Abstract
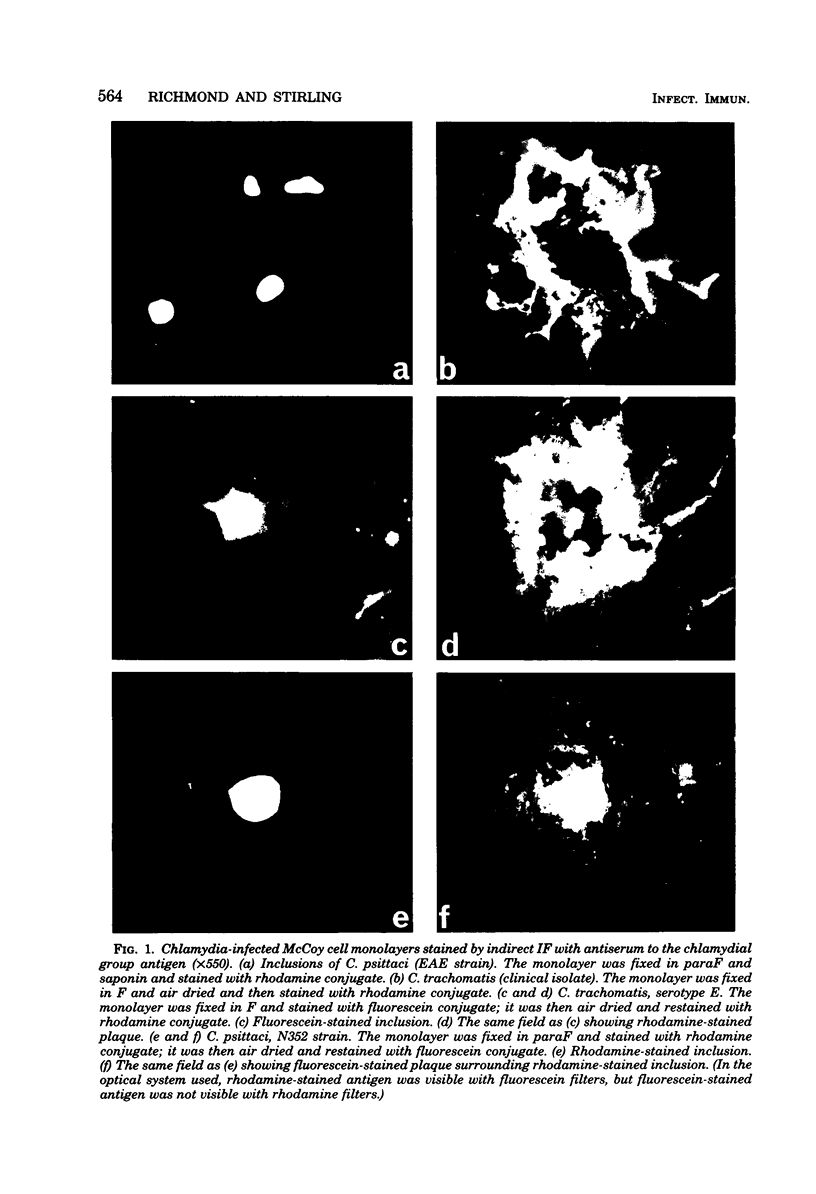
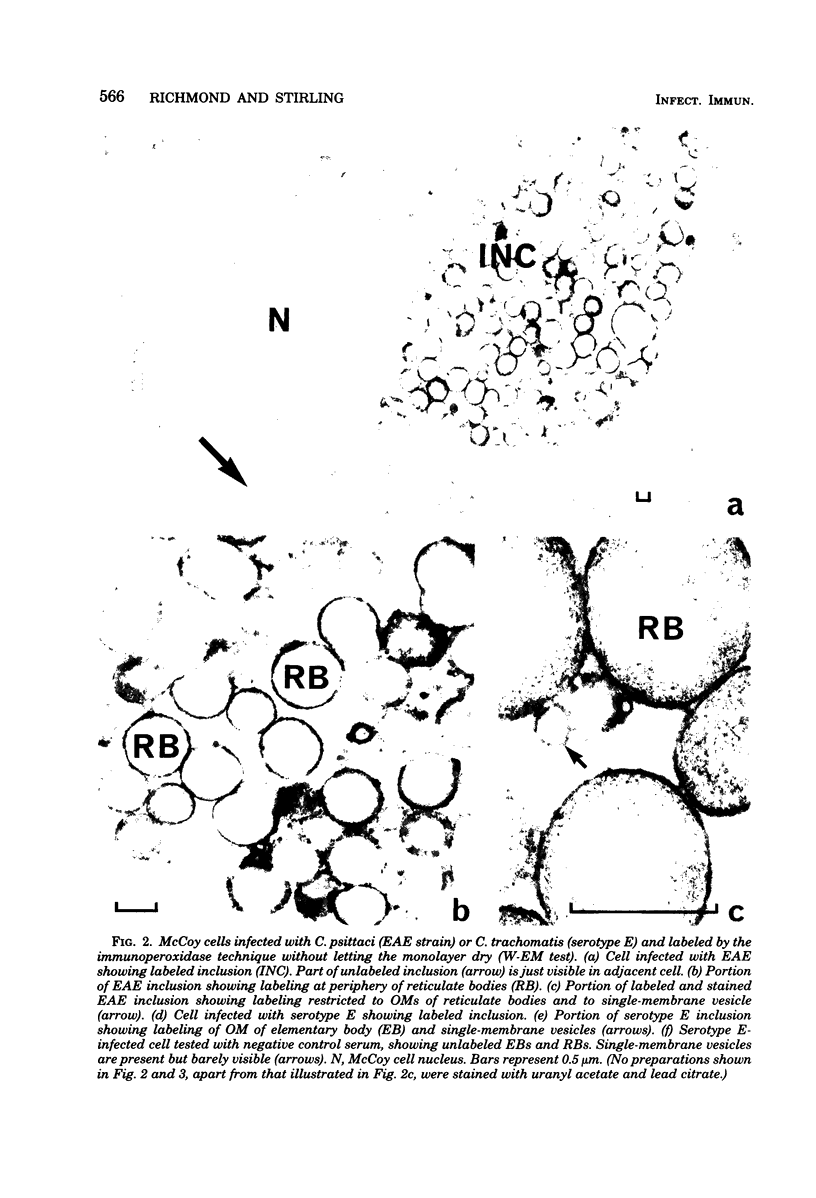
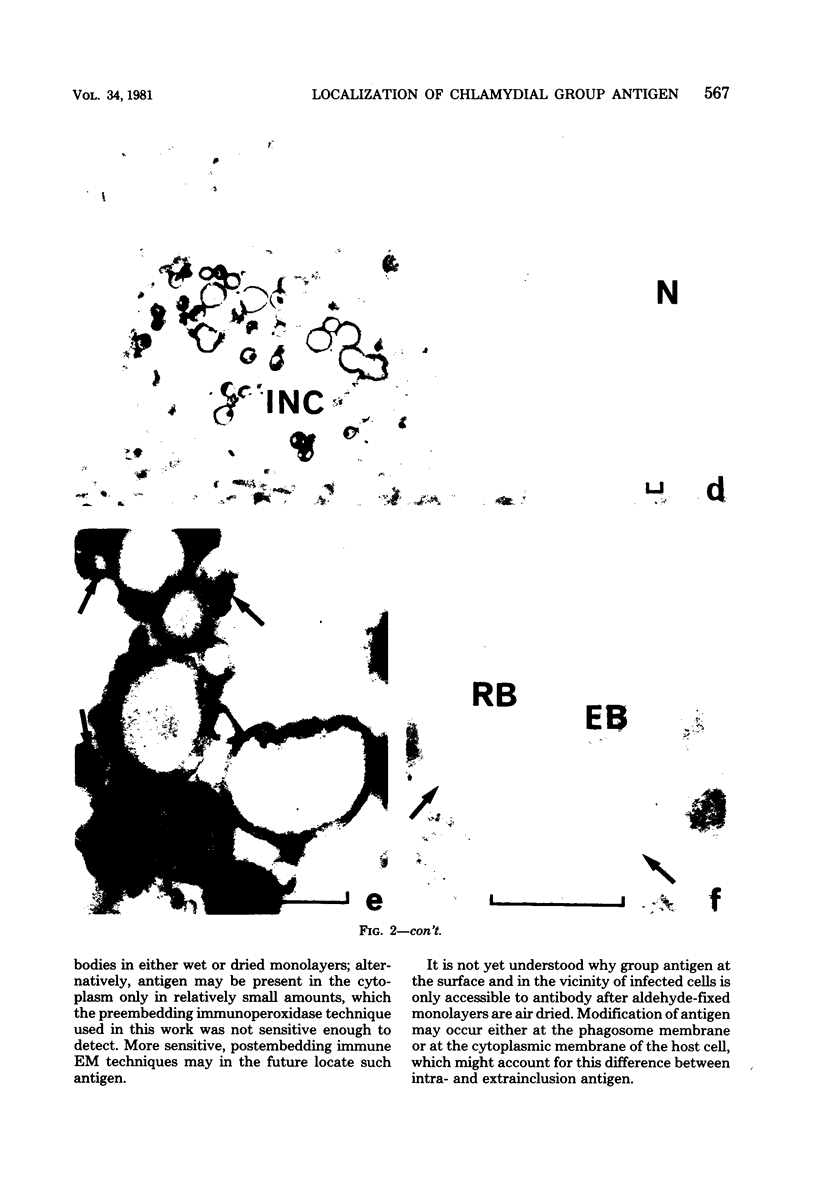
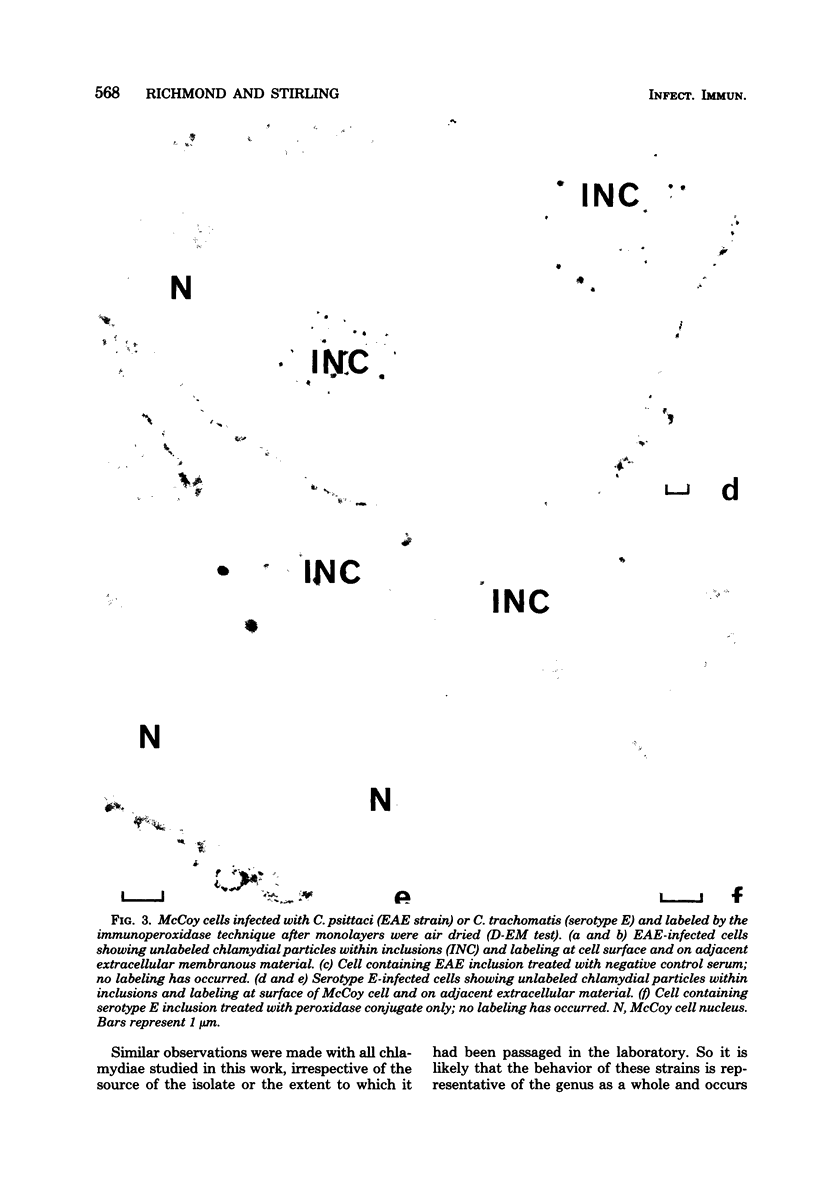
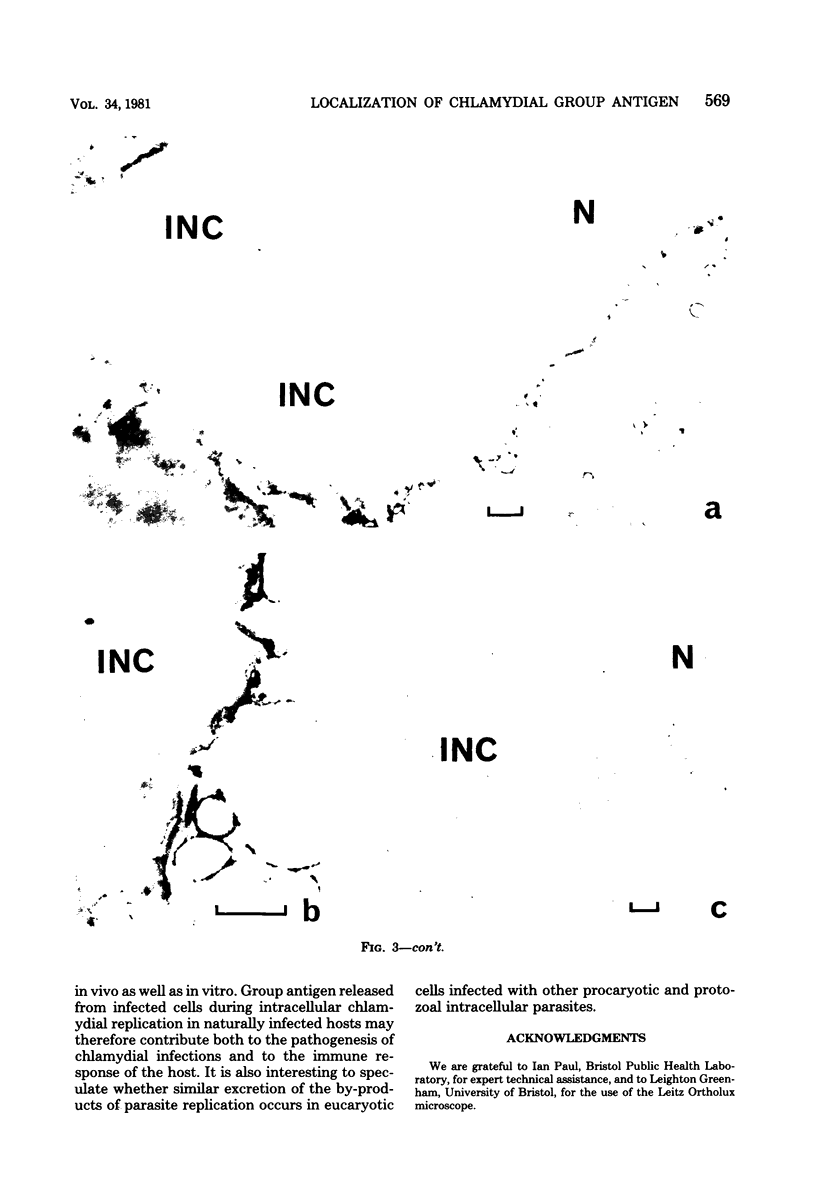
Chlamydial inclusions were demonstrated by indirect immunofluorescence (IF) with antiserum to the chlamydial group antigen when McCoy cell monolayers infected with either Chlamydia trachomatis or Chlamydia psittaci were fixed in formaldehyde or paraformaldehyde, provided the monolayer was not allowed to dry. If these monolayers were then air dried and restained by IF with the same antiserum but with a different fluorescence conjugate, group antigen associated with inclusion-containing McCoy cells but independent of the inclusions was revealed. This antigen was not restricted to infected cells but appeared to radiate out from them, suggesting that group antigen was released from infected cells. Similar host cell-associated antigen could be shown by IF of glutaraldehyde-fixed, air-dried monolayers, but inclusions could not be stained by IF before these preparations were dried, presumably because antibody could not penetrate glutaraldehyde-fixed cells. Electron microscopic immunoperoxidase studies of paraformaldehyde-fixed, wet monolayers located group antigen within inclusions on the outer membrane of chlamydial organisms and on single-membrane vesicles. However, when dried monolayers were labeled with the same immunoperoxidase technique, no intracellular labeling occurred, but dense staining was seen at the surface of infected cells and on adjacent membranous material. These observations are compatible with the postulate that replicating chlamydiae produce outer membrane blebs containing group antigen, which are excreted by the host cells during the chlamydial developmental cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohn W. A fixation method for improved antibody penetration in electron microscopical immunoperoxidase studies. J Histochem Cytochem. 1978 Apr;26(4):293–297. doi: 10.1177/26.4.77869. [DOI] [PubMed] [Google Scholar]
- Bohn W. Electron microscopic immunoperoxidase studies on the accumulation of virus antigen in cells infected with Shope fibroma virus. J Gen Virol. 1980 Feb;46(2):439–447. doi: 10.1099/0022-1317-46-2-439. [DOI] [PubMed] [Google Scholar]
- Chasey D. Investigation of immunoperoxidase-labelled rotavirus in tissue culture by light and electron microscopy. J Gen Virol. 1980 Sep;50(1):195–200. doi: 10.1099/0022-1317-50-1-195. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Poffenroth L., Wilt J. C., Kordová N. Ultrastructural studies of Chlamydia psittaci 6BC in situ in yolk sac explants and L cells: a comparison with gram-negative bacteria. Can J Microbiol. 1975 Oct;21(10):1433–1447. doi: 10.1139/m75-215. [DOI] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir S. P., Boatman E. S. Location of polysaccharide on Chlamydia psittaci by silver-methenamine staining and electron microscopy. J Bacteriol. 1972 Jul;111(1):267–271. doi: 10.1128/jb.111.1.267-271.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir S. P., Hakomori S., Kenny G. E., Grayston J. T. Immunochemical studies on chlamydial group antigen (presence of a 2-keto-3-deoxycarbohydrate as immunodominant group). J Immunol. 1972 Jul;109(1):116–122. [PubMed] [Google Scholar]
- Dhir S. P., Kenny G. E., Grayston J. T. Characterization of the group antigen of Chlamydia trachomatis. Infect Immun. 1971 Dec;4(6):725–730. doi: 10.1128/iai.4.6.725-730.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D., BODENHEIMER T. S., STAGE D. E. THE ULTRASTRUCTURE OF MAUTHNER CELL SYNAPSES AND NODES IN GOLDFISH BRAINS. J Cell Biol. 1963 Oct;19:159–199. doi: 10.1083/jcb.19.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S. J., Caul E. O. Fluorescent antibody studies in chlamydial infections. J Clin Microbiol. 1975 Apr;1(4):345–352. doi: 10.1128/jcm.1.4.345-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMP J. T., McEWEN A. D., WATT J. A. A., NISBET D. I. Enzootic abortion in ewes; transmission of the disease. Vet Rec. 1950 Apr 29;62(17):251–254. doi: 10.1136/vr.62.17.251. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Richmond S. Growth of Chlamydia trachomatis in McCoy cells treated with cytochalasin B. Appl Microbiol. 1974 Dec;28(6):912–914. doi: 10.1128/am.28.6.912-914.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]