Abstract
Background
Rhesus-positive and rhesus-negative persons differ in the presence-absence of highly immunogenic RhD protein on the erythrocyte membrane. This protein is a component of NH3 or CO2 pump whose physiological role is unknown. Several recent studies have shown that RhD positivity protects against effects of latent toxoplasmosis on motor performance and personality. It is not known, however, whether the RhD phenotype modifies exclusively the response of the body to toxoplasmosis or whether it also influences effects of other factors.
Methodology/Principal Findings
In the present cohort study, we searched for the effects of age and smoking on performance, intelligence, personality and self-estimated health and wellness in about 3800 draftees. We found that the positive effect of age on performance and intelligence was stronger in RhD-positive soldiers, while the negative effect of smoking on performance and intelligence was of similar size regardless of the RhD phenotype. The effect of age on four Cattell's personality factors, i.e., dominance (E), radicalism (Q1), self-sentiment integration (Q3), and ergic tension (Q4), and on Cloninger's factor reward dependency (RD) was stronger for RhD-negative than RhD-positive subjects, while the effect of smoking on the number of viral and bacterial diseases was about three times stronger for RhD-negative than RhD-positive subjects.
Conclusions
RhD phenotype modulates the influence not only of latent toxoplasmosis, but also of at least two other potentially detrimental factors, age and smoking, on human behavior and physiology. The negative effect of smoking on health (estimated on the basis of the self-rated number of common viral and bacterial diseases in the past year) was much stronger in RhD-negative than RhD-positive subjects. It is critically needed to confirm the differences in health response to smoking between RhD-positive and RhD-negative subjects by objective medical examination in future studies.
Introduction
About sixteen percent of the population of the Czech Republic express the RhD- negative phenotype, i.e. they have two copies of the null allele of the RHD gene in their genotype. The biological function of the RhD molecule is unknown. Its structure suggests that the molecular complex with RhD protein transports NH3 or CO2 molecules across the erythrocyte cell membrane [1]–[3]. In the RhD negative (rhesus minus) subjects, the product of the particular protein is not synthesized due to a large deletion in the RHD gene. This results in the absence of the D-antigen, probably the most immunogenic epitope on the human red cell membrane [4], [5]. Blood cells of rhesus-positive subjects are therefore a strong antigen for rhesus-negative subjects. Under normal conditions, there are no anti-D antibodies in the serum of RhD-negative subjects. However, after immunization either by transfusion or by delivery of an RhD positive child by an RhD negative mother, large amounts of anti-D antibodies are synthesized by RhD-negative subjects. The presence of these antibodies not only complicates future transfusions and transplantations, but it also represents a strong health risk for delivery of future RhD-positive children.
The existence of genetic polymorphism is an evolutionary enigma since its discovery in the forties of the last century. Theoretically, neither the RhD-negative allele can successfully spread in the RhD positive population nor the RhD-positive allele can spread in the RhD negative population [6], [7]. Before the advent of modern medicine, a positive frequency dependent selection systematically penalized the less abundant allele because lots of children of RhD-negative women in the mostly RhD-positive population as well as children of RhD- positive men in the mostly RhD-positive population were dying of hemolytic anemia. In the past sixty years, several mechanisms explaining the origin and stable existence of RhD polymorphism were suggested and the evidences for them were (nonsystematically) searched for. Presently, the only data-supported explanation of the existence of this polymorphism is the selection in favour of heterozygotes. It was observed that RhD-positive heterozygotes are protected against impairment of psychomotor functions (namely against prolongation of simple reaction times) after Toxoplasma infection [8]. In many countries in Europe, South and Central America, and Africa, more than 50% of the population acquire this parasitic infection during their life [9], [10]. Infected subjects who carry the dormant stages of this protozoan parasite for the rest of life differ from the uninfected ones in the personality profile [11], reaction time [12], secondary sex ratio [13], olfactory preferences [14], risk of schizophrenia [15], [16], brain cancer [17], traffic accidents [18], and suicides [19] [20]. The amount of change usually increases with the duration of toxoplasmosis and most changes were also observed in artificially infected laboratory animals. Several studies have shown that the intensity of toxoplasmosis-associated changes depends on the RhD phenotype of the infected subject. For example, excessive weight gain in Toxoplasma-infected pregnant women, 4.12 kg vs. 2.35 kg in the 16th week of pregnancy, was observed only in RhD-negative women [21] and a 2.4 times higher risk of traffic accidents was also seen in RhD-negative Toxoplasma-infected military drivers [22]. Another studies have reported that also personality [23] and psychomotor differences [8], [24] can be detected mainly in RhD-negative subjects.
It is not clear now whether RhD positivity protects only against the effects of latent toxoplasmosis or whether it also modifies the effects of other factors. Results of a study performed on 300 blood donors suggest that certain personality traits change with age differently in RhD-negative and RhD-positive subjects [23]. Another study has observed that the clinical picture of schizophrenia varies between RhD-negative and RhD-positive patients, namely that the RhD-negative female patients express more severe positive and reality distortion symptoms of the disease (measured with PANSS) and have a longer mean hospital stay than RhD-positive female patients [25]. The main aim of the present study was to systematically search for possible differences in the effects of age and smoking on personality, intelligence, performance, and self-attributed health in a population of 3,820 Czech draftees who were tested by a panel of psychological and performance tests during their entrance examination.
Materials and Methods
Ethics Statement
All participants provided their written informed consent. The recruitment of study subjects and data handling were performed in compliance with the Czech legislation in force and were approved by the Institutional Review Board of the Faculty of Science, Charles University.
Subjects
The study population comprised 3,820 male draftees (mean age 19.73 years, s.d. 1.43) who presented to the Central Military Hospital in Prague for regular entrance psychological examinations between 2000 and 2003 and consented to participate in the research project. The draftees were tested at the beginning of their 1 to1.5-year compulsory military service. In the informed consent form, the draftees were explained the general aim of the project (a study of influence of biological factors on human personality, health, and psychomotor performance) and the need for obtaining their consent to using results of their psychological and clinical examinations. About 80% of the conscripts consented to the use of their test results for the research project purposes and provided 5 ml of blood for RhD phenotype examination and serological testing (the study was part of a more complex project and the subjects were also examined for the presence of anti-Toxoplasma antibodies).
Testing
Cloninger's TCI test [26]and Cattell's 16PF test [27] were used for personality testing. Health and psychic and physical wellness were estimated on the basis of three questions in the anamnestic questionnaire: How often do you catch the flu or common viral or bacterial infections (1- never, 2- about once in five years, 3- about once in two years, 4- about once in a month, 5 more often). Do you usually feel well (in good psychic shape) (1- yes, 2- something between, 3- no). Do you usually feel healthy (in good physical shape) (1- yes, 2- something between, 3- no). A panel of performance tests consisted of Test of attention and short-term memory (TOPP), Numeric Quadrate test of attention and short-term memory (NQ-S), Wiener Matrizen-Test (WMT) [28], and OTIS test of verbal intelligence [29]. All tests except NQ-S are described in [30]. The NQ-S test is a model of searching a target in a rugged visual field. The test is computer administered and evaluated. On the screen, a 10×10 square field containing numbers from 1 to 100 in random positions is shown. The test consists of five subtests and each subtest lasts 6 minutes. During this time individual single-digit and double-digit numbers are presented in the left part of the screen that the proband is supposed to search for. The proband marks the location of the stimulus by typing horizontal and vertical coordinates of the given field. In subtests I, III, and V, the proband is working at his/her own pace: the next stimulus appears only after the previous one is found. In subtests II and IV, the time of the stimulus presentation is limited. This way, it is possible to compare the performance outcomes under time pressure or no time pressure. The method serves for the study of the regulation of cognitive processes under different conditions. The performance is influenced by individual characteristics of visual perception, attention, memory, and load resistance ability. In the present study, we analyzed only two output variables of the test, namely the number of stimuli found under time pressure or no time pressure.
RhD Examination
A standard agglutination method was used for RhD examination. A constant amount of anti-D serum (human monoclonal anti-D reagent; Seraclone®, Immucor Gamma Inc.) was added to a drop of blood on white glass plate. Red cells of RhD-positive subjects were agglutinated within 2–5 minutes.
Statistical Analysis
The Statistica 8.0 and SPSS 16.0 programs were used for statistical testing (t-tests, ordinal regression, and generalized linear model analyses) and checking statistical tests assumptions. The partial Kendall regression was used for non-parametric testing and the Excel spreadsheet for this test [31] can be downloaded at http://web.natur.cuni.cz/flegr/programy.php. All variables including the covariates entered in the respective analyses are specified in the Results section.
Results
Descriptive Statistics
The study population consisted of 3109 RhD-positive and 712 (18.63%) RhD-negative male subjects; however, the particular tests were only passed by a part of probands. One thousand eight hundred and fifteen (1815) RhD-positive and 400 (18.1%) RhD-negative subjects passed the psychomotor performance test NQ-S, 3035 RhD-positive and 695 (18.6%) RhD-negative subjects passed the psychomotor performance test TOP, 761 RhD-positive and 154 (16.8%) RhD-negative subjects passed Cloninger's TCI test, 2331 RhD-positive and 518 (16.8%) RhD-negative subjects passed the nonverbal intelligence test WMT, 532 RhD-positive and 121 (18.5%) RhD-negative subjects passed Cattel's 16PF test, and 2371 RhD-positive and 527 (18.2%) RhD-negative subjects passed the verbal intelligence test OTTIS. Anamnestic data including the information on the current health status and wellness was available for 2038 RhD-positive and 492 RhD-negative subjects. The population contained 1874 smokers and 1142 non-smokers. The descriptive statistics of the tests scores and results of t-test comparison of RhD-positive with RhD-negative subjects and smokers with non-smokers are shown in Table 1.
Table 1. Descriptive statistics of the population and effects of RhD phenotype and smoking on performance, intelligence, wellness, self-rated health, and personality of draftees.
| RhD− | RhD+ | RhD− | RhD+ | nonsmokers | smokers | nonsmokers | smokers | |||
| Mean | Mean | Valid N | Valid N | p | Mean | Mean | Valid N | Valid N | p | |
| Age | 19.76 | 19.72 | 712 | 3109 | 0.509 | 20.09 | 19.53 | 1142 | 1874 | 0.000 |
| Psychical wellness | 1.17 | 1.18 | 492 | 2038 | 0.747 | 1.13 | 1.19 | 828 | 1402 | 0.000 |
| Physical wellness | 1.13 | 1.12 | 492 | 2038 | 0.664 | 1.08 | 1.14 | 828 | 1402 | 0.000 |
| Health | 2.33 | 2.33 | 492 | 2038 | 0.949 | 2.87 | 3.01 | 828 | 1402 | 0.000 |
| NQ test | ||||||||||
| spontaneous rate | 86.55 | 85.39 | 382 | 1717 | 0.281 | 89.55 | 83.75 | 618 | 973 | 0.000 |
| enforced rate | 51.56 | 50.93 | 382 | 1717 | 0.473 | 54.39 | 49.52 | 618 | 973 | 0.000 |
| TOP test | ||||||||||
| 1st minute | 34.93 | 35.04 | 695 | 3035 | 0.772 | 36.18 | 33.97 | 1113 | 1841 | 0.000 |
| 2nd minute | 36.00 | 36.23 | 695 | 3033 | 0.540 | 37.62 | 35.33 | 1111 | 1841 | 0.000 |
| 3rd minute | 36.61 | 36.62 | 694 | 3033 | 0.979 | 38.38 | 36.01 | 1111 | 1841 | 0.000 |
| Intelligence | ||||||||||
| raw score VMS | 14.39 | 14.47 | 518 | 2331 | 0.685 | 15.58 | 13.85 | 789 | 1281 | 0.000 |
| raw score OTIS | 21.34 | 21.25 | 527 | 2371 | 0.729 | 22.50 | 20.65 | 792 | 1291 | 0.000 |
| Cattell 16PF test | ||||||||||
| A | 11.83 | 12.31 | 121 | 532 | 0.189 | 11.38 | 12.04 | 64 | 112 | 0.267 |
| C | 13.80 | 13.68 | 121 | 532 | 0.754 | 13.47 | 13.12 | 64 | 112 | 0.569 |
| E | 12.87 | 12.71 | 121 | 532 | 0.646 | 12.14 | 13.02 | 64 | 112 | 0.078 |
| F | 13.93 | 14.43 | 121 | 532 | 0.194 | 13.52 | 14.90 | 64 | 112 | 0.018 |
| G | 11.61 | 11.21 | 121 | 532 | 0.308 | 11.61 | 10.25 | 64 | 112 | 0.023 |
| H | 12.64 | 12.66 | 121 | 532 | 0.964 | 11.22 | 12.59 | 64 | 112 | 0.111 |
| I | 8.28 | 8.16 | 121 | 532 | 0.761 | 7.88 | 8.35 | 64 | 112 | 0.427 |
| L | 12.05 | 12.44 | 121 | 532 | 0.225 | 11.94 | 12.27 | 64 | 112 | 0.484 |
| M | 8.11 | 8.45 | 121 | 532 | 0.419 | 9.00 | 8.87 | 64 | 112 | 0.850 |
| N | 11.42 | 11.46 | 121 | 532 | 0.923 | 12.09 | 11.61 | 64 | 112 | 0.445 |
| O | 9.64 | 9.78 | 121 | 532 | 0.707 | 10.08 | 9.46 | 64 | 112 | 0.356 |
| Q1 | 14.74 | 15.15 | 121 | 532 | 0.314 | 15.25 | 15.54 | 64 | 112 | 0.628 |
| Q2 | 7.64 | 7.63 | 121 | 532 | 0.981 | 8.44 | 7.84 | 64 | 112 | 0.350 |
| Q3 | 11.61 | 10.86 | 121 | 532 | 0.082 | 11.06 | 10.01 | 64 | 112 | 0.138 |
| Q4 | 8.60 | 8.39 | 121 | 532 | 0.649 | 8.98 | 8.88 | 64 | 112 | 0.887 |
| Cloninger TCI test | ||||||||||
| NS | 18.65 | 19.83 | 154 | 761 | 0.012 | 18.88 | 21.44 | 304 | 461 | 0.000 |
| HA | 14.12 | 14.90 | 154 | 761 | 0.152 | 14.39 | 15.26 | 304 | 461 | 0.060 |
| RD | 14.85 | 14.63 | 154 | 761 | 0.467 | 15.26 | 13.88 | 304 | 461 | 0.000 |
| SD | 26.40 | 26.02 | 154 | 761 | 0.521 | 27.36 | 24.12 | 304 | 461 | 0.000 |
| CO | 29.66 | 28.93 | 154 | 761 | 0.171 | 30.20 | 27.34 | 304 | 461 | 0.000 |
| ST | 13.82 | 14.30 | 154 | 761 | 0.345 | 13.99 | 14.61 | 304 | 461 | 0.151 |
| PE | 4.82 | 4.45 | 154 | 761 | 0.047 | 5.13 | 4.01 | 304 | 461 | 0.000 |
The significant results of t-tests are printed in bold. The performance was measured with the Test of attention and short-term memory (TOPP) and Numeric Quadrate test of attention and short-term memory (NQ-S). Intelligence was estimated with the Wiener Matrizen-Test (WMT) and OTIS test of verbal intelligence. Personality profile was measured with Cattel's 16PF and Cloninger's TCI. Cattel's test measures factors A: affectothymia/schizothymia, C: ego weakness/high ego strength, E: submissiveness/dominance, F: desurgency/surgency, G: low superego strength/high superego strength, H: threctia/parmia, I: harria/premsia, L: alaxia/protension, M: praxernia/autia, N: naivete/shrewdness, O: untroubled adequacy/guilt proneness, Q1: conservatism/radicalism, Q2: group dependency/self sufficiency, Q3: low self-sentiment integration/high strength of self-sentiment, Q4: low ergic tension/high ergic tension. Cloninger's test measures factors NS: novelty seeking, HA: harm avoidance, RD: reward dependence, SD: self-directedness, CO: cooperativeness, ST: self-transcendence, PE: persistence. For self-rating of health (namely the frequency of common diseases), the draftees were asked to use a five-point scale anchored with 1 (very healthy) and 5 (ill more often than once a week). For self-rating of the psychic and physical wellness, they used a three-point scale, 1- I usually feel well, 2- something between, 3- I usually don't feel well.
Effects of RhD, Age, and RhD-Age Interaction on Psychomotor Performance, Intelligence and Personality
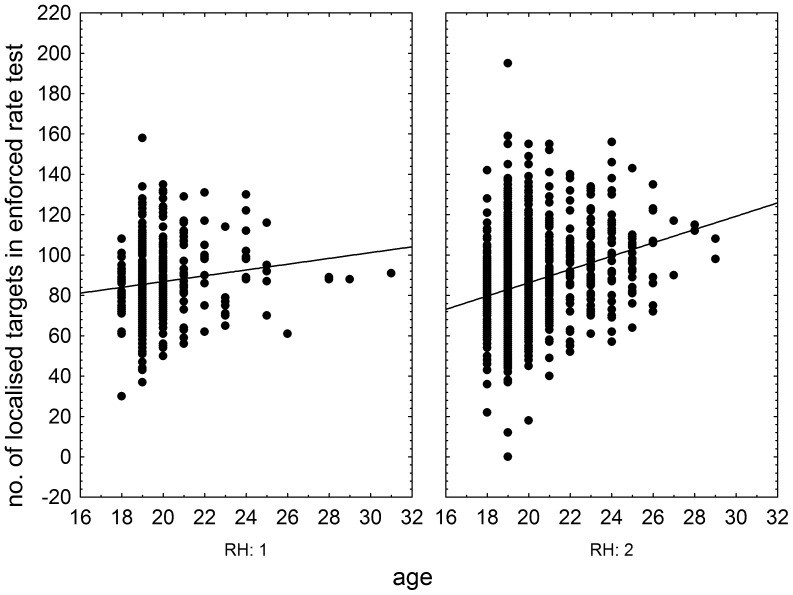
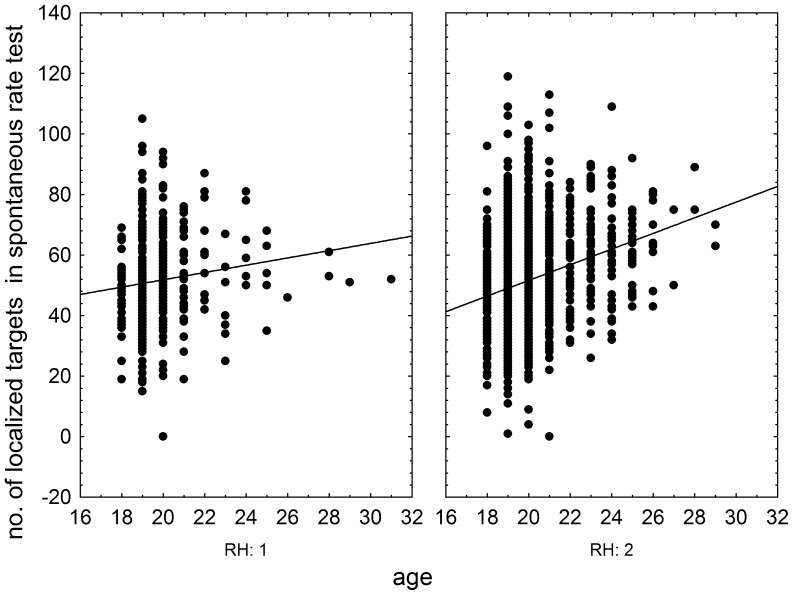
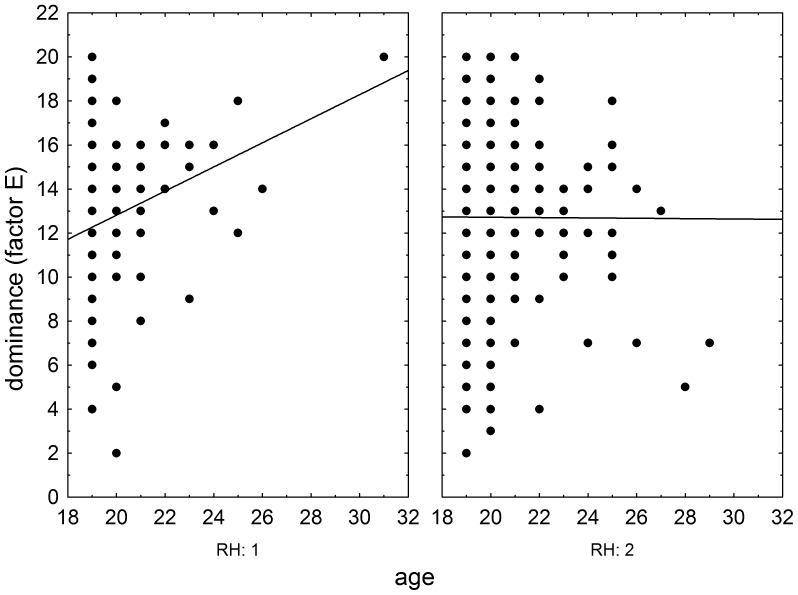
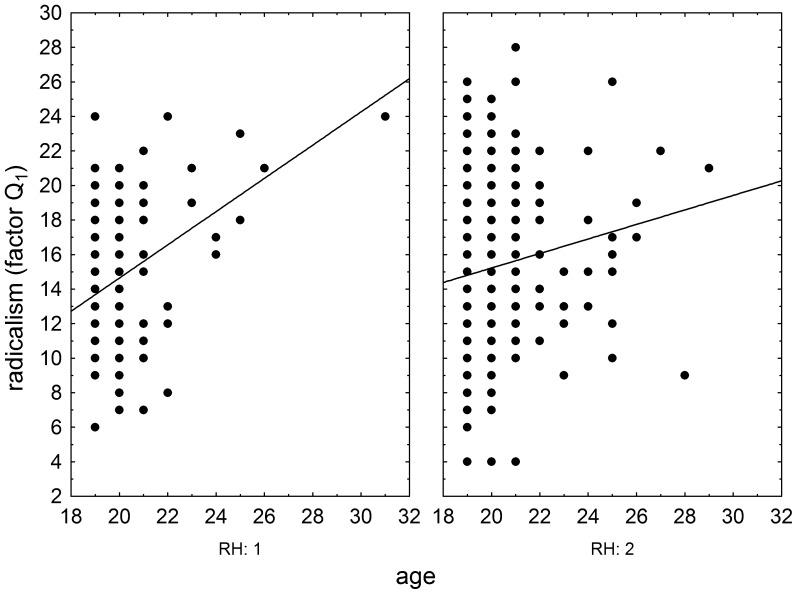
The effects of RhD, age, and RhD-age interaction on psychomotor performance measured by the NQ-70 test were estimated by GLM analysis (full model). The results showed positive effect of RhD positivity (p = 0.003, η2 = 0.004), positive effect of age (p<0.0001, η2 = 0.025), and significant effect of RhD-age (p = 0.004, η2 = 0.004) on the number of targets correctly found in the enforced rate subtest (Figure 1) as well as on correctly found targets in the spontaneous rate subtest (RhD: p = 0.008, η2 = 0.003; age: p<0.0001, η2 = 0.024; RhD-age: p = 0.009, η2 = 0.003), Figure 2. We observed only the positive effect of age on psychomotor performance measured by the TOC test (p<0.0001, η2 = 0.038, repeat measure GLM, with the number of correctly found targets in 1st, 2nd and 3rd minute of the test as the repeated measures). The same was true for intelligence measured by the verbal intelligence test OTIS (RhD: p = 0.787, η2<0.0001; age: p<0.0001, η2 = 0.075, RhD-age: p = 0.791, η2<0.0001, GLM with the number of correctly answered questions as a dependent variable). The nonverbal intelligence test WMT showed positive effect of RhD positivity (p = 0.042, η2 = 0.01), positive effect of age (p<0.0001, η2 = 0.052), and significant effect of RhD-age interaction (p = 0.036, η2 = 0.002) on the number of correctly answered questions. The multivariate GLM with 16 Cattell's personality factors as dependent variables showed highly significant effect of age (p<0.0001, η2 = 0.112) and trends for RhD (p<0.073, η2 = 0.036) and RhD-age interaction (p<0.054, η2 = 0.038). The univariate analyses for particular Cattell's factors showed significant effects of RhD and RhD-age interactions on dominance (E), radicalism (Q1), and strength of ego (Q3), see Figures 3, 4, and 5 and Table 2. Similar analyses of results in Cloninger's TCI test showed only significant effects of age (multivariate analysis and all univariate analyses, except the analysis of effect of age on reward dependency), see Table 2.
Figure 1. Influence of age of RhD-negative and RhD-positive draftees on performance in the enforced rate subtest of the Numeric Quadrate test of attention and short-term memory.
Left and right panels show results (number of correctly localized targets) of RhD-negative and RhD-positive subjects, respectively.
Figure 2. Influence of age of RhD-negative and RhD-positive draftees on performance in the spontaneous rate subtest of the Numeric Quadrate test of attention and short-term memory.
Left and right panels show results (number of correctly localized targets) of RhD-negative and RhD-positive subjects, respectively.
Figure 3. Influence of age of RhD-negative and RhD-positive draftees on Cattel's factor Dominance (E).
Left and right panels show results (raw scores) of RhD-negative an RhD-positive subjects, respectively.
Figure 4. Influence of age of RhD-negative and RhD-positive draftees on Cattel's factor Radicalism (Q1).
Left and right panels show results (raw scores) of RhD-negative and RhD- positive subjects, respectively.
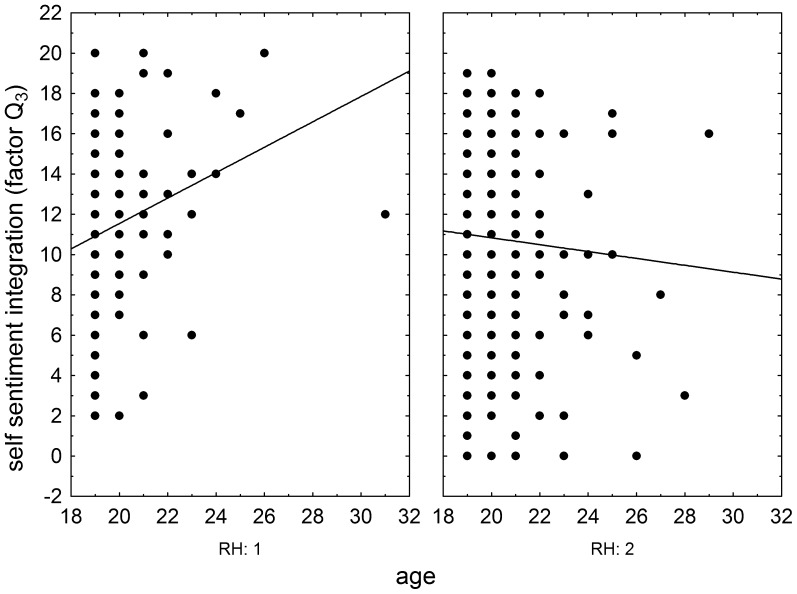
Figure 5. Influence of age of RhD-negative and RhD-positive draftees on Cattel's factor Self- sentiment integration (Q3).
Left and right panels show results (raw scores) of RhD-negative and RhD-positive subjects, respectively.
Table 2. Effects of age, smoking and RhD phenotype on Cattel's personality factors.
| Model 1 | Model 2 | |||||||||
| RhD | age | RhD×age | RhD | smoking | age | RhD×smoking | RhD×age | smoking×age | RhD×smoking×age | |
| A | 0.702 | 0.273 | 0.618 | 0.263 | 0.104 | 0.062 | 0.005 | 0.265 | 0.120 | 0.005 |
| C | 0.262 | 0.002 | 0.257 | 0.170 | 0.242 | 0.008 | 0.363 | 0.170 | 0.252 | 0.328 |
| E | 0.009 | 0.010 | 0.008 | 0.323 | 0.478 | 0.704 | 0.828 | 0.300 | 0.438 | 0.867 |
| F | 0.991 | 0.007 | 0.940 | 0.118 | 0.250 | 0.002 | 0.200 | 0.110 | 0.274 | 0.161 |
| G | 0.139 | 0.002 | 0.122 | 0.105 | 0.541 | 0.023 | 0.014 | 0.091 | 0.487 | 0.015 |
| H | 0.430 | 0.657 | 0.431 | 0.427 | 0.662 | 0.896 | 0.064 | 0.454 | 0.702 | 0.052 |
| I | 0.694 | 0.001 | 0.690 | 0.340 | 0.950 | 0.001 | 0.431 | 0.298 | 0.946 | 0.387 |
| L | 0.644 | 0.276 | 0.719 | 0.672 | 0.225 | 0.210 | 0.491 | 0.767 | 0.224 | 0.522 |
| M | 0.345 | 0.042 | 0.317 | 0.159 | 0.022 | 0.034 | 0.042 | 0.172 | 0.027 | 0.050 |
| N | 0.336 | 0.873 | 0.330 | 0.121 | 0.079 | 1.000 | 0.023 | 0.100 | 0.081 | 0.020 |
| O | 0.337 | 0.137 | 0.324 | 0.242 | 0.058 | 0.360 | 0.270 | 0.245 | 0.049 | 0.273 |
| Q1 | 0.022 | 0.000 | 0.029 | 0.008 | 0.072 | 0.013 | 0.902 | 0.009 | 0.056 | 0.832 |
| Q2 | 0.586 | 0.602 | 0.588 | 0.267 | 0.178 | 0.127 | 0.201 | 0.248 | 0.197 | 0.207 |
| Q3 | 0.004 | 0.084 | 0.003 | 0.014 | 0.810 | 0.181 | 0.279 | 0.009 | 0.759 | 0.288 |
| Q4 | 0.069 | 0.073 | 0.075 | 0.008 | 0.429 | 0.007 | 0.678 | 0.010 | 0.415 | 0.668 |
| NS | 0.632 | 0.040 | 0.771 | 0.847 | 0.646 | 0.025 | 0.380 | 0.983 | 0.855 | 0.295 |
| HA | 0.363 | 0.001 | 0.309 | 0.375 | 0.204 | 0.013 | 0.882 | 0.303 | 0.240 | 0.953 |
| RD | 0.754 | 0.950 | 0.796 | 0.621 | 0.040 | 0.712 | 0.001 | 0.616 | 0.017 | 0.001 |
| SD | 0.475 | 0.000 | 0.450 | 0.366 | 0.545 | 0.000 | 0.507 | 0.328 | 0.733 | 0.457 |
| CO | 0.664 | 0.000 | 0.735 | 0.784 | 0.440 | 0.000 | 0.120 | 0.826 | 0.311 | 0.148 |
| ST | 0.810 | 0.042 | 0.757 | 0.577 | 0.136 | 0.023 | 0.956 | 0.582 | 0.140 | 0.912 |
| PE | 0.781 | 0.004 | 0.670 | 0.642 | 0.999 | 0.042 | 0.757 | 0.521 | 0.703 | 0.735 |
Two independent variables age and RhD phenotype and three independent variables age, smoking, and RhD phenotype were included in Model 1 and Model 2, respectively. The table shows the significance (p) of particular effects. Significant effects are printed in bold. For meanings of particular abbreviations, see the Table 1 legend.
Effects of RhD, Smoking, Age and Interactions between These Variables on Psychomotor Performance, Intelligence, and Personality
The same statistical tests were repeated for independent binary variables RhD and smoking and the independent continuous variable age. The analysis of a full GLM model showed positive effect of RhD positivity (p = 0.003, η2 = 0.006), positive effect of age (p<0.0001, η2 = 0.023), and significant effect of RhD-age interaction (p = 0.005, η2 = 0.005) on the number of targets correctly found in the enforced rate subtest as well as on correctly found targets in the spontaneous rate subtest (RhD: p = 0.038, η2 = 0.003; age: p<0.0001, η2 = 0.024; RhD-age: p = 0.047, η2 = 0.003). The effect of smoking and interactions of smoking with other variables were nonsignificant. Repeated measures GLM analyses of results of the psychomotor performance test TOP showed significant positive effect of age (p<0.0001, η2 = 0.033) and no significant effect of RhD, smoking, or any interactions between these variables. The GLM analyses with the number of correct answers as a dependent variable showed significant negative effect of smoking (p = 0.027, η2 = 0.002), age (p<0.0001, η2 = 0.074), and smoking-age interaction (p = 0.049, η2 = 0.002) on intelligence measured by the verbal intelligence test Otis. A similar analysis for the nonverbal intelligence test WMT showed significant positive effect of age (p<0.0001, η2 = 0.049) and nonsignificant trends for RhD (p = 0.072, η2 = 0.002), and RhD-age interaction (p<0.073, η2 = 0.002). Multivariate analysis of the effect of RhD, age, smoking, and interactions between these variables on Cattell's factors showed only significant effect of RhD positivity (p = 0.007, η2 = 0.18), age (p<0.0001, η2 = 0.264), and RhD-age interaction (p = 0.006, η2 = 0.183). Univariate analyses showed that either the main effects of RhD, smoking, age, or RhD-smoking, RhD-age, and RhD-age-smoking interactions were significant for many of Cattell's factors, see Table 2. The partial Kendall correlation tests with the binary variable smoking as a covariate performed separately for RhD-positive and RhD-negative subjects showed that the correlation between age and Catell's factors was nearly always stronger in RhD negatives. Similarly, the partial Kendall correlation tests with the age as a covariate performed separately for RhD-positive and RhD-negative subjects showed that the correlation between the binary variable smoking and Cattell's factors was much stronger for RhD-negative, than RhD-positive subjects, see Table 3. Multivariate analysis of the effects of RhD, age, smoking, and interactions between these variables on seven Cloninger's TCI factors showed significant effect of age (p<0.0001, η2 = 0.264) and RhD –smoking (p = 0.019, η2 = 0.026) and RhD-smoking-age interaction (p = 0.014, η2 = 0.027). Univariate analyses showed that the effect of age was significant in six of seven Cloninger's factors and the effects of smoking (p = 0.040, η2 = 0.007), RhD-smoking (p = 0.001, η2 = 0.017), smoking-age (p = 0.017, η2 = 0.009), and RhD-smoking-age (p = 0.001, η2 = 0.017) were significant for reward dependency (Tab. 2). The Kendall partial correlation tests with the continuous variable age as a covariate performed separately for RhD-positive and RhD-negative subjects showed that the correlation between smoking and the Cloninger's factors was mostly stronger for RhD negative than RhD positive subjects. Similarly, the Kendall partial correlation tests with the binary variable smoking as a covariate performed separately for RhD-positive and RhD-negative subjects showed that the correlation between age and Cloninger's factors was mostly stronger for RhD-negative than RhD-positive subjects, see Table 3.
Table 3. Effects of age and smoking on Cattel's and Cloninger's personality factors estimated with nonparametric tests for RhD-negative and RhD-positive subjects, respectively.
| age | smoking | |||||||
| Rh posit. | Rh negat. | Rh posit. | Rh negat. | |||||
| Tau | p | Tau | p | Tau | p | Tau | p | |
| A | 0.067 | 0.021 | 0.015 | 0.804 | 0.105 | 0.000 | −0.013 | 0.912 |
| C | 0.069 | 0.018 | 0.093 | 0.130 | 0.011 | 0.841 | −0.104 | 0.395 |
| E | 0.020 | 0.494 | 0.231 | 0.000 | 0.135 | 0.017 | 0.068 | 0.578 |
| F | 0.013 | 0.663 | −0.151 | 0.014 | 0.246 | 0.000 | 0.014 | 0.908 |
| G | 0.010 | 0.723 | 0.144 | 0.019 | −0.157 | 0.006 | −0.052 | 0.669 |
| H | 0.016 | 0.589 | 0.057 | 0.353 | 0.131 | 0.021 | −0.045 | 0.711 |
| I | 0.066 | 0.023 | 0.183 | 0.003 | 0.063 | 0.270 | −0.120 | 0.325 |
| L | 0.034 | 0.241 | 0.091 | 0.138 | 0.053 | 0.354 | −0.043 | 0.726 |
| M | −0.021 | 0.467 | −0.117 | 0.058 | −0.002 | 0.977 | −0.173 | 0.158 |
| N | 0.002 | 0.939 | −0.030 | 0.625 | −0.080 | 0.157 | 0.055 | 0.654 |
| O | −0.045 | 0.124 | −0.070 | 0.252 | −0.079 | 0.161 | −0.072 | 0.558 |
| Q1 | 0.138 | 0.000 | 0.289 | 0.000 | 0.038 | 0.505 | 0.233 | 0.057 |
| Q2 | −0.009 | 0.749 | −0.023 | 0.704 | −0.015 | 0.797 | −0.097 | 0.426 |
| Q3 | −0.034 | 0.242 | 0.180 | 0.003 | −0.123 | 0.029 | −0.060 | 0.625 |
| Q4 | 0.013 | 0.649 | −0.072 | 0.240 | −0.008 | 0.894 | −0.023 | 0.849 |
| NS | −0.034 | 0.164 | −0.060 | 0.271 | 0.221 | 0.000 | 0.041 | 0.532 |
| HA | −0.093 | 0.000 | −0.159 | 0.003 | 0.018 | 0.520 | 0.102 | 0.118 |
| RD | −0.009 | 0.714 | −0.010 | 0.856 | −0.154 | 0.000 | −0.179 | 0.006 |
| SD | 0.084 | 0.001 | 0.157 | 0.004 | −0.173 | 0.000 | −0.082 | 0.213 |
| CO | 0.097 | 0.000 | 0.115 | 0.034 | −0.186 | 0.000 | −0.087 | 0.182 |
| ST | −0.053 | 0.028 | −0.044 | 0.414 | 0.046 | 0.106 | −0.030 | 0.649 |
| PE | 0.038 | 0.112 | 0.117 | 0.031 | −0.199 | 0.000 | −0.191 | 0.003 |
The table shows the significance (p) and strength and sign of particular effects (Tau). The partial Kendall correlation tests were used for the analysis with one confounding variable, either age (when the effect of smoking was studied) or smoking (when the effect of age was studied), being controlled. Positive Tau means that the particular personality trait is higher in older subjects or smokers. Significant effects are printed in bold. For meanings of particular abbreviations, see the Table 1 legend.
Effects of RhD, Smoking, and Interactions between These Variables on Health and Wellness
Ordinal probit regression revealed nearly significant effect of age (p = 0.058) and signifiant effect of smoking-RhD interaction (p = 0.040) on health (number of viral and bacterial diseases in the past year). The partial Kendall non-parametric correlation tests with age as a covariate showed that the correlation between smoking and the number of viral and bacterial diseases in the past year was nearly three times stronger for RhD-negative (Tau = 0.174, p<0.0001) than RhD-positive subjects (Tau = 0.066, p<0.0001). The same ordinal probit regression performed for two ordinal variables, the self-rated current psychical wellness (1 - bad, 2 - something between, 3 - good) and self-rated current physical wellness (1 - bad, 2 - something between, 3 - good) showed no significant effects of the RhD phenotype. However, the partial Kendall correlation (controlled for age) between self-rated psychic and physical wellness and smoking performed separately for RhD-negative and RhD-positive subjects showed stronger negative effects of smoking on RhD-negative (psychical: Tau = 0.099, p = 0.0021, physical: Tau = 0.111, p = 0.0005) than RhD-positive subjects (psychic: Tau = −0.069, p<0.0001, physical: Tau = −0.087, p<0.0001).
Discussion
Our study performed on a cohort of about 3820 draftees detected only two significant main effects of RhD phenotype on results of two psychological, two intelligence and two psychomotor tests. We found that the RhD-positive subjects expressed higher Cloninger's factor novelty seeking and lower Cloninger's persistence than RhD-negative subjects. However, we performed 28 separate tests in total; therefore our two positive results (nonsignificant after the Bonferroni's correction for multiple tests) can be just a statistical artifact. On the other hand, the effect of smoking was strong and was detected in 14 of the 28 subtests. Smoking also negatively correlated with self-rated health and wellness. The most important result of our study was the finding of the influence of RhD phenotype on the effects of age and smoking. The effects of age on four Cattell's personality factors, i.e. dominance (E), radicalism (Q1), self-sentiment integration (Q3), and ergic tension (Q4) and on Cloninger's factor reward dependency were stronger for RhD-negative than RhD-positive subjects and the effect of smoking on the number of viral and bacterial diseases was about three times stronger for RhD-negative than RhD-positive subjects.
It has already been shown that RhD positivity protects against several negative effects of latent toxoplasmosis, namely against prolongation of reaction times [8] [24], increased risk of traffic accidents [22], and excessive increase of body weight in pregnancy [21]. It has also been shown that latent toxoplasmosis has different effects on several Cattell's personality factors, ego strength (C), praxernia (M), and self-sentiment integration (Q3), and Cloninger's reward dependency (RD) in RhD-negative and RhD- positive subjects [23]. The same study has also indicated that the RhD-negative and RhD-positive subjects (blood donors) could differ in the effect of age on two Cattell's personality factors, dominance (E) and shrewdness (N); however, the observed effects were not significant after the Bonferroni correction for multiple tests. In the present study, many tests provided significant results even after the correction for multiple tests.
In contrast with the situation observed in the present study, the effect of age on personality factors was stronger in RhD-positive than RhD-negative blood donors [23]. It must be reminded, however, that the mean age of blood donors was 35.3 years (18–64), while that of draftees was 19.8 years (17–31). It is therefore probable that two qualitatively different processes were monitored in the previous study on blood donors and the present one on draftees – the process of senescence in the blood donor population (which was more evident in RhD-positive subjects) and the process of adolescence in the draftee population (which was more evident in RhD-negative subjects). It is indicative that the effect of age on intelligence was positive in the draftees but negative in the blood donors.
Generally, the statistical tests can estimate the probability of the association between variables; however, no statistical test can identify the causal relationship between the associated variables, i.e., tell what the cause is and what the effect is. There is no doubt that age (either adolescence or senescence) is the cause and not the effect of the observed changes in psychomotor performance, intelligence, and personality. There is only little doubt about the causality behind the observed negative association between health and smoking [32], [33]. However, the associations between smoking and some Cattell's and Cloninger's personality factors can be explained either by the influence of smoking on personality (intelligence, psychomotor performance) or by the influence of personality or intelligence (however, probably not the psychomotor performance) on the probability of starting, continuing or quitting smoking. Theoretically, some still unknown factors, e.g. the size of the place of residence (city, small town, village), that influence both the probability of smoking and personality or performance in various tests, or health and wellness can be responsible for the association between smoking and, for example, nonverbal intelligence or psychomotor performance (or possibly the motivation to succeed in psychomotor tests). It is, however, difficult to explain, by two independent effects of an unknown factor on the probability of smoking and human personality, the association of RhD-smoking interaction with Cloninger's factors affectothymia (p = 0.005), superego strength (p = 0.014), praxernia (p = 0.042), and shrewdness (p = 0.023). We can speculate that among people living in small villages, there could be more smokers and more subjects with lower superego strength and higher shrewdness. At the same time, it is difficult to explain why this is true only for RhD-negative subjects while the opposite (higher superego strength and lower shrewdness) is true for RhD-positive subjects. Probably, the most parsimonious explanation is that not some third factor but the smoking is responsible for the shift in the personality factor related to nonsmokers and the RhD phenotype influences the amount and sometimes also the direction of this shift.
It must be reminded that the design of the present study (and all previous studies on behavioral and physiological effects of RhD phenotype, too) was cross-sectional, not longitudional. Therefore, we cannot tell whether the differences in the performance, intelligence, personality, or health observed between, for example, smokers and nonsmokers are caused by the smoking or whether the populations differ in the probability of starting (quitting or continuing) smoking. Similarly, the observed differences between the younger and older subjects could be either caused by the effect of senescence or adolescence [34]–[38] or it could be just the effect of differences between various age cohorts. Again, the existence of the RhD-smoking and RhD-age interactions (the main subject of the present study) makes the latter explanation of the differences observed (based on the effect of a population or of a cohort) very improbable.
Psychomotor and cognitive performance of subjects is most probably influenced by various confounding factors that have not been monitored in the present study. For example, the alcohol consumption, genotype, and infections can be expected to strongly influence performances as well as personality profile of our subjects. It must be stressed out that the existence of confounding variables cannot be a source of any systematic bias and a cause of false positive results. Still, future studies should aim at better control of these sources of variance to decrease a risk of false negative results.
The mechanism responsible for physiological and behavioural effects of RhD phenotype is unknown. The RhD molecule is part of a molecular complex (RhAG) on the membrane of red cells [4], [5]. Structural data suggest that the complex is a membrane NH3 or possibly CO2 pump with unknown function [1]–[3]. In RhD-negative subjects, the gene RHD is absent in chromosomes of both maternal and paternal origin due to a large deletion and therefore also the RhD molecule is missing and is probably substituted with another related molecule in the complex [39]. RhD-containing and RhD-free molecules may differ in the specificity, activity and most probable also response to regulation signals. The membrane pump could directly or indirectly influence the partial tension of oxygen and water balance in various tissues, including the brain tissue [40], [41]. Various detrimental factors such as infection with the neurotropic pathogen Toxoplasma, senescence, or smoking probably shift the physiological parameters from their optimum to one or to the other side. The absence of the RhD-containing complex either enhances such shift (and makes the RhD-negative subjects more vulnerable to the particular factor) or counterbalances it (and makes the RhD-negative subjects more resistant to the particular factor). Specifically, Prandota (2012) [42] suggested that the absence of RhD-contained complex could be associated with development of brain hypoxia because recent studies showed that the Rhesus-associated glycoprotein (CcEe and D proteins) (RhAG) and water channel aquaporin-1 (AQP1) were equally responsible for the normal CO2 permeability of the red blood cell membrane. In addition, AQP4, the predominant water channel expressed primarily in astrocytes and ependymocytes in the brain, also regulated hypoxia through mediation of bicarbonate transport, and a hypoxia inducible factor binding motif has been identified in the promoter region of AQP4 gene. The lack or deficiency of RhAG proteins in the host red blood cell membrane and an impaired function of AQP1 and AQP4 water/gas channels in the central nervous system could be associated with various degrees of brain hypoxia. The proinflammatory changes in brain tissue associated with hypoxia may therefore overlap chronic subclinical neuroinflammation characteristic for individuals with, for example, chronic T. gondii infection or smokers, thus affecting the intensity of changes in personality found in these persons.
The main conclusion of the present study is that RhD phenotype modulates the influence not only of latent toxoplasmosis, but also of at least two other potentially detrimental factors, age and smoking, on human behavior and physiology. Our data showed that the negative effect of smoking on health (estimated on the basis of the self-rated number of common viral and bacterial diseases in the past year) was much stronger in RhD-negative than RhD-positive subjects. We must stress that the data concerning the health status were based on self-reports only. Theoretically, RhD-positive and RhD-negative subjects could be influenced by smoking in the same way, for example by development of negative bioelectrical status of nasopharyngeal mucosa resulting in easier attachment of some pathogenic bacteria [43] or by modulation of local inflammatory processes [44], [45]; however, the RhD-negative subjects may have higher tendency to report more common diseases in the past year just due to their different personality. It is critically needed to confirm the differences in health response to smoking between RhD-positive and RhD-negative subjects by objective medical examination in the future studies.
Funding Statement
The authors‚ work was supported by the Grand Agency of the Czech Republic (grant number P303/11/1398) and Charles University of Prague (GAUK 18810, grant UNCE 204004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Biver S, Scohy S, Szpirer J, Szpirer C, Andre B, et al. (2006) Physiological Role Of The Putative Ammonium Transporter Rhcg In The Mouse. Transfus Clin Biol 13: 167–168. [DOI] [PubMed] [Google Scholar]
- 2. Kustu S, Inwood W (2006) Biological Gas Channels For Nh3 And Co2: Evidence That Rh (Rhesus) Proteins Are Co2 Channels. Transfus Clin Biol 13: 103–110. [DOI] [PubMed] [Google Scholar]
- 3. Gruswitz F, Chaudhary S, Ho Jd, Schlessinger A, Pezeshki B, et al. (2010) Function Of Human Rh Based On Structure Of Rhcg At 2.1 A. Proc Natl Acad Sci U S A 107: 9638–9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carritt B, Kemp TJ, Poulter M (1997) Evolution Of The Human Rh (Rhesus) Blood Group Genes: A 50 Year Old Prediction (Partially) Fulfilled. Hum Mol Genet 6: 843–850. [DOI] [PubMed] [Google Scholar]
- 5. Flegel WA (2006) Molecular Genetics Of Rh And Its Clinical Application. Transfus Clin Biol 13: 4–12. [DOI] [PubMed] [Google Scholar]
- 6. Fisher RA, Race RR, Taylor Gl (1944) Mutation And The Rhesus Reaction. Nature 153: 106. [Google Scholar]
- 7. Haldane JBS (1944) Mutation And The Rhesus Reaction. Nature 153: 106–106. [Google Scholar]
- 8. Novotná M, Havlíček J, Smith AP, Kolbeková P, Skallová A, et al. (2008) Toxoplasma And Reaction Time: Role Of Toxoplasmosis In The Origin, Preservation And Geographical Distribution Of Rh Blood Group Polymorphism. Parasitology 135: 1253–1261. [DOI] [PubMed] [Google Scholar]
- 9. Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma Gondii: From Animals To Humans. Int J Parasitol 30: 1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dabritz HA, Conrad PA (2010) Cats And Toxoplasma: Implications For Public Health. Zoonoses Public Health 57: 34–52. [DOI] [PubMed] [Google Scholar]
- 11. Lindová J, Příplatová L, Flegr J (2012) Higher Extraversion And Lower Conscientiousness In Humans Infected With Toxoplasma . Eur J Person 26: 285–291. [Google Scholar]
- 12. Havlíček J, Gašová Z, Smith AP, Zvára K, Flegr J (2001) Decrease Of Psychomotor Performance In Subjects With Latent ‘Asymptomatic’ Toxoplasmosis. Parasitology 122: 515–520. [DOI] [PubMed] [Google Scholar]
- 13. Kaňková Š, Šulc J, Nouzová K, Fajfrlik K, Frynta D, et al. (2007) Women Infected With Parasite Toxoplasma Have More Sons. Naturwissenschaften 94: 122–127. [DOI] [PubMed] [Google Scholar]
- 14. Flegr J, Lenochová P, Hodný Z, Vondrová M (2011) Fatal Attraction Phenomenon In Humans: Cat Odour Attractiveness Increased For Toxoplasma-Infected Men While Decreased For Infected Women. Plos Neglect Trop Dis 5: E1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torrey EF, Bartko JJ, Yolken RH (2012) Toxoplasma Gondii And Other Risk Factors For Schizophrenia: An Update. Schizophr Bull 38: 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fekadu A, Shibre T, Cleare AJ (2010) Toxoplasmosis As A Cause For Behaviour Disorders - Overview Of Evidence And Mechanisms. Folia Parasitol 57: 105–113. [DOI] [PubMed] [Google Scholar]
- 17. Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, et al. (2012) Incidence Of Adult Brain Cancers Is Higher In Countries Where The Protozoan Parasite Toxoplasma Gondii Is Common. Biol Lett 8: 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flegr J, Havlíček J, Kodym P, Malý M, Šmahel Z (2002) Increased Risk Of Traffic Accidents In Subjects With Latent Toxoplasmosis: A Retrospective Case-Control Study. Bmc Infect Dis 2: Art–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yagmur F, Yazar S, Temel Ho, Cavusoglu M (2010) May Toxoplasma Gondii Increase Suicide Attempt-Preliminary Results In Turkish Subjects? Forensic Sci Int 199: 15–17. [DOI] [PubMed] [Google Scholar]
- 20. Lester D (2010) Predicting European Suicide Rates With Physiological Indices. Psychol Rep 107: 713–714. [DOI] [PubMed] [Google Scholar]
- 21. Kaňková Š, Šulc J, Flegr J (2010) Increased Pregnancy Weight Gain In Women With Latent Toxoplasmosis And Rhd-Positivity Protection Against This Effect. Parasitology 137: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 22. Flegr J, Klose J, Novotná M, Berenreitterová M, Havlíček J (2009) Increased Incidence Of Traffic Accidents In Toxoplasma-Infected Military Drivers And Protective Effect Rhd Molecule Revealed By A Large-Scale Prospective Cohort Study. Bmc Infect Dis 9: Art. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flegr J, Novotná M, Fialová A, Kolbeková P, Gašová Z (2010) The Influence Of Rhd Phenotype On Toxoplasmosis- And Age-Associated Changes In Personality Profile Of Blood Donors. Folia Parasitol 57: 143–150. [PubMed] [Google Scholar]
- 24. Flegr J, Novotná M, Lindová J, Havlíček J (2008) Neurophysiological Effect Of The Rh Factor. Protective Role Of The Rhd Molecule Against Toxoplasma-Induced Impairment Of Reaction Times In Women. Neuroendocrinol Lett 29: 475–481. [PubMed] [Google Scholar]
- 25. Holub D, Bankovská M, Dragomirecká E, Rodriguez M, Preiss M, et al. (2011) Possible Protective Function Of Rh Factor In Schizophrenia. Psychiatrie 15: 37–42. [Google Scholar]
- 26. Cloninger CR (1994) Temperament And Personality. Curr Opin Neurobiol 4: 266–273. [DOI] [PubMed] [Google Scholar]
- 27.Cattell HEP, Mead AD (2008) The Sixteen Personality Factor Questionnaire (16pf). In: GJ Boyle GM, DH, Editors. The Sage Handbook Of Personality Theory And Assessment. Los Angeles: Sage Publications. Pp. 295–312.
- 28.Formann AK, Piswanger K (1979) Wiener Matrizen-Test. Weinheim: Beltz.
- 29.Otis AS (1954) Otis Quick-Scoring Mental Ability Test, New Edition. Tarrytown-On-Hudson, Ny: Word Book Co.
- 30. Flegr J, Hampl R, Černochová D, Preiss M, Bičíkova M, et al. (2012) The Relation Of Cortisol And Sex Hormone Levels To Results Of Psychological, Performance, Iq And Memory Tests In Military Men And Women. Neuroendocrinol Lett 33: 224–235. [PubMed] [Google Scholar]
- 31. Kaňková Š, Holáň V, Zajícová A, Kodym P, Flegr J (2010) Modulation Of Immunity In Mice With Latent Toxoplasmosis - The Experimental Support For The Immunosupression Hypothesis Of Toxoplasma-Induced Changes In Reproduction Of Mice And Humans. Parasitol Res 107: 1421–1427. [DOI] [PubMed] [Google Scholar]
- 32. Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-Analysis Of The Acute Effects Of Nicotine And Smoking On Human Performance. Psychopharmacology 210: 453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murin S, Bilello KS, Matthay R (2000) Other Smoking-Affected Pulmonary Diseases. Clin Chest Med 21: 121–137. [DOI] [PubMed] [Google Scholar]
- 34. Alkjaer T, Pilegaard M, Bakke M, Jensen BR (2005) Effect Of Aging On Performance, Muscle Activation And Perceived Stress During Mentally Demanding Computer Tasks. Scand J Work Environ Health 31: 152–159. [DOI] [PubMed] [Google Scholar]
- 35. Perrot A, Bertsch J (2007) Role Of Age In Relation Between Two Kinds Of Abilities And Performance In Acquisition Of New Motor Skill. Percept Mot Skills 104: 91–101. [DOI] [PubMed] [Google Scholar]
- 36. Raikkonen K, Forsen T, Henriksson M, Kajantie E, Heinonen K, et al. (2009) Growth Trajectories And Intellectual Abilities In Young Adulthood. Am J Epidemiol 170: 447–455. [DOI] [PubMed] [Google Scholar]
- 37. Biernacki M, Tarnowski A (2011) The Effect Of Age And Personality On The Main Cognitive Processes In Drivers. Int J Occup Med Environ Health 24: 367–379. [DOI] [PubMed] [Google Scholar]
- 38. Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, et al. (2012) Age Group And Sex Differences In Performance On A Computerized Neurocognitive Battery In Children Age 8–21. Neuropsychology 26: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wagner FF, Flegel WA (2000) Rhd Gene Deletion Occurred In The Rhesus Box. Blood 95: 3662–3668. [PubMed] [Google Scholar]
- 40. Prandota J (2004) Possible Pathomechanisms Of Sudden Infant Death Syndrome: Key Role Of Chronic Hypoxia, Infection/Inflammation States, Cytokine Irregularities, And Metabolic Trauma In Genetically Predisposed Infants. Am J Ther 11: 517–546. [DOI] [PubMed] [Google Scholar]
- 41. Prandota J (2010) Migraine Associated With Patent Foramen Ovale May Be Caused By Reactivation Of Cerebral Toxoplasmosis Triggered By Arterial Blood Oxygen Desaturation. Int J Neurosci 120: 81–87. [DOI] [PubMed] [Google Scholar]
- 42.Prandota J (2012) Rhesus-Associated Glycoprotein (Rhag) Phenotype Of The Red Blood Cells Modulates T. Gondii Infection-Associated Psychomotor Performance Reaction Times And Changes In The Human Personality Profile. Impaired Function Of The Co2, Aqp1, And Aqp4 Gas Channels May Cause Hypoxia And Thus Enhance Neuroinflammation In Autistic Individuals. In: Gemma C, Editor. Neuroinflammation: Pathogenesis, Mechanisms And Management. New York: Nova Publishers.
- 43. El Ahmer OR, Essery SD, Saadi AT, Raza MW, Ogilvie MM, et al. (1999) The Effect Of Cigarette Smoke On Adherence Of Respiratory Pathogens To Buccal Epithelial Cells. Fems Immunol Med Microbiol 23: 27–36. [DOI] [PubMed] [Google Scholar]
- 44. Raza MW, Essery SD, Elton RA, Weir DM, Busuttil A, et al. (1999) Exposure To Cigarette Smoke, A Major Risk Factor For Sudden Infant Death Syndrome: Effects Of Cigarette Smoke On Inflammatory Responses To Viral Infection And Bacterial Toxins. Fems Immunol Med Microbiol 25: 145–154. [DOI] [PubMed] [Google Scholar]
- 45. Payne JB, Johnson GK, Reinhardt RA, Dyer JK, Maze CA, et al. (1996) Nicotine Effects On Pge(2) And Il-1 Beta Release By Lps-Treated Human Monocytes. J Periodontal Res 31: 99–104. [DOI] [PubMed] [Google Scholar]