Abstract
Introduction:
Negative mood situations often increase smoking behavior and reward, effects that may be greater among women and smokers low in tolerance for distress.
Methods:
Adult dependent smokers (N = 164; 86 men, 78 women) first completed measures of distress tolerance via self-report and by mirror-tracing and breath-holding tasks. They then participated in 2 virtually identical laboratory sessions, involving induction of negative versus neutral mood (control) via pictorial slides and music. They rated negative affect (NA) before and during mood induction and smoked their preferred brand ad libitum during the last 14 min of mood induction. Our aim was to examine mood effects on NA, smoking reward (“liking”), and smoking intake (puff volume and number) as a function of sex and distress tolerance.
Results:
Negative mood induction increased NA, as planned, and smoking reward and intake compared with neutral mood. Increases in NA and puff volume due to negative mood were greater in women compared with men, as hypothesized, but no main effects of the self-report or behavioral distress tolerance measures were seen in responses to mood induction. However, unexpectedly, lower self-reported distress tolerance was associated with greater smoking intake due to negative (but not neutral) mood in men and generally due to neutral (but not negative) mood in women.
Conclusions:
Negative mood may increase smoking intake more in women compared with men. Yet, low distress tolerance may enhance smoking intake due to negative versus neutral mood differentially between women and men, suggesting that sex and distress tolerance may interact to influence smoking responses to negative mood.
Introduction
Experiencing negative mood promotes smoking persistence (Gehricke et al., 2007), strongly predicts relapse (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004), and may lead to over half of all lapses after smoking quit attempts (Shiffman & Waters, 2004). Similarly, laboratory studies show that negative mood acutely increases craving for cigarettes (Perkins & Grobe, 1992) and amount of smoking behavior (e.g., Conklin & Perkins, 2005; Rose, Ananda, & Jarvik, 1983), although some research shows no such effects of negative mood (e.g., Weinberger & McKee, 2011). Smoking responses to negative mood vary in magnitude between smokers (Gilbert, 1995), and relatively few controlled studies have examined these individual differences.
Increased smoking behavior in response to negative mood may be greater in those less able to handle subjective feelings of negative affect (NA), such as smokers low in distress tolerance (Brown, Lejuez, Kahler, Strong, & Zvolensky, 2005). Low distress tolerance reflects several characteristics related to lack of persistence with difficult or frustrating tasks that elicit psychological or physical discomfort, wanting to do anything to stop distress or feeling upset, etc. (Leyro, Zvolensky, & Bernstein, 2010; Simons & Gaher, 2005). Preventing or relieving feelings of NA long has been viewed as a key factor in smoking persistence, perhaps due to disrupted ability to process information needed for goal-directed behavior arising from the resulting distress (e.g., Baker et al., 2004). For example, smokers who feel less able to tolerate adverse emotional experiences may be more prone to obtain relief by smoking in response to the experience, independent of their degree of tobacco withdrawal or even despite a desire to quit (Brown et al., 2005, 2009).
Perhaps consistent with this notion, smokers lower in distress tolerance report greater NA in general (Abrantes et al., 2008) and increased risk for smoking relapse (e.g., Brown, Lejuez, Kahler, & Strong, 2002; Brown et al., 2009). In one prospective study, smokers who were less persistent in continuing with a frustrating task (mirror tracing) were less likely to remain abstinent 1 year after attempting to quit smoking (Brandon et al., 2003). However, it is not clear that lower distress tolerance increases smoking behavior in response to negative mood that arises from situations other than tobacco abstinence, such as a stressor (Perkins, Karelitz, Giedgowd, Conklin, & Sayette, 2010; see also Baker et al., 2004). Perhaps similarly, research also questions whether nicotine via smoking relieves NA that is due to sources other than abstinence (Kassel, Stroud, & Paronis, 2003) or if NA influences maintenance of smoking in the natural environment (e.g., Shiffman et al., 2002). Therefore, we examined differences in smoking responses to negative mood induction in nonabstinent smokers varying in distress tolerance.
Subject sex also may be an individual difference influencing smoking behavior in response to negative mood. Women may initiate smoking faster than men during negative mood induction (Weinberger & McKee, 2011), although actual smoke intake may not differ by sex (Fucito & Juliano, 2009; Weinberger & McKee, 2011). Other research has shown that women report greater NA responses to overnight abstinence and greater relief of that NA upon smoking a single cigarette compared with men (Xu et al., 2008). Uncertain, then, is whether sex differences may influence actual smoke intake in response to negative mood, especially in environmental situations other than abstinence. Moreover, an interaction of sex and distress tolerance on smoking in response to negative mood is uncertain, although other research suggests that low distress tolerance increases risk of problems with alcohol in men and not women (Simons & Gaher, 2005).
This study examined the separate and combined influences of subject sex and distress tolerance on NA, smoking reward (“liking”), and smoke intake (puff number and puff volume in ml) in response to negative mood induction compared with neutral mood control. We hypothesized that these responses to negative mood would be greater in women and among those lower in distress tolerance. We also examined, but did not hypothesize, the possibility of an interaction of sex with distress tolerance. Although both factors together would be expected to produce the greatest responses to negative mood in women low in distress tolerance, other research on smoking cessation treatment attendance suggests that sex and distress tolerance may interact (MacPherson, Stipleman, Duplinsky, Brown, & Lejuez, 2008).
Methods
Participants
Participants were 164 adults required to smoke 10 or more cigarettes per day for at least 1 year and who met the DSM-IV criteria for tobacco dependence (updated from Breslau, Kilbey, & Andreski, 1994). All were recruited through advertisements in the surrounding community and paid $150 for completing the study (plus a small amount for performance on mirror tracing; see below). Means (±SD) for the 86 men and 78 women, respectively, were 28.6 (±11.2) and 28.3 (±9.9) years for age, 16.7 (±5.8) and 16.2 (±5.0) for cigarettes per day, and 4.4 (±2.2) and 4.8 (±1.9) for Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), indicating moderate dependence. Most participants were Caucasian (76%), with 13% as Black, 3% Asian, about 1% each as Hispanic or Native American, and the remaining (6%) as more than one race. Men and women did not differ on any of these characteristics. Excluded were those wanting to quit smoking and those reporting current or recent (past year) depression or other major psychiatric problems requiring treatment.
Assessment of Distress Tolerance
Because research has suggested differences between self-report and behavioral measures of distress tolerance (e.g., Leyro et al., 2010; McHugh et al., 2011), we assessed two behavioral measures related to distress tolerance, in addition to a self-report measure (Simons & Gaher, 2005). These were mirror-tracing persistence time and breath-holding duration, measures that reflect affective (psychological) and somatic (physical) distress (McHugh et al., 2011) and have been related to likelihood of relapse (Brandon et al., 2003; Hajek, Belcher, & Stapleton, 1987). All were assessed during the initial 2-hr screening session after smoking as usual (see Procedure).
Self-report
The self-reported Distress Tolerance Scale (DTS; Simons & Gaher, 2005) consists of 15 items in four subscales that assessed perceived ability to tolerate emotional distress, subjective appraisal of distress, attention being absorbed by negative emotions, and regulation of efforts to relieve distress. Each item was rated on a 1–5 scale, anchored by strongly agree to strongly disagree, respectively (with 3 anchored by agree and disagree equally) to create a single total DTS score (see Simons & Gaher, 2005).
Behavioral (Mirror Tracing, Breath Holding)
A computerized mirror-tracing task was employed (e.g., McHugh et al., 2011), in which subjects were instructed to use a computer mouse to move a cursor around a monitor to trace over a star shape without leaving the shape’s lines. They first learned the task through simpler “practice” attempts and were told they would earn a small amount of money per second they persisted on the mirror-tracing task, in addition to the $150 for study participation, but could end the task at any time by pressing any key on the keyboard. A buzzing noise was presented when errors were made (i.e., the cursor left the lines shown on the monitor), and the subject had to start the task over. Persistence, adjusted for number of errors, was determined by when the subject pressed the keyboard, since all did so (i.e., no one was able to complete the task). For breath holding, subjects were instructed to hold their breath for as long as they could while wearing a nose clip (to ensure compliance with breath holding). An experimenter assessed the duration with a stopwatch, and monetary reinforcement was not provided for the breath-holding task. These measures were based on procedures described by others (e.g., Brandon et al., 2003; Brown et al., 2009; Hajek et al., 1987; Strong et al., 2003).
Mood Induction
Negative mood was induced by combining pictorial stimulus slides via computer with mood-congruent classical music, with high arousal “negative” slides adapted from the widely used International Affective Picture System (IAPS; Lang, Ohman, & Vaitl, 1988). Neutral mood was induced very similarly, with use of “positive” slides from the IAPS and pleasant music to maintain participants’ generally pleasant mood at baseline (BL) due to their recent smoking (see below). These methods of mood induction maintain standardization of procedures and robustly differ in reported affect, as reported elsewhere (Conklin & Perkins, 2005; Perkins et al., 2008; Perkins et al., 2010).
Negative Affect
Negative affect was assessed via the Mood Form of Diener and Emmons (1984), which contains five Visual Analog Scale items rated from 0 (not at all) to 100 (very much) that yield a NA score. NA scale items are “depressed/blue,” “unhappy,” “frustrated,” “worried/anxious,” and “angry/hostile.” Research has shown the validity of this measure for assessing self-reported NA in response to manipulations of mood conditions similar to those used here (e.g., Conklin & Perkins, 2005; Perkins et al., 2010). Although the Mood Form also assesses positive affect, NA was the focus here because of the objective of comparing responses due to negative mood induction (vs. neutral) under nonabstinent conditions.
Smoking Intake
All smoking was done via the Clinical Research Support System pocket version (CReSS; Borgwaldt KC, Inc., Richmond VA; www.plowshare.com), which allows for assessment of puff volume (in ml) and number, provides puff intake similar to that from smoking without the device, and has been used in many laboratory studies of smoking (e.g., Blank, Disharoon, & Eissenberg, 2009; Perkins et al., 2010). These smoking intake measures are each highly reliable under similar conditions (intraclass correlations of .90 and .92, both p < .001, for puff volume and puff number, respectively; Perkins, Karelitz, Giedgowd, & Conklin, 2011).
Procedure
This study involved three 2-hr sessions for each participant, the first for screening and then two in which negative versus neutral mood induction was manipulated within subjects and in counterbalanced order. At the initial screening session, participants provided informed consent and completed screening procedures and the self-report and behavioral assessments of distress tolerance after having smoked as usual prior to the session. They then engaged in the two virtually identical experimental sessions, varying only in whether negative or neutral (control) mood was induced. Participants smoked as desired prior to each session and one cigarette of their own brand upon arrival. This procedure ensured that negative mood induction could not be attributed to recent smoking abstinence but rather to the mood induction manipulation itself, which was aimed at producing a negative mood situation that was not attributable to smoking abstinence.
Each of the two experimental mood induction sessions began with a BL assessment of NA. Following the first 4 min of induction of negative or neutral mood, NA was assessed to gauge response to mood induction alone (postinduction 1, or PI1), relative to BL. Mood induction continued, and subjects took six puffs on their preferred brand via the CReSS to assess smoking reward by rating the cigarette for liking on a single item from the Cigarette Evaluation Scale (Westman, Behm, & Rose, 1996), “How much did you like the puffs you just took?”, scored 0–100 (anchored by not at all to very much, respectively). This measure of reward has high reliability (e.g., intraclass correlation of .88, p < .001, across four similar sessions in Perkins et al., 2011). These six puffs were taken via computerized instructions on puff number and timing to standardize smoke exposure between sessions (see Perkins et al., 2008). After continuing mood induction an additional 4 min, NA was again assessed to determine responses to this standard amount of smoke exposure (postinduction 2, or PI2). Subjects were then given five of their preferred brand of cigarettes (to make sure they did not run out) and allowed to smoke ad libitum over the last 14 min of the mood induction procedure. Smoking behavior (volume of puff inhalation in ml, puff number) during this period was the measure of smoke intake. NA was assessed a final time at the end of this ad lib smoking period, ending the session (post-ad lib, or PAL). This study was approved by the University of Pittsburgh Institutional Review Board.
Data Analyses
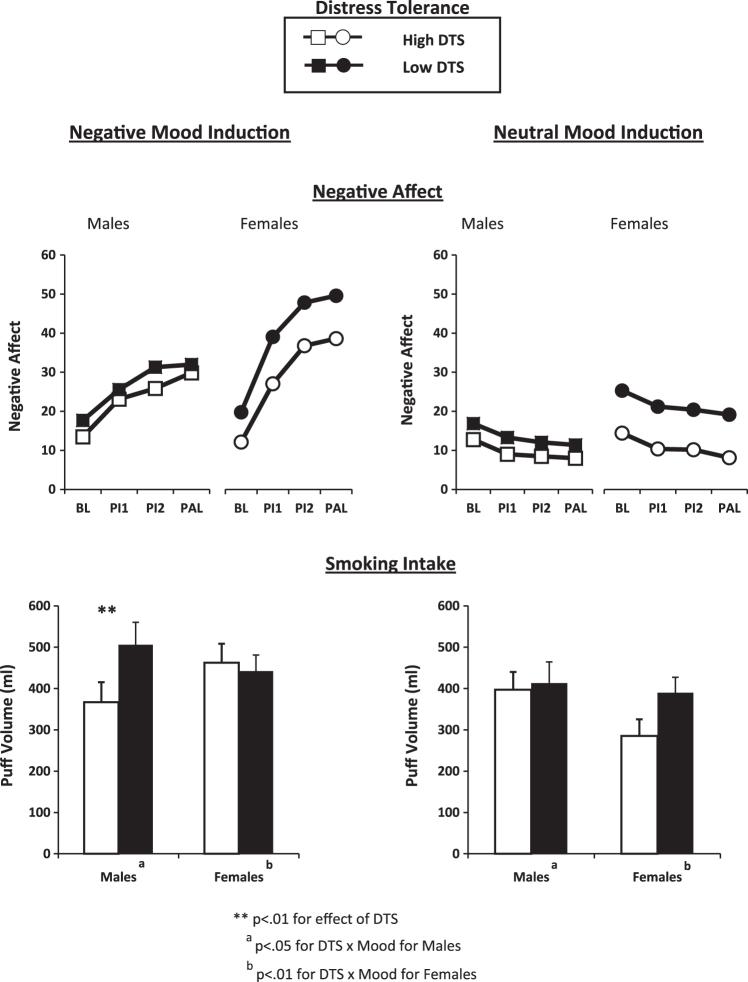
Repeated measures analyses of variance (ANOVAs) first determined NA in response to negative versus neutral mood induction to verify that the mood induction manipulation produced the intended within-subjects difference in NA for men and for women. Similar analyses were conducted for effects of cigarette liking (reward) in response to the six standard puffs (at PI2), and for ad libitum smoking intake (puff volume and number; at PAL), all during mood induction. Analyses of covariance (ANCOVAs) were then used to assess the association of distress tolerance measures with NA, smoking reward, or smoking intake due to mood induction, with the scores for each distress tolerance measure as a continuous factor, and FTND as a covariate. For all analyses, we examined main and interaction effects involving sex, with least-significant difference t tests or other follow-ups performed where appropriate (Huitema, 1980). (For ease of display purposes only, graphs in Figure 2 are presented separately by “high” and “low” scores on DTS, determined by median split separately by sex. As noted, however, all formal data analyses examined the influence of each distress tolerance measure as a continuous variable, and not as a dichotomous split.)
Figure 2.
Negative affect (NA) and smoking intake (puff volume) of men and women in response to negative versus neutral mood induction, by higher versus lower self-reported distress tolerance score (DTS). The main effect of DTS was significant for NA but not other responses. DTS was analyzed as a continuous factor but presented here by higher and lower scores (median split) for clarity of presentation.
Results
NA, smoking reward, and smoking intake are presented in Figure 1 by negative versus neutral mood induction, separately for men and women. Induction of negative mood was clearly demonstrated by the greater NA responses to the negative (vs. neutral) mood condition, F(1, 159) = 65.00, p < .001, for the Mood × Time interaction. Subjects did not differ in NA at BL, prior to negative and neutral mood induction (M ± SE of 15.3 ± 1.2 vs. 17.1 ± 1.4 respectively), and NA increased in response to negative mood induction but not to neutral mood induction (Figure 1), as planned.
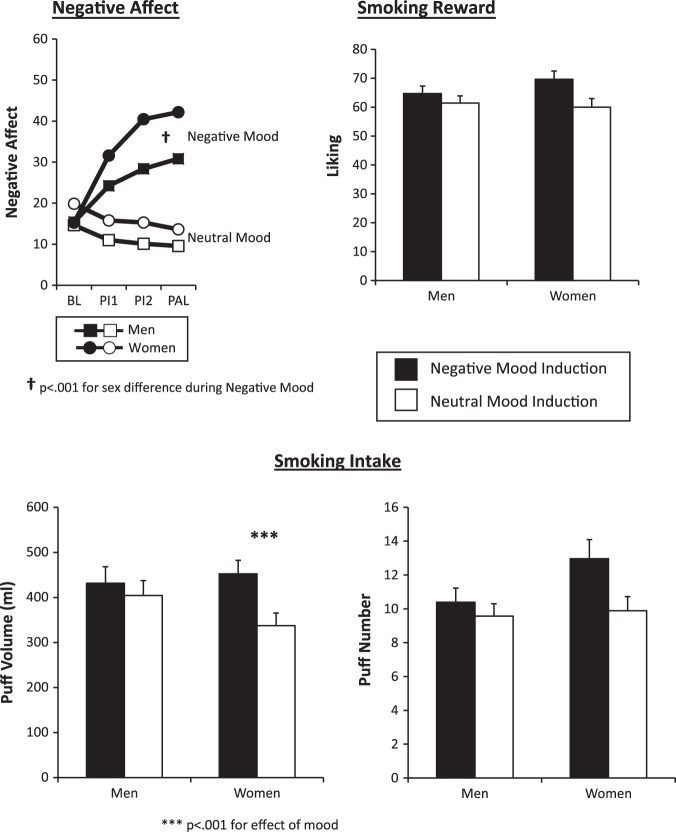
Figure 1.
The effects of negative versus neutral mood induction on negative affect, smoking reward (cigarette “liking”), and smoke intake (puff volume in ml, puff number) for men and women. The main effect of mood condition was significant for each of these responses.
Responses for Men and Women
For NA, the interaction of Sex × Mood × Time was significant, F(3, 159) = 3.93, p < .01, as the increase in NA from BL due to the negative mood induction was greater for women versus men, F(1, 162) = 13.89, p < .001, while there was no sex difference in NA response to neutral mood, F(1, 162) < 1 (Figure 1). Regarding smoking responses to mood, smoking reward (liking) was significantly greater during negative versus neutral mood, F(1, 161) = 12.77, p < .001. This reward effect tended to be greater in women than in men, although the interaction of Sex × Mood was only marginally significant, F(1, 161) = 2.99, p = .086. Smoking intake from the ad libitum period was also greater during negative versus neutral mood, whether measured by M (SE) intake of puff volume in ml (441.5 ± 23.8 vs. 372.5 ± 22.1; F(1, 162) = 9.83, p < .01) or in number of puffs (11.7 ± 0.7 vs. 9.7 ± 0.6, F(1, 162) = 9.00, p < .01). Moreover, the increase in smoke intake due to negative mood was greater in women than men, when assessed by puff volume, F(1, 162) = 3.73, p = .05, although only marginal when assessed by puff number, F(1, 162) = 3.05, p = .083. This Sex × Mood interaction was due to a significant influence of negative mood on increasing puff volume in women, t(77) = 3.50, p < .001, but not in men, t(85) < 1 (Figure 1). Thus, smoking reward and intake were increased by negative (vs. neutral) mood induction, and these smoking effects due to mood tended to be greater in women versus men, similar to the sex difference in NA response to negative mood induction.
In exploratory analyses, we examined whether a greater increase in NA due to negative versus neutral mood was associated with the observed subsequent greater smoking reward or intake due to the different mood induction conditions in all subjects (all r[162], see Figure 1). The increase from BL in NA due to initial negative (vs. neutral) mood induction (before smoking, i.e., BL to PI1) was modestly but significantly correlated with the subsequently greater smoking reward (r = .15, p < .05) and smoking intake (r = .21, p < .01 for puff volume, and r = .19, p < .02 for puff number) due to negative mood. In similar comparisons, the greater smoking reward (at PI2) due to negative (vs. neutral) mood correlated significantly with the corresponding greater smoking puff volume and puff number (at PAL) due to negative mood (r = .40 and .32, respectively, both p < .001).
Responses by Distress Tolerance
Self-report DTS
On the DTS measure, women reported lower tolerance of distress than men, 3.25 (±0.88) versus 3.56 (±0.79), respectively, F(1, 162) = 5.75, p < .02, as reported by others (Simons & Gaher, 2005). NA and puff volume intake responses to mood induction as a function of lower versus higher DTS (analyzed in continuous fashion) are presented by men and women in Figure 2. The main effect of DTS was significant for NA, F(1, 160) = 12.14, p < .001, regardless of mood condition, but the interactions of DTS × Mood and of DTS × Mood × Sex on NA were not significant, both F(1, 160) < 1. The interaction of DTS × Sex also was not significant, F(1, 160) = 1.45, p > .20.
The main and interaction effects of DTS on smoking reward were not significant, all F(1, 160)’s < 1 (and so not shown in Figure 2). However, regarding smoking intake, the interaction of DTS × Mood × Sex was significant for both smoke volume, F(1, 160) = 11.30, p < .001, and puff number, F(1, 160) = 11.05, p < .001. (Because results were essentially the same for both puff volume and puff number, which were highly correlated, r = .87, p < .001, only results for puff volume by DTS are shown in Figure 2.) For men, DTS × Mood was significant for puff volume, F(1, 84) = 4.27, p < .05, and puff number, F(1, 84) = 5.52, p < .05. Similarly, among women, DTS × Mood was also significant for puff volume, F(1, 76) = 8.06, p < .01, and puff number, F(1, 76) = 5.56, p < .05. Unexpectedly, these DTS × Mood interactions tended to differ in opposite directions between men and women. As shown in Figure 2, lower distress tolerance in men increased smoke intake via puff volume due to negative mood, F(1, 84) = 12.15, p < .01, but not neutral mood, F(1, 84) = 2.40, p > .10. By contrast, lower distress tolerance in women marginally increased puff volume due to neutral mood, F(1, 76) = 3.04, p = .085, but had no effect during negative mood, F(1, 76) = 1.10, p > .25. (Note that the only exception between these puff number and puff volume results was that lower distress tolerance in women did significantly increase puff number due to neutral mood, F(1, 76) = 7.56, p < .01.
Behavioral Tasks
In separate analyses, the mirror-tracing and breath-holding tasks examining distress tolerance were also analyzed for associations with these responses to negative mood induction. None of the dependent measures of NA, smoking reward, or smoke intake (puff volume or number) were influenced by scores on mirror tracing (all F’s < 1.11, all p > .29) or breath holding (all F’s < 1.31, all p > 0.25). Mean (±SD) responses for men versus women, respectively, were 347.7 ± 444.6 versus 276.7 ± 464.5 s for mirror-tracing persistence and 57.2 ± 17.7 versus 49.5 ±18.2 s for breath-holding duration, although no sex differences were significant. These responses are comparable with those reported in other research (e.g., McHugh et al., 2011). For all 164 participants, breath-holding duration was significantly correlated with both mirror-tracing time (r = .36, p < .001) and with self-report DTS score (r = .18, p < .02), but mirror tracing was not significantly correlated with DTS score (r = .05, p > .10).
Discussion
Results of our overall findings due to mood induction, excluding the individual difference factors of interest, demonstrated that negative mood increases smoking reward and smoking intake, consistent with some similar studies (Conklin & Perkins, 2005) but not others (e.g., Weinberger & McKee, 2011). As shown in Figure 1, our results are also consistent with prior findings that smoking behavior during causes of negative mood other than tobacco abstinence fails to result in subsequent relief of NA (e.g., Perkins et al., 2010; see also Baker et al., 2004 and Kassel et al., 2003).
Regarding the factors of primary interest, we first discuss the findings due to subject sex and then those due to distress tolerance, since the latter also clearly involved sex differences. Importantly, NA and smoke intake responses to negative mood were generally greater in women compared with men, as hypothesized. Because exploratory analyses showed that the increase in NA due to negative mood was correlated with subsequent smoking reward and reward was correlated with subsequent smoke intake, the greater smoking response to negative mood in women versus men may stem from their greater NA response to negative mood. The sex differences in NA responses may be consistent with prior research indicating that compared with men, women report more severe NA in response to overnight tobacco abstinence (Xu et al., 2008) and that they are less able to manage NA during cessation (McKee, O’Malley, Salovey, Krishnan-Sarin, & Mazure, 2005). However, the fact that our mood induction procedure intentionally avoided effects due to tobacco abstinence indicates that this sex difference in the NA responses of smokers may extend to other causes of negative mood situations.
The smoking intake results may expand the breadth of sex differences in responses to negative versus neutral mood by suggesting that women increase smoke intake more than men under such conditions, which has often not been found in between-groups studies of mood (Fucito & Juliano, 2009; Weinberger & McKee, 2011). Our use of a within-subjects design may have enhanced the power to detect mood differences by sex, since each subject acted as his or her own control between mood conditions. On the other hand, future research should examine whether similar sex differences in smoking responses are observed with other specific causes of negative mood to determine the generalizability of our sex difference findings.
Our results for distress tolerance, by contrast, were very different than those above for sex differences alone. We found that NA during either mood induction condition was greater for those lower in distress tolerance as assessed by the self-report DTS (Figure 2), similar to other research (Abrantes et al., 2008). Yet, we found no differences due to DTS or the interaction of DTS by sex in the NA response to negative mood per se. So, contrary to our hypothesis, lower distress tolerance, whether assessed by the self-report or behavioral tasks, did not increase NA or smoking responses specifically to this nonabstinent cause of negative mood induction (compared with neutral mood), consistent with some prior research (Perkins et al., 2010).
Notably, however, smoke intake responses to mood tended to run in opposing directions when we combined the factors of DTS and subject sex. The interaction of DTS by mood on smoke intake (puff volume and number) was significant for men and for women but in contrasting manner. While lower distress tolerance in men was related to greater smoke intake during negative mood and not neutral mood, generally the opposite interaction was observed in women. For women, lower distress tolerance was related to greater puff volume (marginally) and puff number (significantly) during neutral mood and not at all related during negative mood. One potential implication of these results is that distress tolerance and subject sex do not combine in additive fashion to increase smoking responses to negative mood.
An explanation for this pattern of sex differences in the way distress tolerance influences smoking responses to mood is not obvious, particularly since the increase in smoke intake due to negative mood was significantly greater in women compared with men, collapsing across DTS (bottom of Figure 1). Women lower in distress tolerance may be more likely to smoke maximally during either mood condition when given the opportunity (i.e., “ceiling effect”). If so, a further increase in smoking due to negative mood might be difficult within a prescribed period of time (e.g., the 14-min ad libitum smoking period of this study). By comparison, women higher in distress tolerance may generally smoke less during neutral mood, allowing greater opportunity to increase smoke intake during the negative mood condition (see bottom of Figure 2). Further research should examine different intensities of negative mood induction, as well as different types and durations of induction procedures, to determine whether the sex difference in effects of distress tolerance on smoking may depend on severity or type of negative mood induction and the duration of smoking access.
Regarding the different assessments of distress tolerance, we found no effects of distress tolerance as measured by the behavioral tasks of mirror-tracing and breath-holding duration compared with some smoking intake results for men and women assessed by the DTS self-report measure of distress tolerance. In addition, although breath-holding duration was correlated with mirror-tracing and self-reported DTS score, DTS and mirror tracing were not significantly correlated, confirming a clear difference between self-report and some task measures of distress tolerance (McHugh et al., 2011).
One clear strength of this study was the greater statistical power due to use of a within-subjects design, to allow for stronger comparison of responses due to negative versus neutral mood induction. A similar strength was use of a fairly large sample, so that we could examine sex differences and distress tolerance, separately and combined, on responses to mood. Third, smokers smoked their own nicotine cigarette brand in unblinded fashion to better capture how these participants might respond to negative mood in the natural environment. Our use of both self-report and behavioral measures of distress tolerance may also be a strength (McHugh et al., 2011), although distress tolerance may have had greater effects on smoking responses to mood if it had been assessed with other measures (e.g., Brown et al., 2009).
Limitations of this research include the possibility that stronger effects of distress tolerance on smoking responses to negative mood may have been found in smokers with current mood dysregulation problems (e.g., major depression, panic disorder; see Fucito & Juliano, 2009) or in smokers preparing to quit (Brown et al., 2009). Also, as noted, our results could differ if smoking is assessed over a longer duration of exposure to negative mood or if negative mood varies in intensity or is caused by other types of situations. Results may differ as well for smokers who are older or generally higher in dependence than our sample or if subjects’ distress tolerance level is assessed after a period of tobacco abstinence rather than smoking as usual (Bernstein, Trafton, Ilgen, & Zvolensky, 2008). Finally, although smoking here involved access to one’s own brand without blinding, to increase generalizability to smoking in the natural environment, findings could differ if smokers are blind to cigarette brand.
In conclusion, NA and smoking in response to experiencing negative versus neutral mood may be greater in women than men. Low distress tolerance was related to heightened NA under both mood conditions. Although distress tolerance may not have effects on the responses of all smokers to this negative mood induction, it may moderate smoking responses to mood conditions differently between women and men. Thus, the influences of subject sex and distress tolerance on smoking responses to negative mood may not be additive but rather interact in unexpected fashion.
Funding
This research was supported by National Institutes of Health Grants DA027449 and DA031218.
Declaration of Interests
No authors have any disclosures.
Acknowledgments
The authors thank David Strong, Ph.D., for providing information on assessing distress tolerance via behavioral tasks.
References
- Abrantes AM, Strong DR, Lejuez CW, Kahler CW, Carpenter LL, Price LH, et al. The role of negative affect in risk of early lapse among low distress tolerance smokers. Addictive Behaviors. 2008;33:1394–1401. doi: 10.1016/j.addbeh.2008.06.018. doi:10.1016/j.addbeh.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;1:33–51. doi: 10.1037/0033-295X.111.1.33. doi:10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Trafton J, Ilgen M, Zvolensky MJ. An evaluation of the role of smoking context on a biobehavioral index of distress tolerance. Addictive Behaviors. 2008;33:1409–1415. doi: 10.1016/j.addbeh.2008.06.003. doi:10.1016/j.addbeh.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation. Nicotine & Tobacco Research. 2009;11:896–903. doi: 10.1093/ntr/ntp083. doi:10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology. 2003;112:448–456. doi: 10.1037/0021-843x.112.3.448. doi:10.1037/0021-843X.112.3.448. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. DSM-IIIR nicotine dependence in young adults: Prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. doi: 10.1111/j.1360-0443.1994.tb00960.x. doi:10.1111/j.1360-0443.1994.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111:180–185. doi:10.1037//0021-843X.111.1.180. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clinical Psychology Review. 2005;25:713–733. doi: 10.1016/j.cpr.2005.05.003. doi:10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, et al. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11:439–502. doi: 10.1093/ntr/ntp041. doi:10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. Journal of Abnormal Psychology. 2005;114:153–164. doi: 10.1037/0021-843X.114.1.153. doi:10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA. The independence of positive and negative affect. Journal of Personality and Social Psychology. 1984;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. doi:10.1037/0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Juliano LM. Depression moderates smoking behavior in response to sad mood induction. Psychology of Addictive Behaviors. 2009;23:546–551. doi: 10.1037/a0016529. doi:10.1037/a0016529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehricke J.-G., Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, et al. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine & Tobacco Research. 2007;9(Suppl. 4):S523–S536. doi: 10.1080/14622200701685039. doi:10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Gilbert DG. Smoking: Individual differences, psychopathology, and emotion. London: Taylor and Francis; 1995. [Google Scholar]
- Hajek P, Belcher M, Stapleton J. Breath-holding endurance as a predictor of success in smoking cessation. Addictive Behaviors. 1987;12:285–288. doi: 10.1016/0306-4603(87)90041-4. doi:10.1016/0306-4603(87)90041-4. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K.-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. ISSN: 09520481. [DOI] [PubMed] [Google Scholar]
- Huitema BE. The analysis of covariance and alternatives. New York: John Wiley & Sons; 1980. [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. doi:10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Ohman A, Vaitl D. The International affective picture system. [Photographic slides] Gainesville, FL: Center for the Study of Emotion and Attention, University of Florida; 1988. [Google Scholar]
- Leyro TM, Zvolensky MJ, Bernstein A. Distress tolerance and psychopathological symptoms and disorders: A review of the empirical literature among adults. Psychological Bulletin. 2010;136:576–600. doi: 10.1037/a0019712. doi:10.1037/a0019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Stipleman BA, Duplinsky M, Brown RA, Lejuez CW. Distress tolerance and pre-smoking treatment attrition: Examination of moderating relationships. Addictive Behaviors. 2008;33:1385–1393. doi: 10.1016/j.addbeh.2008.07.001. doi:10.1016/j.addbeh.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Daughters SB, Lejuez CW, Murray HW, Hearon BA, Gorka SM, et al. Shared variance among self-report and behavioral measures of distress intolerance. Cognitive Therapy and Research. 2011;35:266–275. doi: 10.1007/s10608-010-9295-1. doi:10.1007/s10608-010-9295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O’Malley SS, Salovey P, Krishnan-Sarin S, Mazure C. Perceived risk and benefits of smoking cessation: Gender-specific predictors of motivation and treatment outcome. Addictive Behaviors. 2005;30:423–425. doi: 10.1016/j.addbeh.2004.05.027. doi:10.1016/j.addbeh.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. British Journal of Addiction. 1992;87:1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. ISSN: 09520481. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Ciccocioppo M, Conklin C, Milanak M, Grottenthaler A, Sayette M. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. Journal of Abnormal Psychology. 2008;117:79–93. doi: 10.1037/0021-843X.117.1.79. doi:10.1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine & Tobacco Research. 2011 doi: 10.1093/ntr/ntr150. doi:10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology. 2010;210:25–34. doi: 10.1007/s00213-010-1811-1. doi:10.1007/s00213-010-1811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addictive Behaviors. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. doi:10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, et al. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. doi:10.1037/0021-843X.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. doi:10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Simons JS, Gaher RM. The Distress tolerance scale: Development and validation of a self-report measure. Motivation and Emotion. 2005;29:83–102. doi:10.1007/s11031-005-7955-3. [Google Scholar]
- Strong DR, Lejuez CW, Daughters S, Marinello M, Kahler CW, Brown R. The computerized mirror tracing task version 1. 2003. Unpublished Manual. [Google Scholar]
- Weinberger AH, McKee SA. Gender differences in smoking following an implicit mood induction. Nicotine & Tobacco Research. 2011;13 doi: 10.1093/ntr/ntr198. doi:10.1093/ntr/ntr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology Biochemistry and Behavior. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. doi:10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Xu JS, Azizian A, Monterosso J, Domier C, Brody A, London E, et al. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine & Tobacco Research. 2008;10:1653–1661. doi: 10.1080/14622200802412929. doi:10.1080/14622200802412929. [DOI] [PMC free article] [PubMed] [Google Scholar]