Abstract
We used a model involving acute and latent herpes simplex virus (HSV) infections of mouse superior cervical ganglia to assess in vivo neuronal infections with two thymidine kinase-deficient (TK-) mutants of HSV type 1. Despite replication of the TK- HSV strains at the site of inoculation in the eyes, little if any viral replication occurred in the superior cervical ganglia, as assessed by the viral titers of ganglion homogenates, viral antigens in tissue sections, and histopathological evidence of cytopathology or inflammation. Cyclophosphamide-induced immunosuppression and treatment with 6-hydroxydopamine, which enhanced productive infections of superior cervical ganglia with TK+ HSV did not induce TK- HSV ganglionic infections. Latent TK- HSV infections were not detected by cocultivation of ganglion explants. Efforts to infect ganglia in culture after they were removed from animals indicated that superior cervical ganglia, and particularly their neuronal elements, resisted productive TK- HSV infection. These results supported the hypothesis that viral thymidine kinase facilitates acute and reactivated productive HSV infections of neurons.
Full text
PDF

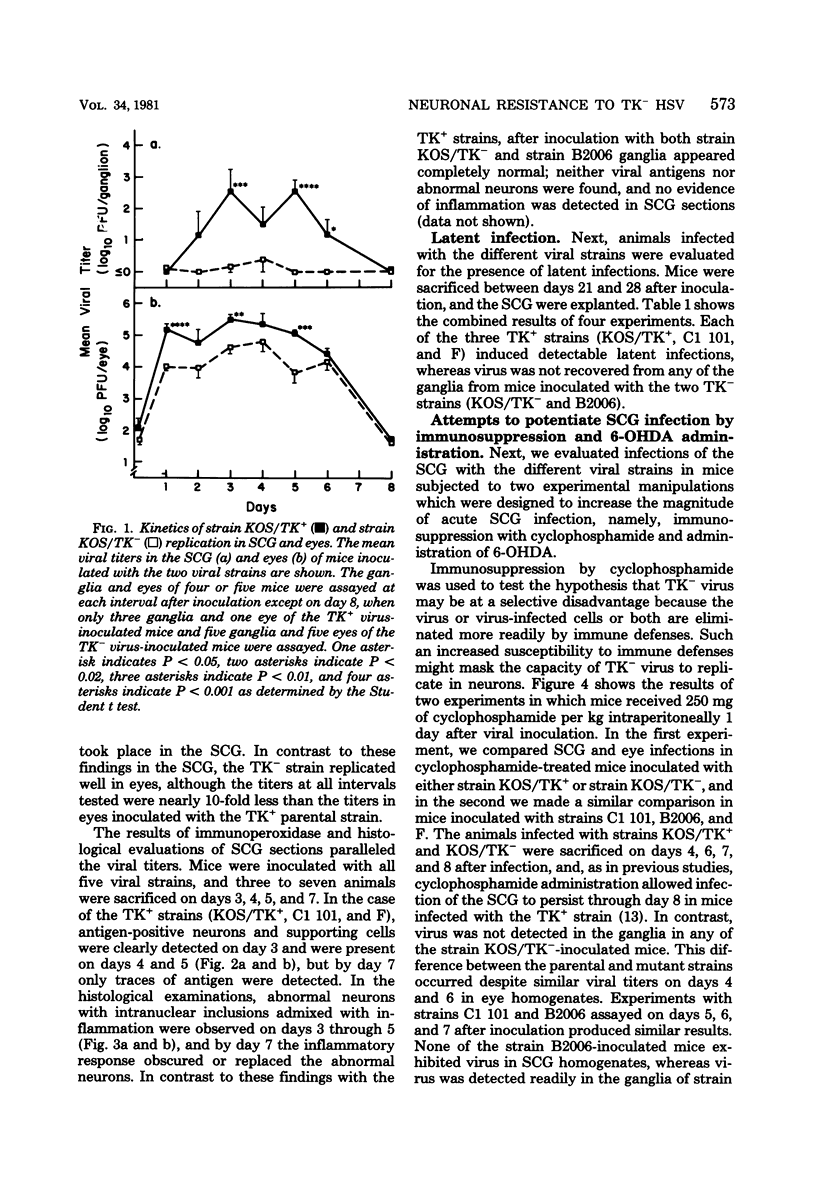

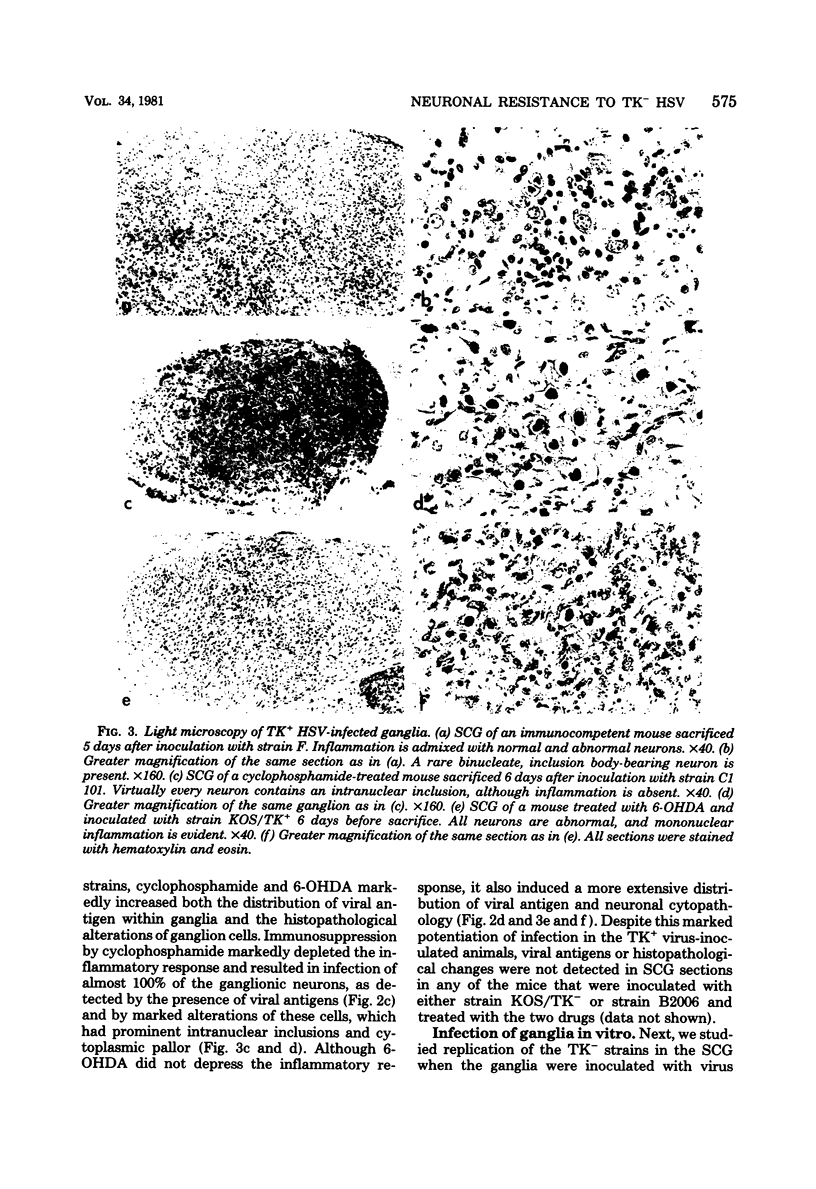
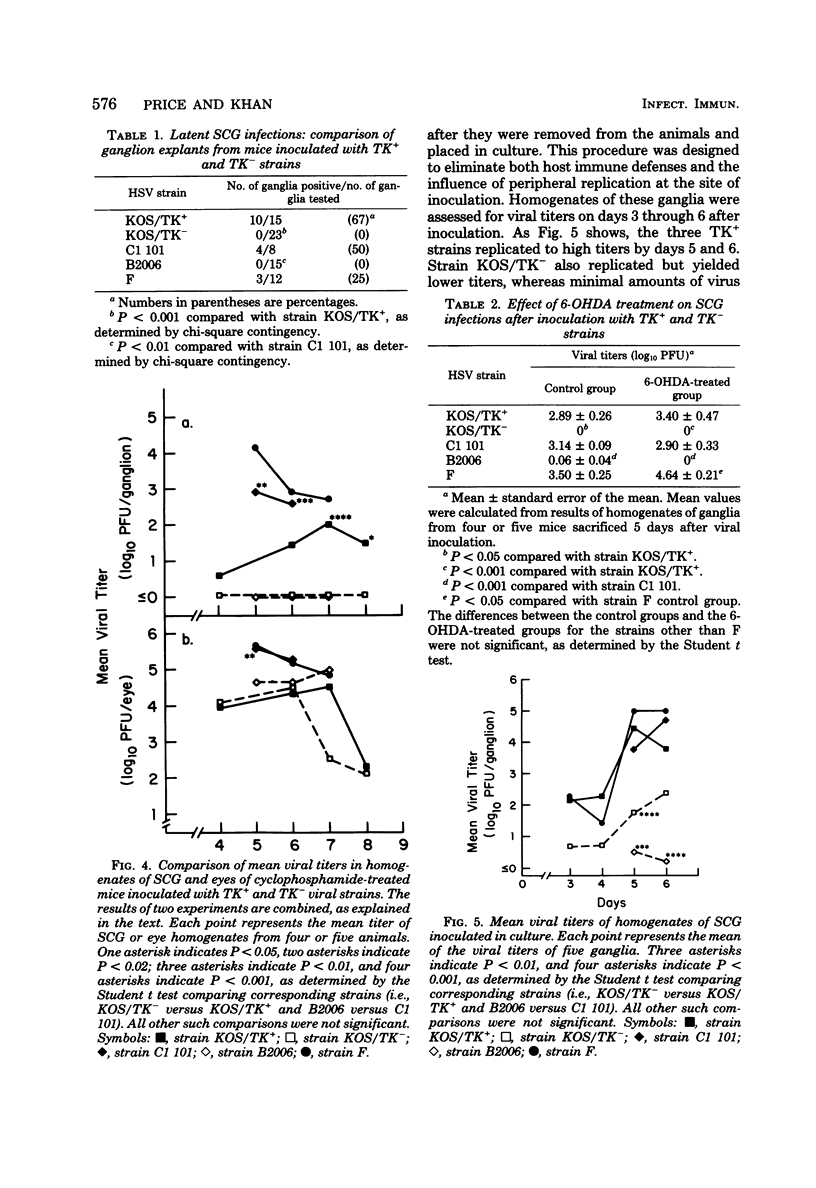

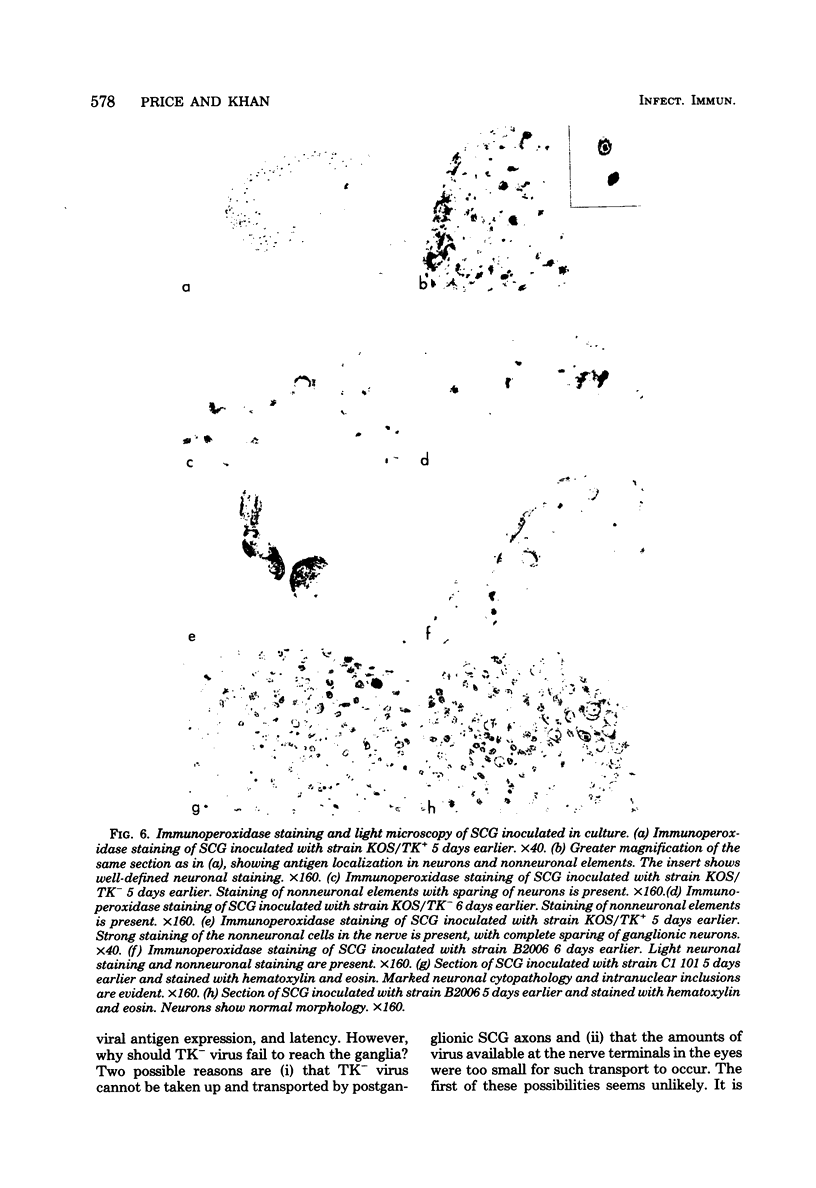


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R. Herpes simplex virus infection of nervous tissue in animals and man. Prog Med Virol. 1975;20:1–26. [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus-specific polypeptides studied by polyacrylamide gel electrophoresis of immune precipitates. J Gen Virol. 1974 Feb;22(2):171–185. doi: 10.1099/0022-1317-22-2-171. [DOI] [PubMed] [Google Scholar]
- Horten B., Price R. W., Jimenez D. Multifocal varicella-zoster virus leukoencephalitis temporally remote from herpes zoster. Ann Neurol. 1981 Mar;9(3):251–266. doi: 10.1002/ana.410090308. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Viral-induced thymidine kinase isozymes. Prog Med Virol. 1975;21:13–34. [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Iltis J. P., Rapp F. Differential effect of arabinofuranosylthymine of the replication of human herpesviruses. J Virol. 1977 Sep;23(3):679–684. doi: 10.1128/jvi.23.3.679-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W. 6-Hydroxydopamine potentiates acute herpes simplex virus infection of the superior cervical ganglion in mice. Science. 1979 Aug 3;205(4405):518–520. doi: 10.1126/science.221984. [DOI] [PubMed] [Google Scholar]
- Price R. W., Schmitz J. Reactivation of latent herpes simplex virus infection of the autonomic nervous system by postganglionic neurectomy. Infect Immun. 1978 Feb;19(2):523–532. doi: 10.1128/iai.19.2.523-532.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Schmitz J. Route of infection, systemic host resistance, and integrity of ganglionic axons influence acute and latent herpes simplex virus infection of the superior cervical ganglion. Infect Immun. 1979 Feb;23(2):373–383. doi: 10.1128/iai.23.2.373-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Wildy P. Deoxypyrimidine kinases of herpes simplex viruses types 1 and 2: comparison of serological and structural properties. J Gen Virol. 1975 Feb;26(2):159–170. doi: 10.1099/0022-1317-26-2-159. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]