Abstract
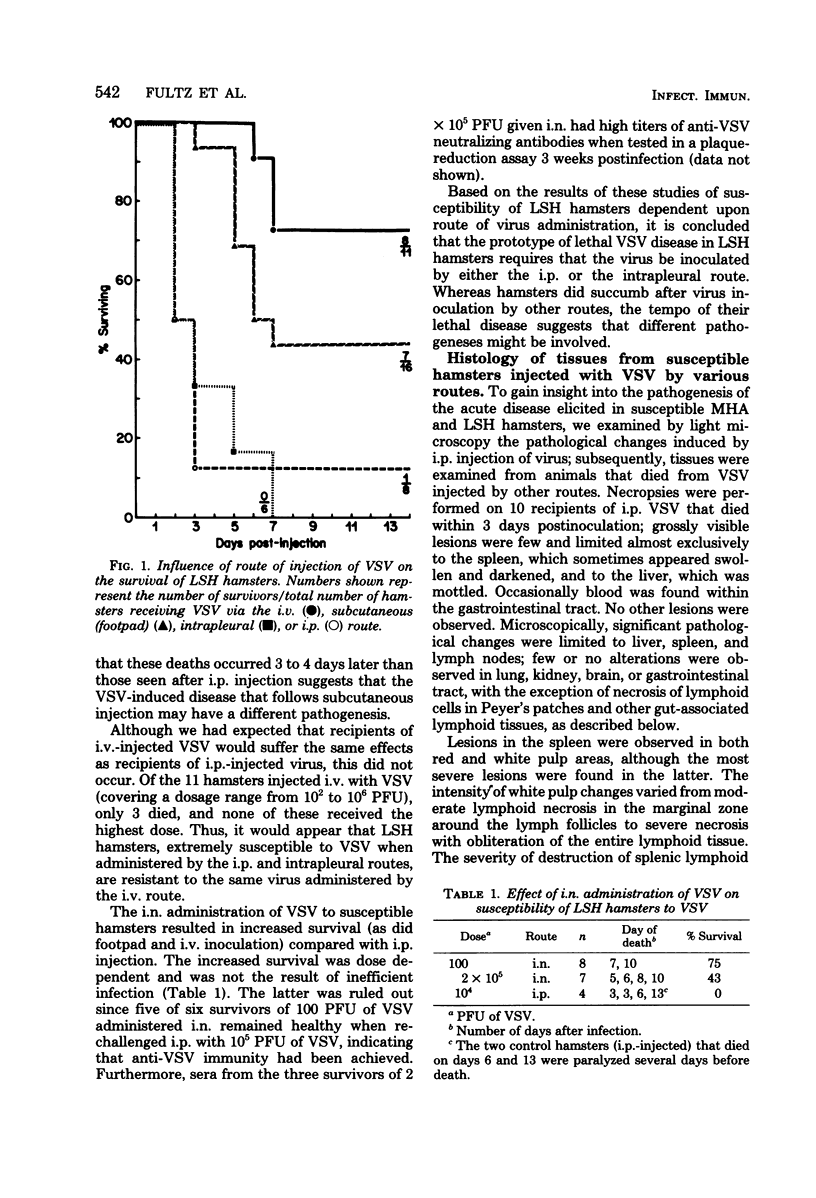
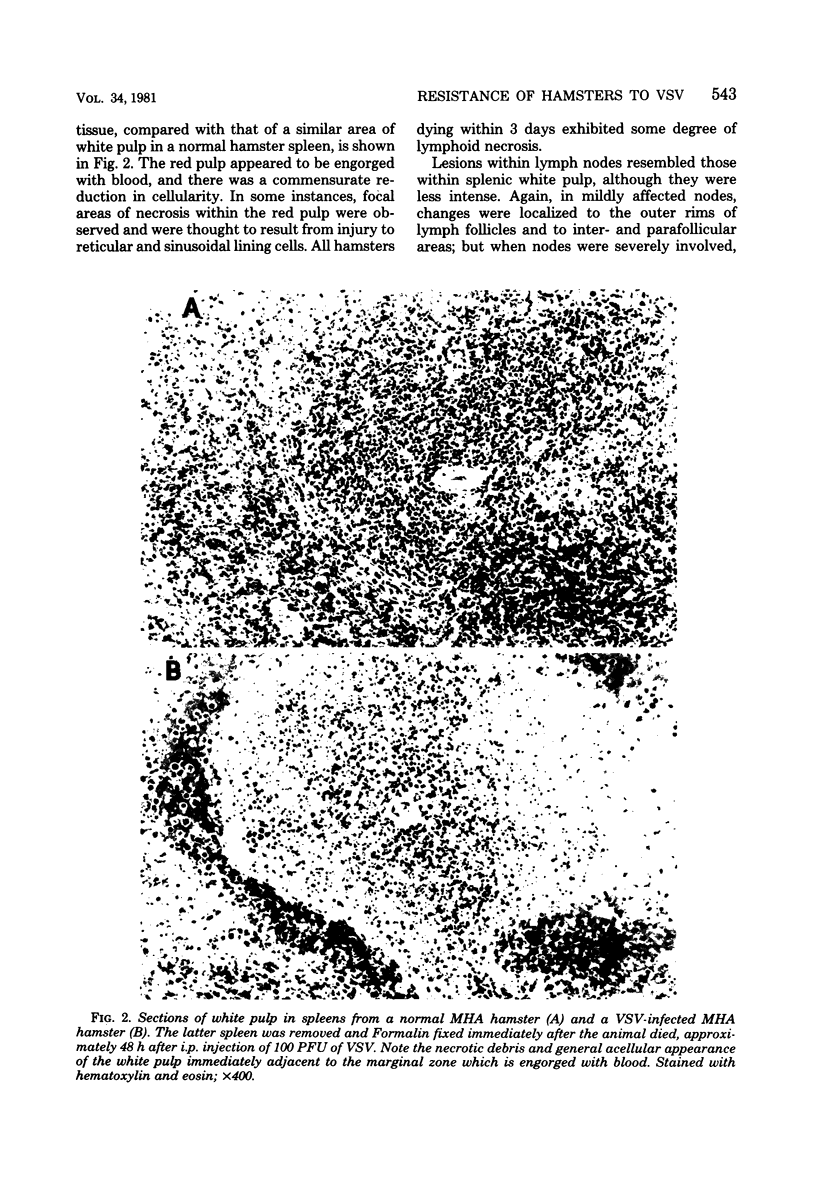
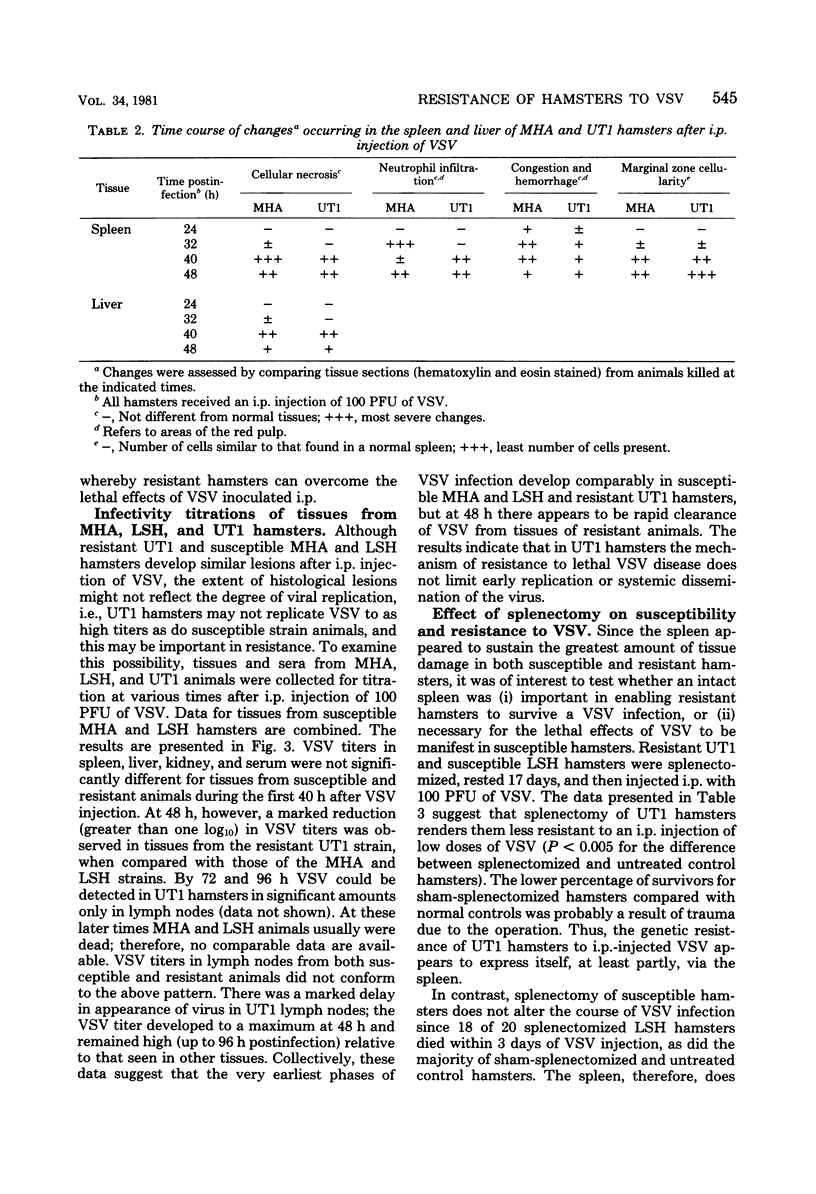
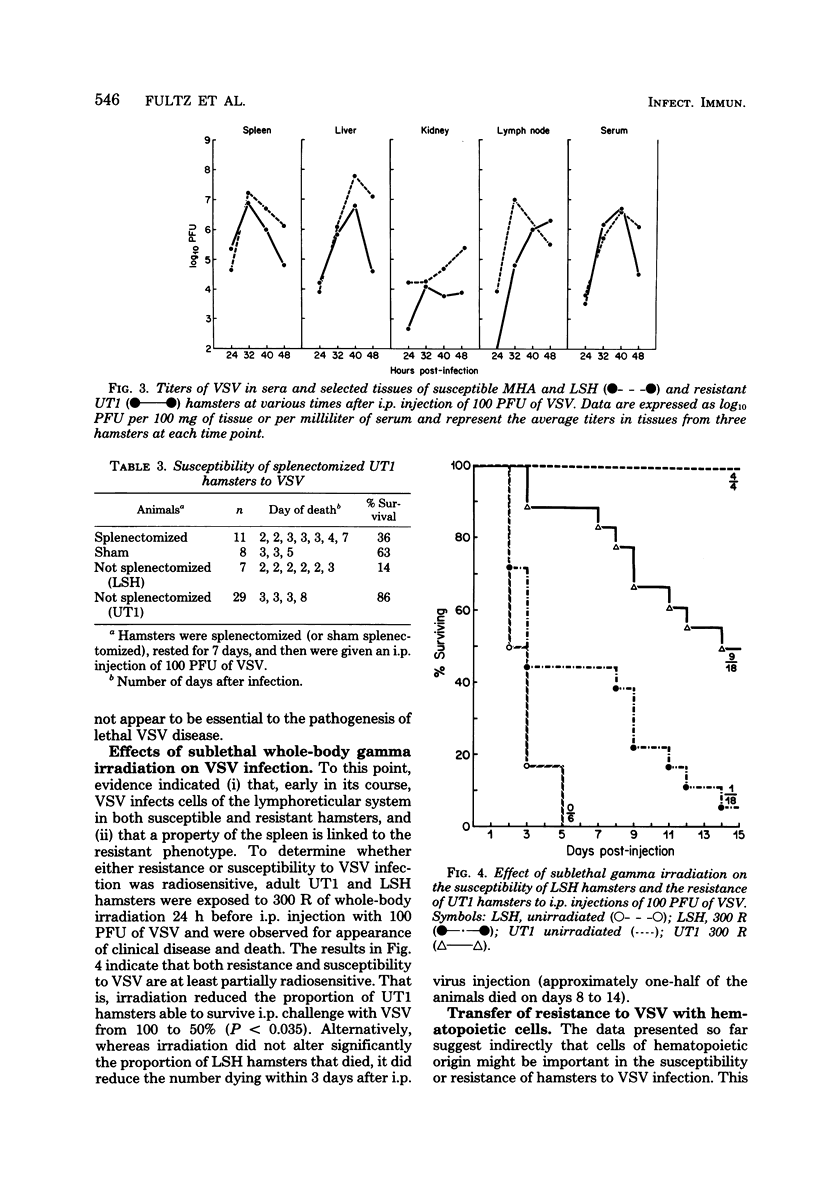
Susceptibility of Syrian hamsters of the inbred LSH and MHA strains to injection of as few as 10 plaque-forming units of vesicular stomatitis virus (VSV) was shown to occur only after intraperitoneal and intrapleural injection and not after injection of VSV intravenously, intranasally, or in the footpads. Despite the fact that fewer LSH hamsters died when VSV was injected via the latter routes, the histopathology of the VSV-induced disease at early times after infection was identical irrespective of the route of virus administration. Histological examination of tissues at various times after administration of VSV by the various routes revealed that VSV exhibited tropism for lymphoreticular tissue, with the greatest amount of necrosis in the splenic periarteriolar lymphoid sheath. A similar pattern also was observed in VSV-infected tissues from genetically resistant UT1 hamsters. Infectivity titrations of various tissues at different times after intraperitoneal injection of VSV revealed that resistant UT1 hamsters began to clear virus from tissues between 40 and 48 h postinfection, whereas virus titers remained high in susceptible animals. Resistance of UT1 hamsters appeared to require an intact spleen since survival of splenectomized animals was less than that of sham-splenectomized UT1 controls. Sublethal whole-body irradiation was also able to reduce resistance of UT1 hamsters (survival was reduced from 100 to 50%). Bone marrow cells from resistant (UT1 X LSH) F1 females were transferred into lethally irradiated susceptible LSH hamsters, and hematopoietic chimeras were produced. After intraperitoneal injection of 100 plaque-forming units of VSV, all of the female chimeras survived, but only 33% of male chimeras survived. These data indicate that resistance to VSV in Syrian hamsters is mediated, at least partially, by cells of hematopoietic origin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin F. J., Scherer W. F. Studies of viral virulence. I. Growth and histopathology of virulent and attenuated strains of Venezuelan encephalitis virus in hamsters. Am J Pathol. 1971 Feb;62(2):195–210. [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Rawls W. E. Variation between strains of hamsters in the lethality of Pichinde virus infections. Infect Immun. 1977 May;16(2):413–421. doi: 10.1128/iai.16.2.413-421.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Falke D., Rowe W. P. Die Erkrankung der Maus durch das Virus der Stomatitis vesicularis. I. Die Ausbreitung des Virus in Abhängigkeit vom Alter der Maus. Arch Gesamte Virusforsch. 1965;17(5):549–559. [PubMed] [Google Scholar]
- Fultz P. N., Shadduck J. A., Kang C. Y., Streilein J. W. Genetic analysis of resistance to lethal infections of vesicular stomatitis virus in Syrian hamsters. Infect Immun. 1981 Jun;32(3):1007–1013. doi: 10.1128/iai.32.3.1007-1013.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee S. R., Chan M. A., Clark D. A., Rawls W. E. Susceptibility to fatal Pichinde virus infection in the Syrian hamster. Adv Exp Med Biol. 1981;134:327–338. doi: 10.1007/978-1-4757-0495-2_29. [DOI] [PubMed] [Google Scholar]
- Gee S. R., Clark D. A., Rawls W. E. Differences between Syrian hamster strains in natural killer cell activity induced by infection with Pichinde virus. J Immunol. 1979 Dec;123(6):2618–2626. [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Gorelkin L., Jahrling P. B. Virus-initiated septic shock. Acute death of Venezuelan encephalitis virus-infected hamsters. Lab Invest. 1975 Jan;32(1):78–85. [PubMed] [Google Scholar]
- Jahrling P. B., Navarro E., Scherer W. F. Interferon induction and sensitivity as correlates to virulence of Venezuelan encephalitis viruses for hamsters. Arch Virol. 1976;51(1-2):23–35. doi: 10.1007/BF01317831. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Bloom B. R., Jones C., Holland J., Reid L. M. Mechanism of rejection of virus persistently infected tumor cells by athymic nude mice. J Exp Med. 1979 May 1;149(5):1117–1133. doi: 10.1084/jem.149.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Reid L., Cantor H., Lengyel P., Bloom B. R. Mode of regulation of natural killer cell activity by interferon. J Exp Med. 1980 Jul 1;152(1):124–137. doi: 10.1084/jem.152.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Buchmeier M. J., Rawls W. E. The reticuloendothelium as the target in a virus infection. Pichinde virus pathogenesis in two strains of hamsters. Lab Invest. 1977 Nov;37(5):502–515. [PubMed] [Google Scholar]
- Oehler J. R., Herberman R. B. Natural cell-mediated cytotoxicity in rats. III. Effects of immunopharmacologic treatments on natural reactivity and on reactivity augmented by polyinosinic-polycytidylic acid. Int J Cancer. 1978 Feb 15;21(2):221–229. doi: 10.1002/ijc.2910210214. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Lindsay L. R., Nunn M. E., Holden H. T., Herberman R. B. Natural cell-mediated cytotoxicity in rats. II. In vivo augmentation of NK-cell activity. Int J Cancer. 1978 Feb 15;21(2):210–220. doi: 10.1002/ijc.2910210213. [DOI] [PubMed] [Google Scholar]
- SCHELL K. Studies on the innate resistance of mice to infection with mousepox. II. Route of inoculation and resistance; and some observations on the inheritance of resistance. Aust J Exp Biol Med Sci. 1960 Aug;38:289–299. doi: 10.1038/icb.1960.30. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Goldberg V. J. On the mechanism of neurotropism of vesicular stomatitis virus in newborn hamsters. Studies with temperature-sensitive mutants. J Gen Virol. 1975 Dec;29(3):281–296. doi: 10.1099/0022-1317-29-3-281. [DOI] [PubMed] [Google Scholar]
- Stein-Streilein J., Frazier J. L., Gross G. N., Hart D. A. Splenic influence on the development of a local pulmonary immune response. Infect Immun. 1979 Apr;24(1):139–144. doi: 10.1128/iai.24.1.139-144.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolan H. W. Susceptibility of the Syrian hamster to virus infection. Fed Proc. 1978 May 15;37(7):2065–2068. [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Harrison A., Murphy K., Flemister M., Murphy F. A. Lymphoreticular and myeloid pathogenesis of Venezuelan equine encephalitis in hamsters. Am J Pathol. 1976 Aug;84(2):351–370. [PMC free article] [PubMed] [Google Scholar]



