA) and P values were calculated (P representing the probability that two means are not significantly different).
Microscopy
Fluorescence imaging to visualize GFP in insect cells was performed using a microscope (Provis AX70, Olympus, Hyde Park, NJ) equipped with filters HQ480/40 and HQ510LP. For photography Kodak EPJ-320T film was used.
RESULTS
Cloning of the skt1 cDNA from Potato
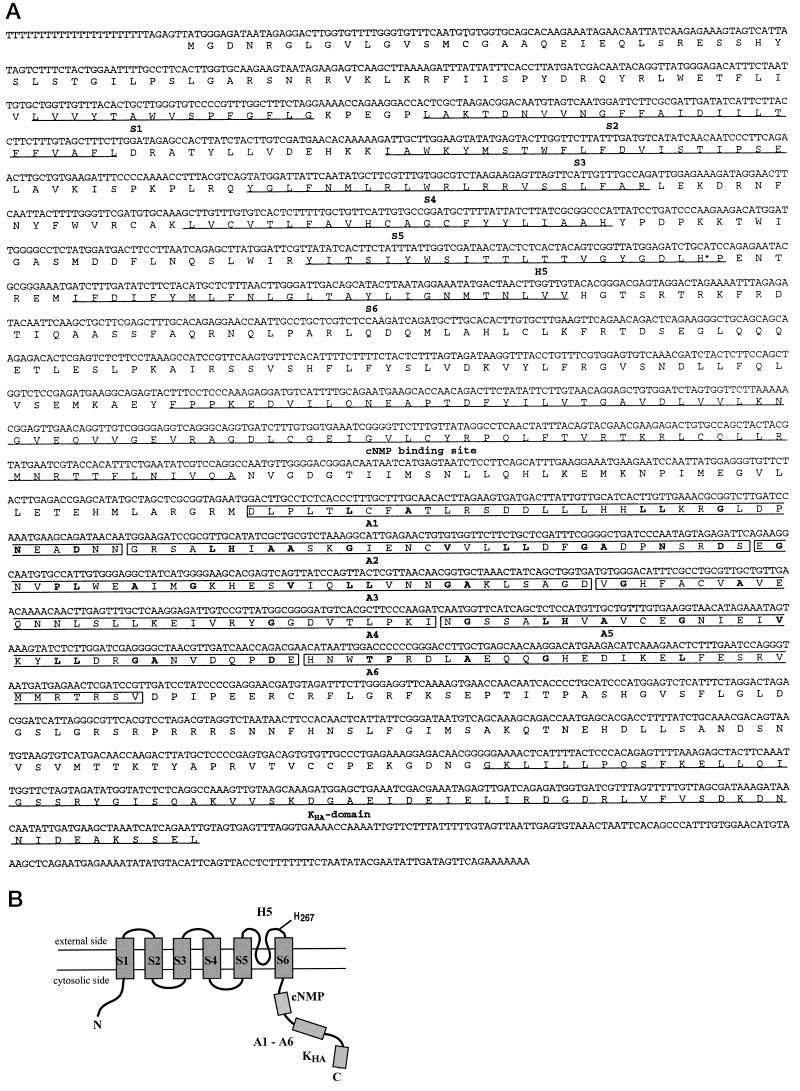
We have constructed a λ ZAP II cDNA library from epidermal fragments of potato leaves highly enriched for stomatal guard cells (Müller-Röber et al., 1995). Screening of this library with an akt1 probe (see Methods) resulted in the identification of several cross-hybridizing phage clones. The nucleotide sequence of the longest cDNA insert (skt1) had a length of 2846 nucleotides with an open reading frame located between nucleotides 29 (ATG) and 2680 (TAG) coding for an 883-amino acid polypeptide (92 kD) (see Fig. 1A).
Figure 1.
(On facing page.)Sequence and structure of SKT1. S1 to S6, Transmembrane segments; H5, pore region; cNMP, putative cyclic nucleotide-binding site; A1 to A6, ankyrin-like repeats; KHA, C-terminal homology domain. A, Nucleotide sequence of the skt1 cDNA and deduced amino acid sequence of the SKT1 protein. Transmembrane segments, H5 region, cNMP, and the interaction domain KHA are underlined. The ankyrin-like repeats are boxed and respective conserved amino acids (Bennett, 1992) are in boldface. The conserved His residue His-267 involved in pH sensing (Hoth et al., 1997) is marked by an asterisk. B, Structural model of the SKT1 protein.
The deduced SKT1 protein contains several structural domains that are common to plant K+in channels (see Fig. 1, A and B): (a) six putative transmembrane domains (S1–S6), as deduced from hydropathy blots (not shown); (b) a K+-selective pore-forming domain, H5; (c) a putative cyclic nucleotide-binding domain; (d) six ankyrin repeat sequences that are also present in AKT1, AKT2/3, SKT2, and SKT3, but not in KAT1 or KST1 (these domains have been proposed to bind to cytoskeletal proteins [compare with Cao et al., 1995]); and (e) a C-terminal interaction domain present in all cloned plant K+in channels (Ehrhardt et al., 1997). Table I shows identity scores between the potato protein and other plant K+in channel proteins previously published. SKT1 is most closely related to Arabidopsis AKT1, with 73.1% identical (85.6% similar) amino acids.
Table I.
Identity scores between SKT1 and other potato and Arabidopsis K+in channel proteins
| Protein | KST1 (688 aaa) | KAT1 (677 aa) | KAT2b(565 aa) | AKT1 (857 aa) | AKT2c(802 aa) | SKT2 (845 aa) | SKT3b(469 aa) |
|---|---|---|---|---|---|---|---|
| SKT1 | 52.4 | 52.8 | 52.1 | 73.1 | 45.9 | 43.7 | 36.8 |
| (881 aa) | (70.8) | (70.9) | (71.1) | (85.6) | (65.9) | (64.6) | (60.3) |
Sequence homologies were determined via pairwise sequence alignments using the BESTFIT program of the GCG package. Sizes (no. of amino acids) are given for the individual proteins. Numbers indicate percentage of identical and similar (in parentheses) amino acids. The GenBank accession numbers are as follows: SKT1, X86021; KST1, X79779; KAT1, M86990; KAT2, U25694; AKT1, U06745; AKT2, U40154; SKT2, Y09699; and SKT3, Y09818.
aa, Amino acids.
Only partial sequence available.
AKT2 (Cao et al., 1995) is identical to AKT3 (Ketchum and Slayman, 1996).
Functional Expression in Baculovirus-Infected Insect Cells Reveals SKT1 to Be a Second K+in Channel from Potato
Injection of skt1 cRNA into Xenopus oocytes did not lead to a functional expression of the SKT1 protein (Dreyer et al., 1997; and S. Zimmermann, I. Talke, and B. Müller-Röber, unpublished data). Furthermore, SKT1 was not able to complement the CY162 yeast mutant (Anderson et al., 1992) deficient for the K+ uptake systems TRK1 and TRK2 (B. Müller-Röber, unpublished data). Therefore, we focused on the expression of SKT1 in the baculovirus/insect cell system.
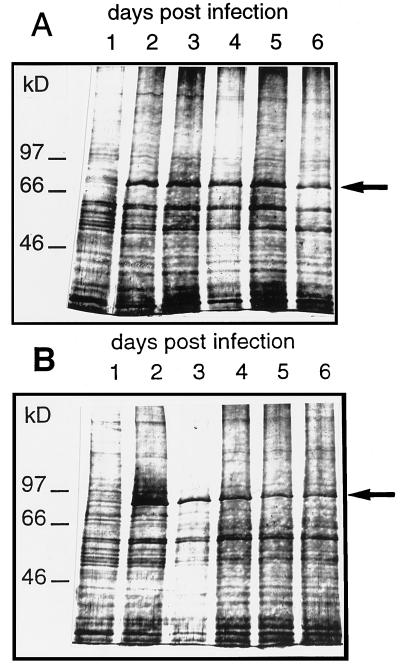
To test for protein expression total membrane fractions were prepared from insect cells at d 1 to 6 after infection and separated on SDS-polyacrylamide gels (Fig. 2). Already 2 d after infection a protein with a molecular weight corresponding to that of SKT1 appeared (Fig. 2B). The amount of this protein did not change significantly during d 2 to 6 after infection, indicating that insect cells have a protein-synthesizing capacity that allows a maximal level of expression of a channel protein within the first 2 d. Similar observations were made for KST1 (see Fig. 2A).
Figure 2.
SDS-polyacrylamide gel showing the presence of KST1 (A) and SKT1 (B) protein in membrane preparations of vir-KST1- and vir-SKT1-infected insect cells. Membrane proteins were isolated from insect cells 1 to 6 d after infection and after electrophoresis visualized by silver staining (typical examples from two independent experiments). The novel proteins KST1 (79 kD) and SKT1 (92 kD), appearing at d 2 after infection, are indicated by arrows.
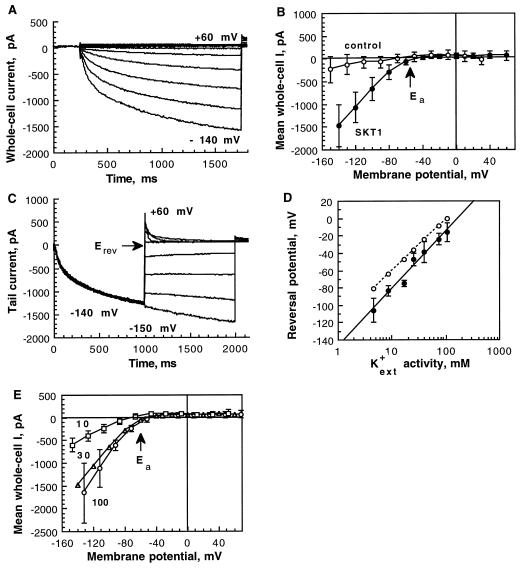
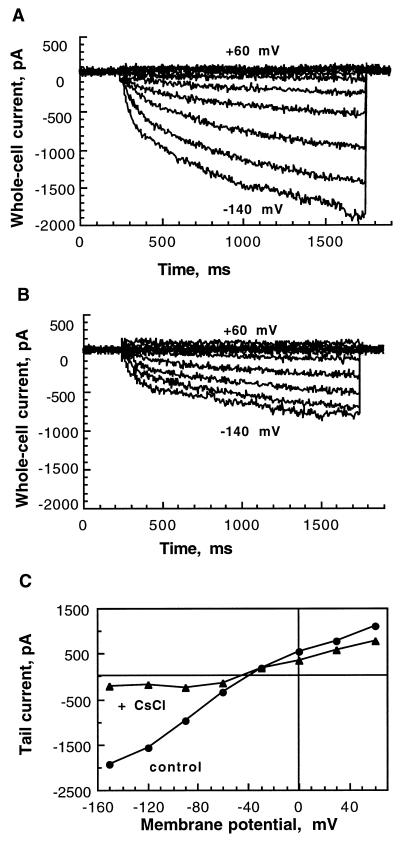
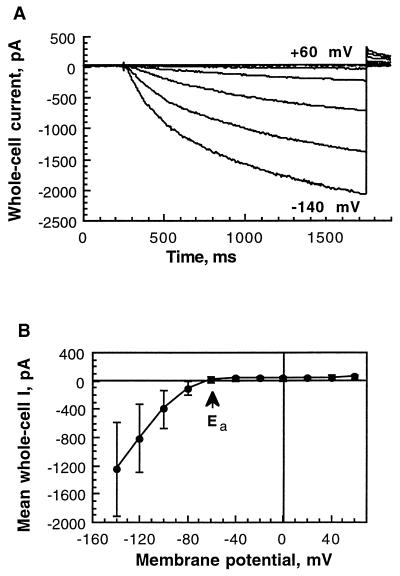
Insect cells that were infected by the recombinant baculovirus vir-SKT1 were assayed for K+ inward currents 2 d after infection in the whole-cell configuration of the patch-clamp method. In standard solutions ([K+ext] = 30 mm, [K+in] = 150 mm), hyperpolarizing pulses elicited slowly activating negative currents (Fig. 3A). The activation kinetics could be fitted by a multiexponential function. Half-activation times, representing the time in which half-maximal amplitudes of the “quasi”-steady-state currents were reached, were voltage dependent for each individual cell and were found on average to be 389 ± 116 ms at −100 mV, and 259 ± 86 ms at −140 mV (n = 10), respectively (significance of voltage-dependent activation was tested; P = 0.01, paired Student's t test P < 1 × 10−5). Inactivation of SKT1-mediated currents was not observed under the applied conditions. The current-voltage relation (Fig. 3B) of the mean current at the end of the applied voltage pulses as shown in Figure 3A of vir-SKT1-infected Sf9 cells (n = 10) was compared with that of control currents of uninfected cells (n = 10). Uninfected Sf9 cells as well as cells expressing another protein (i.e. the “two-pore” channel KCO1; Czempinski et al., 1997) displayed only small currents at highly hyperpolarized potentials. SKT1 activated at membrane potentials more negative than the Ea at around −60 mV.
Figure 3.
Electrophysiological characteristics of SKT1 expressed in insect cells. The cells were measured in the whole-cell mode 2 d after infection. A, Hyperpolarization-activated SKT1 currents for one representative vir-SKT1-infected Sf9 cell. Voltage pulses (1.5 s) were applied every 10 s from +60 to −140 mV in 20-mV increments from a holding potential of −30 mV. B, Current-voltage relation of the mean quasi-steady-state currents at the end of the 1.5-s voltage pulses derived from control insect cells (n = 10; not infected) and Sf9 cells expressing SKT1 (n = 10). The Ea was approximately −60 mV. C, SKT1 tail currents elicited by double-voltage pulses. Inward currents were activated at −140 mV (1100 ms) from a holding potential of −40 mV. Current transients in response to a second voltage pulse (900 ms) from −150 to +60 mV in 30-mV increments were recorded. D, Erev of SKT1 at different external K+ concentrations (•; n = 4–13). Semilogarithmically displayed data points were linearly fitted. The measured Erev values are in good accordance with the theoretical Nernst potentials for K+ (○). E, SKT1 mean current-voltage relations in different external K+ concentrations: 10 mm (□; n = 4), 30 mm (▵; n = 10), and 100 mm (○; n = 4) K-gluconate. Error bars for 30 mm are left out for clearness (see B). Note that the Ea did not change significantly.
Standard solutions contained the impermeable anion gluconate as the counterion for K+, thus suggesting that K+ is the main carrier of the observed currents elicited by hyperpolarizing pulses. Also, the Erev determined in double-pulse experiments (−47 ± 7 mV; n = 13) was in good accordance with the Nernst potential for K+ (−42 mV; Fig. 3C). Furthermore, we analyzed SKT1-mediated currents in different external K+ concentrations. The Erev from tail-current experiments for [K+ext] of 5 to 150 mm followed approximately the calculated value of the Nernst potential for K+ (Fig. 3D). The increase of [K+ext] by a factor of 10 changed the Erev by 58 mV, as expected from the Nernst equation, indicating permeability for K+. The Ea of SKT1 was not significantly dependent on different external K+ concentrations (Fig. 3E). Replacing gluconate in the external bath solution with chloride had no influence on the voltage-dependent SKT1 current (n = 5; data not shown), demonstrating the cation permeability of SKT1. Further measurements of SKT1 currents in bath solutions containing monovalent cations replacing K+ demonstrated that SKT1 is a highly selective K+ channel. Chloride salts of K+ (n = 5), NH4 (n = 6), Na+ (n = 4), and Li+ (n = 3) were tested. Currents were strongly reduced in the K+-free bath solutions and relative permeabilities were determined: K+ (1) > NH4+ (0.02) ≫ Na+, Li+ (<0.01).
To test whether divalent cations, i.e. Mg2+ or Ca2+, might be responsible for the rectification as described for animal K+ inward rectifiers (Matsuda, 1991), we performed experiments with bath and pipette solutions depleted of Mg2+ and Ca2+. As in standard conditions (see Fig. 3, A and B), only hyperpolarizing potentials activated the inwardly rectifying SKT1 currents (n = 5; data not shown), arguing for intrinsic mechanisms of voltage sensing and regulation.
Cytosolic compounds, like nucleotides, are known to influence the activity of ion channels. Because our standard pipette solutions contained ATP, we also checked SKT1 activity in the absence of internal ATP. ATP-free pipette solutions caused a slow decrease of hyperpolarization-activated SKT1 currents within 5 to 10 min (n = 3; data not shown), as was observed for KST1 expressed in oocytes (Müller-Röber et al., 1995) or in insect cells (see below). The long lag time until observation of significant effects may be the result of the time required to equilibrate the cytosol with the ATP-free pipette solution. In addition, high-affinity ATP-binding or phosphorylation/dephosphorylation events might also be considered.
Taken together, these results confirm that SKT1 could be functionally expressed in the plasma membrane of baculovirus-infected insect cells and that it is indeed the second K+in channel cloned from potato.
External pH and Cs+ Affect SKT1 Channel Activity
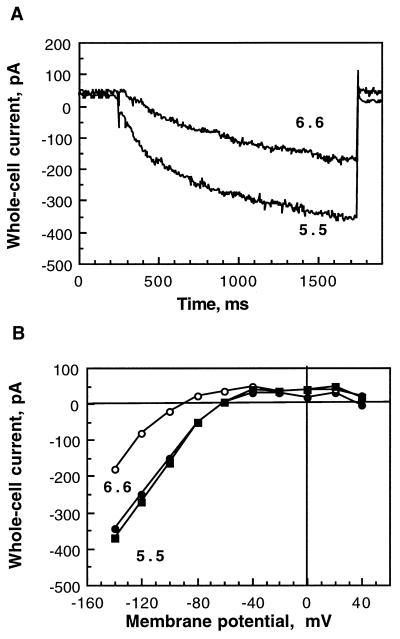
It has previously been shown that external His is conserved in K+in channels from higher plants and that it mediates pH sensitivity of KST1 (Hoth et al., 1997). SKT1 also possesses the conserved His (His-267) in the outer pore region (see Fig. 1, A and B), suggesting a possible pH regulation. The external pH turned out to regulate the current amplitudes of SKT1 in insect cells (Fig. 4). The current activating at −140 mV was reduced by 56 ± 12% (n = 3) by increasing the external pH from 5.5 to 6.6 (Fig. 4A). Furthermore, the regulation of SKT1 by external pH was found to be reversible, and repeated acidification caused a new current increase (Fig. 4B).
Figure 4.
Dependence of SKT1 currents on external pH. A, Decrease of current amplitude for a representative cell at −140 mV by alkalinization of the external pH from 5.5 to 6.6. B, Current-voltage relationships for the cell shown in A at pH 5.5 (•), pH 6.6 (○), and finally pH 5.5 (▪), demonstrating reversibility of the pH regulation.
To characterize SKT1 pharmacologically, we tested Cs+ as an inhibitor of plant K+ channels (Tester, 1990). Application of Cs+ in the bath solution caused a decrease of the K+ inward current within a few minutes (Fig. 5, A and B). Inhibition was determined at −130 mV at different external Cs+ concentrations and the dose-response curve (see Fig. 6B) revealed an IC50 of 105 μm. Tail-current experiments (n = 4) revealed that the inhibition was dependent on applied potentials (Fig. 5C), suggesting a voltage-dependent block. We found an inhibition of tail-current amplitudes at −90 mV of 51 ± 17%, but at −120 mV the inhibition was 75 ± 10% (inhibition significantly different; P = 0.06, paired Student's t test P = 0.02). The blocking Cs+ ion might be more attracted into the channel pore at hyperpolarized potentials but more easily liberated at depolarizing potentials.
Figure 5.
Cs+ inhibition of SKT1-mediated K+in currents. A, Control currents of a representative vir-SKT1-infected insect cell (for voltage pulses, see Fig. 3A legend). B, Inhibition of SKT1 current by external addition of 100 μm Cs+. C, Voltage-dependent inhibition of SKT1 tail currents of another cell by 100 μm Cs+ (for voltage pulses, see Fig. 3C legend).
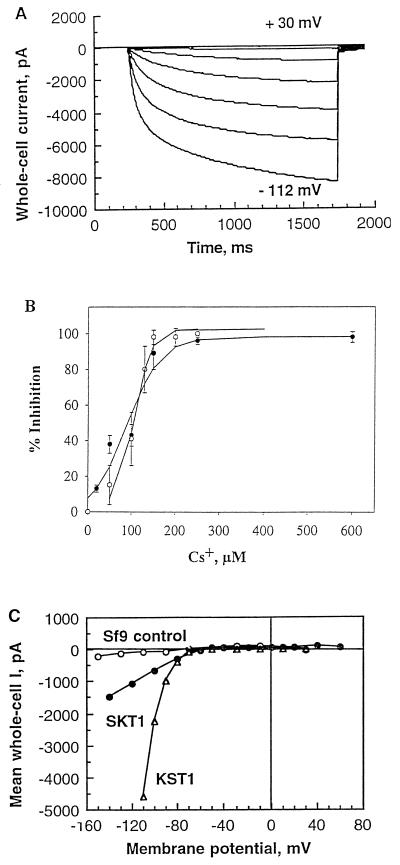
Figure 6.
Characteristics of KST1 expressed in insect cells in comparison with SKT1. A, Hyperpolarization-activated KST1 currents shown for one representative vir-KST1-infected Sf21 cell. Voltage pulses (1.5 s) were applied every 10 s from +60 to −112 mV. B, Dose-response curve of SKT1 and KST1 current inhibition by externally applied Cs+. Percentage of inhibition was determined for quasi-steady-state currents at −130 mV and fitted by a logistic function. ○, SKT1; •, KST1 (n = 3–7). Note that concentrations for half-maximal inhibition were nearly identical for SKT1 (IC50 = 105 μm) and KST1 (IC50 = 90 μm). C, Voltage-dependent mean currents in control cells (○; n = 10), vir-SKT1-infected cells (•; n = 10), and vir-KST1-infected cells (▵; n = 13). Note the marked difference in absolute current amplitudes in SKT1- and KST1-expressing cells (significance tested for −100 mV; P < 0.009).
Our data concerning external modulation of SKT1 activity demonstrate a physiologically interesting pH dependence as well as a voltage-dependent Cs+ sensitivity that might be of interest in obtaining a pharmacological fingerprint of the different members of plant K+in channels (see Discussion).
SKT1 and KST1, Two K+in Channels from Potato: Similarities and Differences
Sf9 cells that were infected with vir-KST1 displayed large, whole-cell currents activating at membrane potentials slightly more negative than for SKT1 (at around −80 mV; Fig. 6, A and C). Inwardly rectifying currents elicited by hyperpolarizing pulses activated with voltage-dependent, slow kinetics. Tail-current analysis confirmed the K+ selectivity of KST1. Erev values in standard solutions (−41 ± 4 mV; n = 8) were in good accordance with the Nernst potential for K+ (−42 mV) (compare with Ehrhardt et al., 1997). These data from insect cells corresponded well with those from voltage-clamp experiments on KST1-expressing oocytes (Müller-Röber et al., 1995). The high selectivity of SKT1 for K+ (see relative permeability values above) was similarly found for KST1 in oocytes. In contrast to SKT1, activation times could be fitted by two exponentials instead of multiple exponentials. Half-activation times were determined at −100 and −140 mV with 217 ± 61 and 122 ± 52 ms (n = 7), respectively. KST1 activation was therefore faster (P < 0.003) than SKT1 activation in the same expression system (see above), and also faster than KST1 activation determined in oocytes (−170 ± 40 ms at −180 mV; Müller-Röber et al., 1995). Note, however, that the analysis of activation kinetics is limited because of voltage errors caused by large current amplitudes.
The pH dependence of KST1 was initially studied in experiments on oocytes (Müller-Röber et al., 1995) and further confirmed by mutational analysis of conserved His's (Hoth et al., 1997). We found that KST1 currents were also reversibly regulated by external pH modulation in insect cells, as shown for SKT1 (see Fig. 4). Alkalinization of the pH from 5.5 to 6.6 decreased KST1 currents by more than 50% (n = 3). Experiments without cytosolic ATP demonstrated a slow decrease of KST1 currents in insect cells, confirming results obtained on oocytes on the single-channel level (Müller-Röber et al., 1995). As in SKT1-expressing insect cells, we observed a rundown of KST1 activity within 5 to 10 min with the ATP-free pipette solution, resulting in about a 50% current decrease after 10 min of the whole-cell configuration (n = 4). Thus, SKT1 and KST1 are both regulated by modulation of external pH and are dependent on the presence of internal ATP.
As previously observed in oocytes, application of 0.5 and 1 mm Cs+ to KST1-expressing insect cells caused strong inhibition of the inward current, and the voltage-dependent block was pronounced in tail-current experiments (data not shown). In contrast to pharmacological results obtained on oocytes (600 μm in oocytes; Müller-Röber et al., 1995), however, we determined a much lower IC50 of 90 μm for Cs+ in the baculovirus/insect cell system. In this system, the Cs+ sensitivities of SKT1 and KST1 turned out to be almost identical (Fig. 6B).
So far, both channels from potato were shown to be inward rectifiers with very similar characteristics. Nevertheless, slight differences in Ea and activation kinetics were detected (see above). Much more obvious was a discrepancy in the amplitudes of SKT1- or KST1-mediated currents, as shown in the current-voltage relation (Fig. 6C). Furthermore, almost all of the vir-KST1-infected cells exhibited K+in currents, whereas K+in currents were detectable in only 30 to 50% of vir-SKT1-infected cells.
Use of GFP to Visualize Targeting of SKT1 and KST1 in Insect Cells
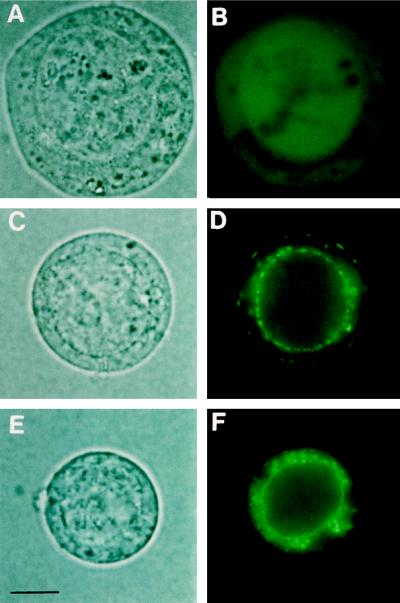
Even though both SKT1 and KST1 proteins were easily detected in silver-stained SDS-polyacrylamide gels of crude microsomal fractions of insect cells (see Fig. 2), the electrophysiological results suggested a difference of functionally active channels located to the plasma membrane. To further analyze the subcellular localization and amount of recombinant protein in insect cells, the GFP was used as a marker. Expression of GFP alone gave a diffuse overall fluorescence within the insect cells (Fig. 7, A and B), whereas expression of KST1 linked to GFP resulted in fluorescent signals within the plasma membrane as well as fluorescence in endogenous membranes (Fig. 7, C and D; see also Ehrhardt et al., 1997). In contrast, expression of a GFP-SKT1 fusion protein led to strong signals only within inner membranes. No fluorescence could be detected in the outer plasma membrane by standard fluorescence microscopy (Fig. 7, E and F) or laser-scanning confocal microscopy (not shown). Note, however, that SKT1-like currents identical in voltage dependence and mean current amplitudes (n = 3) were also measured in vir-GFP-SKT1-infected cells (Fig. 8). A slight difference between SKT1- and GFP-SKT1-expressing cells was detected only with respect to activation kinetics (compare Figs. 3A and 8A).
Figure 7.
SKT1 and KST1 expression in insect cells visualized by GFP. Microscopic images representing >90% of insect cells expressing GFP or GFP fusion proteins as seen under normal light and UV light in at least eight independent infections (bar = 3.5 μm). Note that insect cells infected with recombinant virus show distinct signs of infection, such as a swollen cell body and a clearly visible swollen nucleus. The size of the cells is variable (8–15 μm) and does not depend on the virus used for infection. A and B, GFP. The protein is equally distributed in the cytosol, as can be seen by a diffuse overall fluorescence. C and D, GFP-KST1 fusion. The protein is clearly localized in cellular membranes. In the plasma membrane, GFP-KST1 appears as fluorescent patches most likely consisting of several clustered channel proteins (compare with Ehrhardt et al., 1997). E and F, GFP-SKT1 fusion protein. The channel protein can be seen only in internal cellular membranes, especially around the nucleus. No fluorescence can be detected in the plasma membrane.
Figure 8.
Functional activity of GFP-SKT1. A, Current response of a GFP-SKT1-expressing insect cell. For voltage pulse protocol, see legend to Figure 3A. B, Current-voltage relation of the mean quasi-steady-state currents (n = 3) at the end of the 1.5-s voltage pulses. The Ea of approximately −60 mV is indicated.
These results suggest that the targeting of SKT1 might be affected in vir-SKT1-infected insect cells, preventing the insertion of detectable amounts of fluorescently labeled channel protein, although K+ currents were resolved with the more sensitive patch-clamp recordings. Taken together, our observations might explain the observed differences in current amplitudes and occurrence (see Fig. 6C).
The skt1 Gene Is Expressed in Roots and Epidermal Fragments of Potato Plants
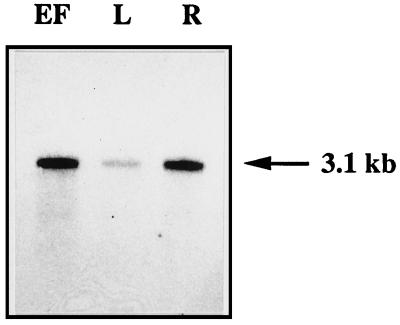
RNA was isolated from various tissues of greenhouse-grown potato plants and analyzed for skt1 mRNA expression. When total RNA was used for RNA-blot experiments, only very faint skt1 signals were detected in leaves, epidermal fragments, and roots after several days of exposure to x-ray films (data not shown). We therefore isolated poly(A+) RNA and found the strongest expression in epidermal fragments and in roots, whereas lower transcript levels were detected in mature leaves (Fig. 9). It is interesting that the homologous Arabidopsis akt1 gene was reported to be expressed in roots and in hydathodes (Lagarde et al., 1996), indicating that variations in the expression patterns may exist between these plant species. Because the epidermal fragments used in our experiments are highly enriched for guard cells (Kopka et al., 1997), it is possible that skt1 is expressed in this cell type and may contribute to stomatal function.
Figure 9.
mRNA expression of skt1 in potato tissues. EF, Epidermal fragments; L, whole leaves; and R, roots. The size of the transcript is indicated at the right of the figure.
DISCUSSION
The molecular and electrophysiological characterization of K+ channels from various plant species, including crops, is required to characterize the full range of K+ transport mechanisms in relation to turgor regulation, nutrient uptake, and nutrient transport, as well as to elucidate the structure-function relationship of these channel proteins. Functional expression of plant K+ channels in heterologous systems has been demonstrated mainly for K+ channels from the genetic model plant A. thaliana (e.g. Anderson et al., 1992; Sentenac et al., 1992; Ketchum and Slayman, 1996; Czempinski et al., 1997). To our knowledge, the only reported exception is the K+in channel KST1 from potato, which was shown by in situ hybridization to be expressed in leaf guard cells (Müller-Röber et al., 1995). Here we describe the cloning and functional characterization of another K+in channel from potato, SKT1, which on the amino acid level is most similar to Arabidopsis AKT1. Initial attempts to express SKT1 functionally in Xenopus oocytes were not successful, a phenomenon that has also been observed for AKT1. Surprisingly, however, coinjection of skt1 and akt1 cRNAs into Xenopus oocytes resulted in strong K+in currents upon membrane hyperpolarization, indicating that SKT1 and AKT1 subunits were able to associate and form electrophysiologically active K+in channels in this heterologous expression system (Dreyer et al., 1997). We therefore evaluated whether the baculovirus/insect cell system would be suitable to determine the properties of a channel solely composed of SKT1 polypeptides. Insect cells have been used in many cases in the animal field to study channel properties; however, they have only more recently been used for expression of the plant K+in channel proteins KAT1 (Marten et al., 1996), AKT1 (Gaymard et al., 1996), and KST1 (Ehrhardt et al., 1997). Furthermore, modified KST1 proteins, e.g. those harboring N-terminal extensions (GFP or polyhistidine tags [Ehrhardt et al., 1997; T. Ehrhardt, S. Zimmermann, and B. Müller-Röber, unpublished data]), could be functionally expressed in insect cells. Here we demonstrated that another channel protein, SKT1 from potato, can be studied electrophysiologically in these cells.
The expression of plant ion channels in baculovirus-infected insect cells makes it possible to directly compare channels of the KAT family with AKT-type channels. We showed that SKT1 represents a K+in-selective channel that is activated with slow kinetics by hyperpolarizing voltage pulses, indicating that overall properties of SKT1 were similar to those described for KST1 expressed in oocytes (Müller-Röber et al., 1995) and in insect cells (this report; see also Ehrhardt et al., 1997).
Plant growth and activity of K+ channels are extremely sensitive to extracellular Cs+ (e.g. Tester, 1990; Sheahan et al., 1993). Previously, KAT1 and KST1 channels expressed in Xenopus oocytes were investigated with respect to block by Cs+ ions (Hedrich et al., 1995; Müller-Röber et al., 1995; Véry et al., 1995). Here we showed that both potato channels, SKT1 and KST1, exhibited a similar voltage-dependent inhibition by Cs+ (around 100 μm at −130 mV) when expressed in insect cells. In contrast, much higher concentrations of Cs+ were needed to achieve half-maximal inhibition of KST1-mediated currents in Xenopus oocytes (IC50 = 600 μm at −180 mV; Müller-Röber et al., 1995). Similarly, expression of Arabidopsis KAT1 in oocytes required 335 μm Cs+ (at −180 mV; Becker et al., 1996) or 500 μm Cs+ (at −135 mV; Ichida and Schroeder, 1996) for half-maximal inhibition.
Recently, Dreyer et al. (1997) found a strongly increased sensitivity toward Cs+ (IC50 = 40 μm at −150 mV) of K+in currents in Xenopus oocytes upon coinjection of kst1 and akt1 cRNA, and an even higher Cs+ sensitivity, with an IC50 of 15 μm, was observed upon coinjection of skt1 and akt1 cRNAs. For AKT1 expressed in yeast a Cs+ block with an IC50 of approximately 20 μm was found recently by Bertl et al. (1997). The reasons for the differences in observed Cs+ sensitivities in the various systems are not clear at present. For oocytes, Véry et al. (1994) proposed that the level of expression of KAT1 influences the apparent inhibition by Cs+. In these experiments injection of lower amounts of kat1 cRNA resulted in smaller K+ currents that were more strongly inhibited by Cs+. Alternatively, one can speculate that in vivo properties of K+in channels of the KAT family are modified by subunits of the AKT family. The recent observation that electrically active K+in channels can form by oligomerization of members of the two families supports such a possibility (compare with Dreyer et al., 1997). It is interesting that IC50 values of 40 and 64 μm (determined at −200 mV) were obtained for K+in currents in isolated guard cell protoplasts from Vicia faba and potato, respectively (compare with Hedrich and Dietrich, 1996). Our results obtained here for SKT1 and KST1 expressed in baculovirus-infected insect cells indicate a Cs+ sensitivity that, at least for KST1, is close to the value determined in vivo in guard-cell protoplasts.
Another aspect of particular interest is the observation of pH-dependent regulation of plant K+in channels. We have recently shown that KST1 is activated by acidic pH values of the external medium (Müller-Röber et al., 1995), and that an extracellular His residue, which is highly conserved in all plant K+in channel proteins molecularly identified to date, contributes to pH sensitivity (Hoth et al., 1997). Similarly, KAT1, after expression in oocytes, has been shown to be activated by a shift in external pH from 6.5 to 5.0 (Véry et al., 1995). In guard cells, where KST1 (Müller-Röber et al., 1995) and KAT1 (Nakamura et al., 1995) are expressed, initiation of stomatal opening involves an activation of the plasma membrane proton pump (H+-ATPase), leading to membrane hyperpolarization and acidification of the extracellular space (Shimazaki et al., 1986), thereby activating K+in channels (Blatt and Thiel, 1993). In this work we could demonstrate the dependence of current activity on external pH also for SKT1, thereby extending this effect to members of the AKT family. Although the exact cell type in which the skt1 gene is expressed is not known at present, we have shown here that skt1 mRNA is detectable in roots (and in leaf epidermal fragments). Similarly, the akt1 gene has been shown to be expressed in roots of A. thaliana (Basset et al., 1995; Lagarde et al., 1996), consistent with a role of AKT1, and also of SKT1, in K+ uptake from the soil. The observation that SKT1 is reversibly activated by a shift to more acidic pH values indicates that also in the cellular environment of the plant tissue (e.g. in roots) this channel might be activated by a combination of membrane hyperpolarization and extracellular acidification. Previous physiological experiments have indeed indicated that uptake of K+ is driven by an electrogenic extrusion of H+, e.g. in barley roots (Behl and Raschke, 1987).
Although SKT1 and KST1 exhibited similar functional properties in insect cells, we found an important difference in targeting efficiency to the insect cell's plasma membrane, visualized by fusions to GFP. An appreciable proportion of the GFP signal was detected in the plasma membrane upon fusion of GFP to KST1 (see Fig. 7D; see also Ehrhardt et al., 1997). In contrast, we never detected GFP signals in the plasma membrane when the reporter protein was fused to SKT1, even when sensitive confocal microscopy was used, although high amounts of GFP-SKT1 fusion proteins localized to intracellular membranes (see Fig. 7F). These data indicate very inefficient targeting of the GFP-SKT1 protein to the plasma membrane of insect cells. A similar result was obtained by Gaymard et al. (1996) for AKT1 in immunogold studies. At present, we cannot explain the differences in plasma membrane targeting. However, one might speculate that insufficient targeting of AKT-like channels to the plasma membrane is responsible for unsuccessful attempts to express these proteins in Xenopus oocytes.
The presence of multiple K+in channels in higher plants raises questions about their cellular distribution and functional roles. More physiological evidence is needed to determine whether functionally similar ion channels contribute to tissue- and cell-type-specific ion fluxes in planta or whether they are coexpressed and possibly cooperate in plant cell membranes.
ACKNOWLEDGMENTS
The authors thank Dr. Katrin Czempinski and Dr. Gunnar Plesch for critical comments on the manuscript.
Abbreviations:
- Ea
activation potential
- Erev
reversal potential
- GFP
green fluorescence protein
- IC50
inhibitor concentration for 50% inhibition
- [K+ext]
external K+ concentration
- [K+in]
internal K+ concentration
- K+in channel
inwardly rectifying K+ channel
Footnotes
The accession number for the nucleotide sequence of skt1 described in this article is X86021.
LITERATURE CITED
- Amasino RM. Acceleration of nucleic acid hybridisation rate by polyethylene glycol. Anal Biochem. 1986;152:304–307. doi: 10.1016/0003-2697(86)90413-6. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset M, Conjelo G, Lepetit M, Fourcroy P, Sentenac H. Organization and expression of the gene coding for the potassium transport system AKT1 of Arabidopsis thaliana. Plant Mol Biol. 1995;29:947–958. doi: 10.1007/BF00014968. [DOI] [PubMed] [Google Scholar]
- Becker D, Dreyer I, Hoth S, Reid JD, Busch H, Lehnen M, Palme K, Hedrich R. Changes in voltage activation, Cs+ sensitivity, and ion permeability in H5 mutants of the plant K+ channel KAT1. Proc Natl Acad Sci USA. 1996;93:8123–8128. doi: 10.1073/pnas.93.15.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl R, Raschke K. Close coupling between extrusion of H+ and K+ by barley roots. Planta. 1987;172:531–538. doi: 10.1007/BF00393871. [DOI] [PubMed] [Google Scholar]
- Bennett V. Ankyrins: adaptors between diverse plasma membrane proteins and the cytoplasm. J Biol Chem. 1992;267:8703–8706. [PubMed] [Google Scholar]
- Bertl A, Reid JD, Sentenac H, Slayman CL. Functional comparison of plant inward-rectifier channels expressed in yeast. J Exp Bot. 1997;48:405–413. doi: 10.1093/jxb/48.Special_Issue.405. [DOI] [PubMed] [Google Scholar]
- Blatt MR, Thiel G. Hormonal control of ion channel gating. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:543–567. [Google Scholar]
- Boorer KJ, Loo DDF, Wright EM. Steady-state and presteady-state kinetics of the H+-hexose cotransporter (STP1) from Arabidopsis thaliana expressed in Xenopus oocytes. J Biol Chem. 1994;269:20417–20424. [PubMed] [Google Scholar]
- Bustos MM, Luckow VA, Griffing LR, Summers MD, Hall TC. Expression, glycosylation and secretion of phaseolin in a baculovirus system. Plant Mol Biol. 1988;10:475–488. doi: 10.1007/BF00033603. [DOI] [PubMed] [Google Scholar]
- Butt AD, Blatt MR, Ainsworth CC. Expression, evolution and genomic complexity of potassium ion channel genes of Arabidopsis thaliana. J Plant Physiol. 1997;150:652–660. [Google Scholar]
- Cao Y, Ward JM, Kelly WB, Ichida AM, Gaber RF, Anderson JA, Uozumi N, Schroeder JI, Crawford NM. Multiple genes, tissue specificity, and expression-dependent modulation contribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 1995;109:1093–1106. doi: 10.1104/pp.109.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coté GG. Signal transduction in leaf movement. Plant Physiol. 1995;109:729–734. doi: 10.1104/pp.109.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czempinski K, Zimmermann S, Ehrhardt T, Müller-Röber B. New structure and function in plant K+ channels: KCO1, an outward rectifier with a steep Ca2+-dependency. EMBO J. 1997;16:2565–2575. doi: 10.1093/emboj/16.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer I, Antunes S, Hoshi T, Müller-Röber B, Palme K, Pongs O, Reintanz B, Hedrich R. Plant K+ channel α-subunits assemble indiscriminately. Biophys J. 1997;72:2143–2150. doi: 10.1016/S0006-3495(97)78857-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt T, Zimmermann S, Müller-Röber B. Association of plant K+in channels is mediated by conserved C-termini and does not affect channel assembly. FEBS Lett. 1997;409:166–170. doi: 10.1016/s0014-5793(97)00502-4. [DOI] [PubMed] [Google Scholar]
- Gaymard F, Cerutti M, Horeau C, Lemaillet G, Urbach S, Ravallec M, Devauchelle G, Sentenac H, Thibaud JB. The baculovirus/insect cell system as an alternative to Xenopus oocytes. J Biol Chem. 1996;271:22863–22870. doi: 10.1074/jbc.271.37.22863. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:228–232. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Becker D. Green circuits: the potential of plant specific ion channels. Plant Mol Biol. 1994;26:1637–1650. doi: 10.1007/BF00016494. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Dietrich P. Plant K+ channels: similarity and diversity. Bot Acta. 1996;109:94–101. [Google Scholar]
- Hedrich R, Moran O, Conti F, Busch H, Becker D, Gambale F, Dreyer I, Küch A, Neuwinger K, Palme K. Inward rectifier potassium channels in plants differ from their animal counterparts in response to voltage and channel modulators. Eur Biophys J. 1995;24:107–115. doi: 10.1007/BF00211406. [DOI] [PubMed] [Google Scholar]
- Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth S, Dreyer I, Dietrich P, Becker D, Müller-Röber B, Hedrich R. Molecular basis of plant-specific acid activation of K+ uptake channels. Proc Natl Acad Sci USA. 1997;94:4806–4810. doi: 10.1073/pnas.94.9.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida AM, Schroeder JI. Increased resistance to extracellular cation block by mutation of the pore domain of the Arabidopsis inward-rectifying K+ channel KAT1. J Membr Biol. 1996;151:53–62. doi: 10.1007/s002329900057. [DOI] [PubMed] [Google Scholar]
- Ketchum KA, Slayman CW. Isolation of an ion channel gene from Arabidopsis thaliana using the H5 signature sequence from voltage-dependent K+ channels. FEBS Lett. 1996;378:19–26. doi: 10.1016/0014-5793(95)01417-9. [DOI] [PubMed] [Google Scholar]
- King LA, Possee RD. The Baculovirus Expression System: A Laboratory Guide. London: Chapman and Hall; 1992. [Google Scholar]
- Kopka J, Provart NJ, Müller-Röber B. Potato guard cells respond to drying soil by a complex change in the expression of genes related to carbon metabolism and turgor regulation. Plant J. 1997;11:871–882. doi: 10.1046/j.1365-313x.1997.11040871.x. [DOI] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J. 1996;9:195–203. doi: 10.1046/j.1365-313x.1996.09020195.x. [DOI] [PubMed] [Google Scholar]
- Landschütze V, Müller-Röber B, Willmitzer L. Mitochondrial citrate synthase from potato: predominant expression in mature leaves and young flower buds. Planta. 1995;196:756–764. [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:21–26. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Luckow VA, Summers MD. Trends in the development of baculovirus expression vectors. Bio/Technology. 1988;6:47–55. [Google Scholar]
- Macdonald H, Henderson J, Napier RM, Venis MA, Hawes C, Lazarus CM. Authentic processing and targeting of active maize auxin-binding protein in the baculovirus expression system. Plant Physiol. 1994;105:1049–1057. doi: 10.1104/pp.105.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten I, Gaymard F, Lemaillet G, Thibaud JB, Sentenac H, Hedrich R. Functional expression of the plant K+ channel KAT1 in insect cells. FEBS Lett. 1996;380:229–232. doi: 10.1016/0014-5793(96)00042-7. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Magnesium gating of the inwardly rectifying K+ channel. Annu Rev Physiol. 1991;53:289–298. doi: 10.1146/annurev.ph.53.030191.001445. [DOI] [PubMed] [Google Scholar]
- Maurel C, Reizer J, Schroeder JI, Chrispeels MJ. The vacuolar membrane protein gamma-tip creates water specific channels in Xenopus oocytes. EMBO J. 1993;12:2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Röber B, Ellenberg J, Provart N, Willmitzer L, Busch H, Becker D, Dietrich P, Hoth S, Hedrich R. Cloning and electrophysiological analysis of KST1, an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai A, Suzuki K, Ward E, Moyer M, Hashimoto M, Mano J, Ohta D, Scheidegger A. Overexpression of plant histidinol dehydrogenase using a baculovirus vector system. Arch Biochem Biophys. 1992;295:235–239. doi: 10.1016/0003-9861(92)90512-u. [DOI] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WL, Jr, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch-clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Robinson RA, Stokes RH. Electrolyte Solutions. London: Butterworths; 1965. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Ward JM, Gassmann W. Perspective on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Sheahan JJ, Ribeiro-Neto L, Sussman MR. Plant J. 1993;3:647–656. [Google Scholar]
- Shimazaki K, Iino M, Zeiger E. Nature. 1986;319:324–326. [Google Scholar]
- Sigel E. Use of Xenopus oocytes for the functional expression of plasma membrane proteins. J Membr Biol. 1990;117:201–221. doi: 10.1007/BF01868451. [DOI] [PubMed] [Google Scholar]
- Tester M. Plant ion channels: whole-cell and single-channel studies. New Phytol. 1990;114:305–340. doi: 10.1111/j.1469-8137.1990.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Vernet T, Tssier DC, Richardson C, Laliberté F, Khouri HE, Bell AW, Storer AC, Thomas DY. Secretion of functional papain precursor from insect cells. J Biol Chem. 1990;265:16661–16666. [PubMed] [Google Scholar]
- Véry AA, Bosseux C, Gaymard F, Sentenac H, Thibaud JB. Level of expression in Xenopus oocytes affects some characteristics of a plant inward-rectifying voltage-gated K+ channel. Pflugers Arch. 1994;428:422–424. doi: 10.1007/BF00724528. [DOI] [PubMed] [Google Scholar]
- Véry AA, Gaymard F, Bosseux C, Sentenac H, Thibaud JB. Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J. 1995;7:321–332. doi: 10.1046/j.1365-313x.1995.7020321.x. [DOI] [PubMed] [Google Scholar]
- Yamada S, Katsuhara M, Kelly WB, Michalowski CB, Bohnert HJ. A family of transcripts encoding water channel proteins: tissue-specific expression in the common ice plant. Plant Cell. 1995;7:1129–1142. doi: 10.1105/tpc.7.8.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]