Abstract
Extracellular matrix (ECM) production is critical to preserve the function and integrity of mature blood vessels. Toward the engineering of blood vessels, studies have centered on ECM production by supporting cells, whereas few studies implicate endothelial cells (ECs) with ECM synthesis. Here, we elucidate variations between cultured human arterial, venous, and progenitor ECs with respect to ECM deposition assembly, composition, and response to biomolecular and physiological factors. Our studies reveal that progenitor ECs, endothelial colony-forming cells (ECFCs), deposit collagen IV, fibronectin, and laminin that assemble to an organized weblike structure, as confirmed by decellularized cultures. Mature ECs only express these ECM proteins intracellularly. ECFC-derived ECM is abrogated in response to TGFβ signaling inhibition and actin cytoskeleton disruption. Hypoxic (1%) and physiological (5%) O2 tension stimulate ECM deposition from mature ECs. Interestingly, deposition of collagen I is observed only under 5% O2 tension. ECM production from all ECs is found to be regulated by hypoxia-inducible factors 1α and 2α but differentially in the different cell lines. Collectively, we suggest that ECM deposition and assembly by ECs is dependent on maturation stage and oxygen supply and that these findings can be harnessed to advance engineered vascular therapeutics.—Kusuma, S., Zhao, S., Gerecht, S. The extracellular matrix is a novel attribute of endothelial progenitors and of hypoxic mature endothelial cells.
Keywords: vasculature, collagen I, collagen IV, hypoxia-inducible factors
Endothelial cells (ECs) form the dynamic inner lining of blood vessels; this interior layer plays a pivotal role in providing necessary nutrients, facilitating gas exchange, and maintaining homeostasis, among many other functionalities. Stem cells aid the growth and repair of blood vessels; specifically, endothelial progenitor cells (EPCs) that circulate in the bloodstream can be endogenously triggered to hone to sites of ischemia or form vasculatures (1). Endothelial colony-forming cells (ECFCs) are a class of circulating EPCs mainly known for their high proliferation capabilities and contribution to functional vessels (2–4). Because of their regenerative capacity, EPCs have been widely studied for the purpose of developing vascular therapeutics (5–9).
Concentrically surrounding the endothelial layer is its extracellular matrix (ECM), which is critical to preserve the function and integrity of blood vessels as well as engineered vascular substitutes (10, 11). The vascular basement membrane, which largely comprises collagens, laminins, fibronectin, heparin sulfate proteoglycans, and entactin (12), is known to be a crucial mediator of cell behavior during angiogenesis, the growth of new blood vessels (13).
Among myriad growth factors and biochemical signals required for angiogenesis and vessel maturation, transforming growth factor-β (TGFβ), in particular, stimulates ECM deposition to stabilize nascent vessels (14–16). Hypoxia contributes to the complex interplay between vascular cells and their ECM (17). It critically regulates blood vessel growth in ischemic, injured, or cancerous tissue by increasing the expression of numerous genes involved in vessel formation and maturation. Hypoxia-inducible factors 1α and 2α (HIF1α and HIF2α), isoforms of the HIFα transcription factor, accumulate greatly under hypoxic conditions to maintain oxygen homeostasis and play an important role in hypoxia-driven angiogenesis, which is vital for ECM formation in tissue repair (18–22). As such, it has been an emerging focus of vascular tissue engineering.
The ECM of tissues has recently been of immense relevance with the advent of transplantable decellularized tissues that have served as functional and more biomimetic tissue replacements (23, 24). These scaffolds, which are isolated from various cell types and primarily composed of ECM proteins, provide a bioinstructive template for reseeded autologous cells (25–30). The examination of decellularized ECM of human arteries and veins has primarily focused on the evaluation of mechanical properties, longevity, and ability to support recellularization to engineer functional vessels (29–31).
Specific to blood vessels, studies have centered on ECM production by pericytes (32, 33), vascular smooth muscle cells (SMCs; ref. 34), and fibroblasts (35, 36). Studies associating ECs with their ECM have largely deduced that the endothelium modulates its ECM through secreted factors (37, 38), but only few studies unequivocally implicate ECs with ECM synthesis (39). To the best of our knowledge, the endothelial contribution to blood vessel ECM in humans has not been rigorously evaluated, although it has the potential to advance vascular therapeutics.
In the present study, we hypothesize that ECM produced by the endothelial lining is dependent on the maturation stage of the cells and the environmental cues acting on them. For this, we determined the ECM characteristics of human umbilical artery ECs (HUAECs), human umbilical vein ECs (HUVECs), and ECFCs to elucidate variations in ECM composition and response to physiological and biomolecular factors. We anticipate that elucidation of ECM variations between ECFCs, arterial ECs, and venous ECs provides the framework for the development of more suitable vascular tissue engineered substitutes.
MATERIALS AND METHODS
Cell culture
ECFCs (Lonza, Walkersville, MD, USA) were cultured according to manufacturer's instructions in endothelial growth medium 2 (EGM2; Lonza) containing 10% fetal bovine serum (FBS) on type I collagen (BD Biosciences, Franklin Lakes, NJ, USA). HUAECs and HUVECs (Promocell, Heidelberg, Germany) were cultured following ECFC culture conditions to exclude the possibility that variable culture conditions could affect cell behavior. Medium was changed every other day. Cells were passaged every 3–4 d with 0.05% trypsin/0.1% ethylenediaminetetraacetic acid (EDTA; Invitrogen, Carlsbad, CA, USA), and maintained in a humidified incubator at 37°C in 5% CO2 atmosphere. For all experiments, cells were seeded at 8000 cells/cm2 on 4-well chamber slides (Sigma, Allentown, PA, USA) and coated with collagen I in EGM2 as described above. When indicated, EGM2 was supplemented with 10% FBS (for a total of 20%), or higher cell densities (16,000/cm2) were seeded. Medium was changed every other day. For TGFβ inhibitor or Rho-associated kinase (ROCK) inhibitor studies, ECFCs were cultured in EGM2 supplemented with 20 μM TGFβ inhibitor, SB431542 (Tocris, Minneapolis, MN, USA), or 20 μM ROCK inhibitor, Y-27632 (Sigma).
Decellularization of cultures
Cultures were decellularized on the basis of a previously established protocol (40). Briefly, cells were decellularized by washing with PBS and incubating with 0.1% Triton in 50 mM NH4OH. To ensure complete cellular removal, cultures were gently washed with PBS and examined via light microscopy.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed as described previously (41). Briefly, total RNA was isolated from cells using TRIzol (Invitrogen), according to the manufacturer's instructions. Total RNA was quantified using an ultraviolet spectrophotometer and validated for having no DNA contamination. RNA (1 μg/sample) was transcribed using reverse transcriptase M-MLV and oligo(dT) primers (both from Promega, Madison, WI, USA), according to the manufacturer's instructions. We used TaqMan Universal PCR MasterMix and Gene Expression Assay (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions, for genes mentioned in the text in addition to β-ACTIN and HPRT1. The TaqMan PCR step was performed with a StepOne Real-Time PCR system (Applied Biosystems), according to the manufacturer's instructions. The relative expressions of these genes were normalized to HPRT1 or β-ACTIN amount in the same cDNA by using the manufacturer's standard curve method. For each primer set, the comparative computerized tomography method was used to calculate the amplification differences between different samples. The values for experiments were averaged and graphed with standard deviations.
Flow cytometry
Flow cytometry was performed as described previously (41, 42). Briefly, 250,000 live cells in 100 μl 0.1% bovine serum albumin (BSA; Sigma) in PBS were incubated with conjugated antibodies vascular endothelial cadherin–phycoerythrin (VEcad-PE), cluster of differentiation 31 (CD31)-PE, kinase domain receptor (KDR)-PE, CD146-PE, or platelet-derived growth factor receptor β (PDGFRβ)-PE (all from BD). Cells were washed twice with 0.1% BSA and strained through a 0.22-μm filter to eliminate cell clumps. User guide instructions were followed to complete the flow cytometry analysis on a BD FACSCalibur flow cytometer. Forward-side scatterplots were used to exclude dead cells. All analyses were done using IgG-PE (BD) isotype control. Quantification was performed using Cyflogic 1.2.1 (http://www.cyflogic.com) to calculate geometric means and percentage coefficient of variation (CV) of fluorescent peaks.
Immunofluorescence microscopy
After 1, 4, or 7 d, cultures were prepared for immunofluorescence microscopy, as described previously (41–43). Briefly, cells were fixed using 3.7% paraformaldehyde; incubated in 1% BSA, followed by 0.1% Triton-X; and stained with mouse anti-human collagen I (1:100; Abcam, Cambridge, MA, USA), mouse anti-human collagen IV (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-human fibronectin (1:100; Sigma), and rabbit anti-human laminin (1:100; Abcam) for 90 min minimum. The cells were washed with PBS 3 times, and incubated with the appropriate fluorophore—anti-mouse FITC (1:50; Sigma), anti-rabbit Alexa Fluor 488 (1:1000; Invitrogen), or anti-rabbit Alexa Fluor 647 (1:250; Invitrogen)—as well as Phalloidin-546 (1:100; Invitrogen) for 1 h. Cells were washed, counterstained with DAPI to visualize cell nuclei, and imaged using an Olympus BX60 microscope (Olympus, Tokyo, Japan).
Western blot analysis
Western blot was performed as described previously (40). Cell lysates were prepared in a Tris-Triton-X buffer (1% Triton-X, 150 mM NaCl, and 50 mM Tris, pH 7.5) with 1× protease inhibitor cocktail (Thermo Scientific, Waltham, MA, USA), and total protein was quantified via a DC protein assay (Bio-Rad, Hercules, CA, USA). Protein (10 μg/well) was loaded into a 4–20% SDS-PAGE gel (Bio-Rad). Proteins were transferred to nitrocellulose membranes, blocked for 1 h in 3% nonfat milk, and incubated overnight at 4°C and with constant shaking with primary antibodies: rabbit anti-human collagen IV, mouse anti-human fibronectin, and rabbit anti-human laminin (all from Abcam). Membranes were washed, incubated with either anti-rabbit HRP (1:1000) or anti-mouse HRP (1:3000; both from Cell Signaling Technology, Danvers, MA, USA), washed again, and developed using enhanced chemiluminescence (Thermo Scientific). Membranes were visualized using the ChemiDoc XRS+ System (Bio-Rad). Images were acquired using Bio-Rad Quantity One software. Western blots were quantified using Image J (U.S. National Institutes of Health, Bethesda, MD, USA), and data are presented as relative density normalized to GAPDH.
Hypoxia
Hypoxia experiments were conducted as described previously (44). Briefly, cells were cultured in a hermetically sealed, humidified modular incubator chamber (Billups-Rothenberg, Del Mar, CA, USA), which was flushed with an appropriate gas mixture (a 1% O2/5% CO2/N2 balance or 5% O2/5% CO2/N2 balance) 3 times every 30 min at the beginning of each experiment. A plastic Petri dish containing 10 ml of sterile water on the chamber bottom maintained humidity during experiments. Cells were cultured in excess medium (to prevent medium depletion) and allowed to attach in atmospheric O2 for 4–6 h. Percentage area covered by ECM was evaluated from 10 nonoverlapping images (×20) using ImageJ. The images were thresholded to eliminate background. ECM coverage was assessed using the measurements function tool.
siRNA transfection
Cells were transfected with siGenome SmartPool human HIF1α and HIF2α (Dharmacon, Lafayette, CO, USA), using the manufacturer's protocol as we previously described (9, 45). Cells were seeded on a 24-well plate and treated with 50 nM siRNA. mRNA analysis was performed after 24 h. Confirmed transfected cells were used for experiments after 48 h.
Statistical analysis
Flow cytometry and RT-PCR of the three cell types, immunofluorescence of cellular and decellular samples, and inhibition studies were all performed on n ≥ 3 with duplicates. Western blot analysis was performed on n = 2. ANOVA tests were performed with a Bonferroni or Dunnett post hoc test where appropriate (Prism 4.02; GraphPad, La Jolla, CA, USA). Significance levels were set at P < 0.05, P < 0.01, and P < 0.001. All graphical data were reported.
RESULTS
Evaluating ECFCs, HUAECs, and HUVECs
ECFCs, HUAECs, and HUVECs are commonly studied in vascular engineering and regenerative medicine applications (46–49). Thus, understanding inherent differences between these cell types can yield important advancements in vascular regenerative medicine. To evaluate these differences, we examined general EC marker expression as well as specific lineage marker expression.
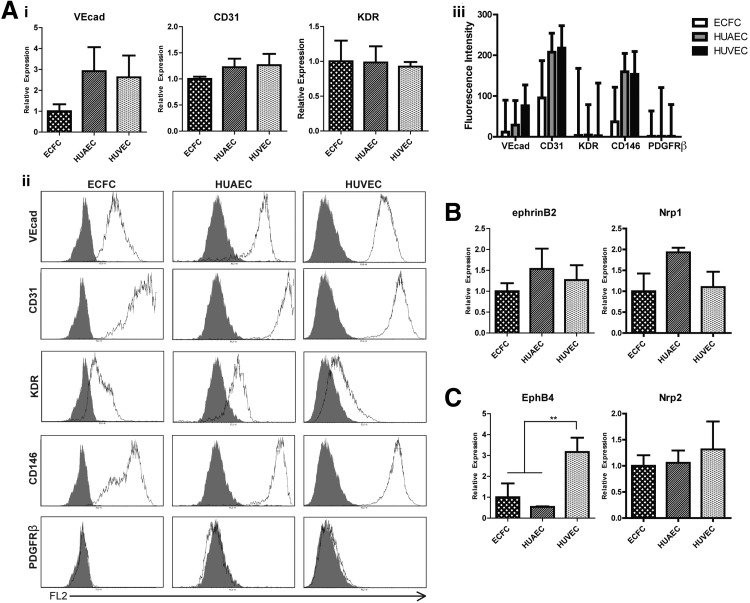
We first sought to characterize inherent differences between the cells with respect to EC marker expression. We found that HUAECs and HUVECs express CD31 and VEcad at similar mRNA levels, which are greater than those of ECFCs (∼3-fold greater expression of VECAD and ∼1.5 greater expression of CD31; Fig. 1Ai). Expression of kinase domain receptor (KDR) is similar between all three cell types. Flow cytometry analysis revealed further differences at the protein level (Fig. 1Aii). The normalized mean fluorescence intensities of VEcad, CD31, and CD146 in HUAECs and HUVECs were greater than those for ECFCs (Fig. 1Aiii). All cells were negative for the SMC marker, PDGFRβ. The percentage CV, which represents the width of the fluorescent peak, was noticeably larger for CD31, KDR, and CD146 in ECFCs, suggesting that there is a wide distribution of expression of these proteins on the progenitor EC population.
Figure 1.
Comparison of ECFCs, HUAECs, and HUVECs. A) i) Quantitative RT-PCR analysis of EC markers. ii) Flow cytometry analysis of EC markers (shade indicates isotype control). iii) Mean fluorescent intensity of EC marker expression normalized to isotype control. Graphed values represent mean ± %CV fluorescent intensity. B, C) Quantitative RT-PCR analysis of arterial markers (B) and venous markers (C). **P <0.01.
As expected, mRNA of arterial markers ephrinB2 and Nrp1 are expressed in greater amounts in HUAECs (Fig. 1B), and venous markers EphB4 and Nrp2 are expressed in greater amounts in HUVECs (Fig. 1C). These data correlate with numerous previous findings confirming the expression of these markers restricted to each particular lineage (50–52); however, we did not see statistically significant differences between these markers in the tested cell types except for EphB4.
ECM composition and protein expression patterns
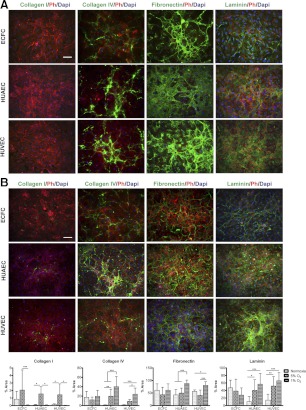
To elucidate whether the tested cell types possessed different ECM characteristics with respect to quantity and composition, we examined the expression of matrix proteins collagen I, collagen IV, fibronectin, and laminin. Samples were analyzed via immunofluorescence microscopy after 1, 4, and 7 d.
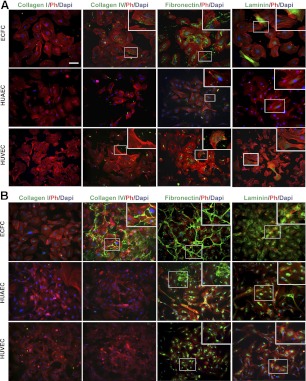
After 1 d, ECFCs produced fibrous collagen IV, which was primarily confined within the cells (Fig. 2A), but no collagen I was detected. The majority of fibronectin appeared within the cells' cytoplasm, yet trace amounts were clearly visible as deposited matrix outside the cells. Laminin was expressed intracellularly in high amounts in particular cells, but this expression pattern was not homogeneous within the cultures. Similar to ECFCs, HUAECs and HUVECs did not produce collagen I after 1 d. HUAECs and HUVECs produced scant amounts of fibrous collagen IV, which was primarily observed extracellularly. Fibronectin was clearly visible within the cells, as well as in trace amounts extracellularly, in both HUAEC and HUVEC cultures. After 4 d, ECM protein characteristics of cultures were markedly distinct from one another (Fig. 2B). Collagen I remained undetected in all cultures; furthermore, cells cultured on gelatin did not deposit collagen I (data not shown). However, both collagen IV and fibronectin expression not only increased strikingly in ECFC cultures, but also assembled into an organized weblike deposition. Laminin expression greatly increased and appeared to form a network-like pattern between cells. Unlike fibronectin and collagen IV, some laminin was still visible within cells. HUAEC and HUVEC cultures had an increased expression of fibronectin and laminin; however, the majority of both matrix proteins were restricted to intracellular regions. Few cells within HUAEC cultures produced fibrous fibronectin, which appeared outside the cells in small sections throughout the confluent cell layer. The intricate expression patterns of fibronectin, collagen IV, and laminin in ECFC cultures continued to be observed after 7 d in culture (data not shown). We further examined whether ECM deposition can be attributed to culture conditions, including serum concentrations and cell growth rates and densities. We observed an increase in the intracellular expression of fibronectin by mature ECs grown in high serum (20%) concentrations or when plated at higher densities, while no change in the deposition of fibronectin was observed (Supplemental Fig. S1A, B). The intracellular fibronectin increase is likely due to additional fibronectin and TGFβ in the serum and increased cell-cell contacts (53). Changes in collagen IV expression and deposition were insignificant in both cases (Supplemental Fig. S1A, B). We also found that the cell growth is comparable among the three cells types (Supplemental Fig. S1C). Collectively, ECFCs seem to produce and deposit ECM proteins in noticeably different patterns compared to mature ECs. Western blot analysis demonstrated that collagen IV, laminin, and fibronectin were visible in ECFCs and HUAECs but less pronounced in HUVECs. We speculate that much of the ECM proteins from HUAECs observed via the blotting assay reflects the intracellular protein expression seen in the corresponding HUAEC immunofluorescence images (Supplemental Fig. S1D).
Figure 2.
Expression of ECM proteins. ECM protein expression after 1 d (A) and 4 d (B). ECM proteins, F-actin (Ph, phalloidin), and nuclei (DAPI) are in green, red, and blue, respectively. Inset: high-magnification view of boxed area. Scale bar =50 μm.
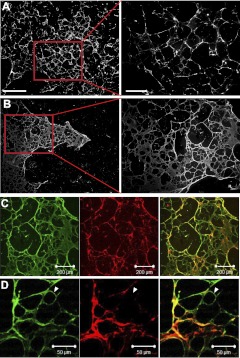
To confirm extracellular deposition and assembly of ECM proteins, cultures of ECFCs, HUAECs, and HUVECs were decellularized after 4 d of culture. Immunofluorescent images of decellularized cultures confirmed our aforementioned findings (Fig. 3). An intricate network of fibronectin and collagen IV was seen in decellularized ECFC cultures (Fig. 3A, B), but not in HUAECs or HUVECs. Few instances of fibronectin expression were detected in decellularized HUAEC cultures (Supplemental Fig. S1E); however, this was not observed in HUVEC cultures (not shown).
Figure 3.
Decellularized ECFC matrices. A, B) Collagen IV (A) and fibronectin (B) production by ECFCs after 4 d. Red outline delineates magnified right panel. Scale bars = 100 μm (left panels); 500 μm (right panels). C, D) Confocal microscopy images of fibronectin (green) and collagen IV (red) matrix produced by ECFCs after 4 d. White arrowheads indicate an area in the ECM made up of only fibronectin.
Spatial organization of ECFC-derived ECM
We sought to further interrogate the ECM produced by ECFCs by examining the spatial relationship between collagen IV and fibronectin on decellularized cultures after 4 d. Previous work examining the relationship between deposited fibronectin and other ECM proteins by fibroblasts revealed that fibronectin is a signal initiator of matrix production (54). Four-day cultures of ECFCs were examined for overlap of fibronectin with collagen IV proteins. Because both matrix proteins appeared in an intricate network-like fashion, we speculated that fibronectin may precede and dictate collagen IV alignment. Three-dimensional projections acquired from confocal microscopy revealed that fibronectin deposition preceded collagen IV deposition, evidenced by areas of ECM that were only occupied by fibronectin (Fig. 3C, D, white arrows). Collagen IV was only visible on top of deposited fibronectin.
Chemical mediators of ECM production
ECM deposition is typically regulated via integrin attachments to the cell's cytoskeleton and is greatly increased in response to TGFβ signaling. We hypothesized that ECM deposition by our ECFC cultures is mediated by TGFβ signaling and cytoskeletal formations. When a TGFβ inhibitor, SB431542, was added to ECFC cultures for 4 d, we found that the network formation of collagen IV and fibronectin previously observed was completely abrogated (Fig. 4A). Both fibronectin and collagen IV were still deposited by ECFCs; however, the intricate network formation observed in control conditions was not observed.
Figure 4.
TGFβ and cytoskeleton effects in ECFC-derived ECM. Effect of TGFβ inhibition (A) and Rho kinase inhibition (B) on ECM production by ECFCs. Scale bar = 100 μm.
When the cytoskeletons of ECFCs were disrupted via a specific Rho-associated kinase inhibitor, Y-27632, phalloidin staining revealed that the cytoskeleton structure was lost (Fig. 4B). Fibronectin expression was visibly reduced and appeared to be intracellular. Collagen IV was still deposited, however, without any distinct fibrous organization. These studies indicate that ECFC-derived ECM production and deposition is regulated by TGFβ signaling and cytoskeletal formations.
Regulation of ECM production by low oxygen tension
ECM production under 1% O2 oxygen tension
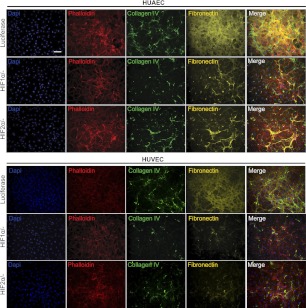
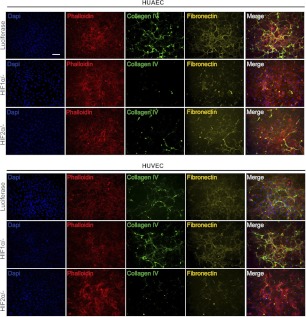
With the prominence of hypoxia in cancerous, ischemic, and injured tissues, we next examined whether ECM deposition is affected by low oxygen (i.e., 1 and 5%) environments. Exposure to hypoxic conditions (1% O2) did not alter the deposition of ECM proteins by ECFCs, which already produced abundant ECM under normoxic conditions. Remarkably, mature ECs deposited collagen IV, fibronectin, and laminin when exposed to 1% O2 for 4 d (Fig. 5A), reminiscent of the ECM produced by ECFCs under atmospheric condition. We observed that this dramatic change in ECM production occurred largely with respect to deposition of a fibrous mesh. Investigation into cell growth kinetics revealed that hypoxic conditions yielded an increased proliferation of mature ECs but not of ECFCs (Supplemental Fig. S1C). To assess ECM production as well as its heterogeneity, we quantified the percentage of area occupied by each ECM protein under the various oxygen tensions (Fig. 5C).
Figure 5.
Regulation of ECM production by oxygen tension. Effect of 1% O2 (A) and 5% O2 (B) on ECM protein expression from ECFCs, HUAECs, and HUVECs. Ph, phalloidin. Scale bar = 100 μm. C) Quantification of percentage area coverage of ECM deposition from tested conditions. *P < 0.05, **P < 0.01, ***P < 0.001.
In HUAEC cultures, collagen IV and fibronectin appeared mesh-like, and the deposited matrix had extensive coverage. Little fibronectin was observed within the cells. HUVEC cultures also increased deposited collagen IV and fibronectin under 1% O2; however, fibronectin was visible both within the cells and as deposited matrix. Laminin appeared as a fibrous mesh in both HUAEC and HUVEC cultures and was much more pronounced in its deposition compared to normoxic conditions. Western blot confirmed that ECM production was increased under 1% O2 conditions (Supplemental Fig. S2A). Collagen I was not detected in any of the cultures.
ECM production under 5% O2 oxygen tension
Under more physiological conditions (5% O2), ECFCs produced and deposited ECM proteins in similar amounts as compared to normoxic conditions (Fig. 5B, C). Mature ECs increased ECM protein production and deposition as well as cell proliferation under 5% O2 as compared to normoxic conditions (Supplemental Figs. S1C and S2A). Collagen IV was increased in mature EC cultures compared to normoxic conditions but appeared similar to that observed under 1% O2 conditions. Although fibronectin appeared as a fibrous mesh under 1% O2 conditions, it was more assembled into a weblike and sprawling organization under 5% conditions; 5% O2-induced fibronectin expression was still greater than deposited fibronectin under normoxic conditions. Laminin production took the form of a fibrous mesh in both HUAEC and HUVEC cultures, similar to that observed under 1% O2 conditions. Quantitative comparison of the percentage of area occupied by these ECM proteins reveals that collagen IV, laminin, and fibronectin production progressively increased with decreasing oxygen tension in mature ECs (Fig. 5C). Production of these three ECM proteins by ECFCs did not vary significantly between different oxygen tensions.
Perhaps the most striking ECM response to 5% O2 was the cells' ability to produce collagen I, which was not observed at either normoxic or 1% O2 conditions. In all three tested cell types, collagen I was observed under 5% O2 and took on a fibrous organization, though scant and heterogeneous in its distribution. Quantitative comparison of the percentage of area occupied by collagen I revealed that collagen I production was not homogenous throughout individual samples (Fig. 5C), as collagen I expression was pronounced in some areas of the samples but scant in others. This heterogeneous expression is reflected in the large sd in the percentage of area occupied by 5% O2-induced collagen I expression in all cell types.
Collectively, these data imply that ECM production, deposition, and assembly by ECFCs, HUAECs, and HUVECs are regulated by oxygen tension. We do not attribute the increased ECM deposition to increased cell density alone, based on the results of the aforementioned studies, which revealed that higher-density mature EC cultures did not increase ECM protein deposition or assembly.
HIF pathways in ECM production
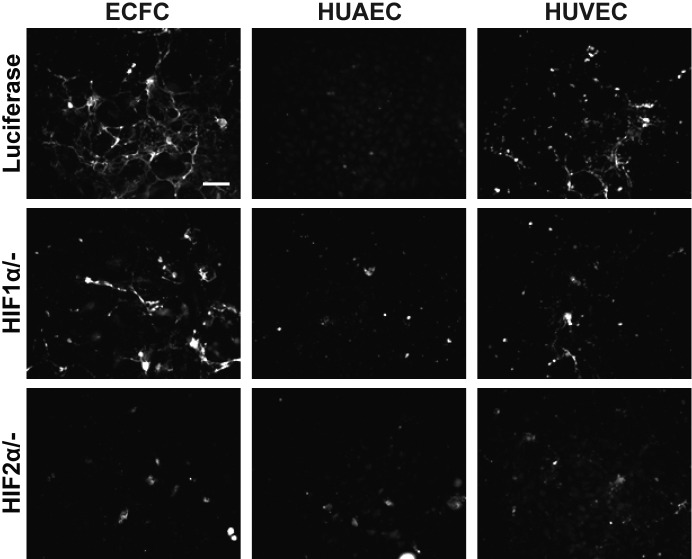
To elucidate the underlying mechanisms regulating ECM deposition in response to low oxygen tension, we studied the roles of HIF1α and HIF2α. We focused on expression of collagens and fibronectin due to their distinct weblike organization under low oxygen tension. Using siRNA (Supplemental Fig. S3), we observed that HIF1α primarily influenced collagen IV and fibronectin deposition by mature ECs under 1% O2 conditions (Fig. 6). HUAECs and HUVECs treated with siRNA for HIF1α exhibited a drastic decrease in collagen IV production. HIF2α-knockdown cells produced some collagen IV, though not to the diminutive levels seen in HIF1α-knockdown cells. Fibronectin production followed the trends of collagen IV production; fibronectin production was most greatly reduced in HIF1α-knockdown cells.
Figure 6.
HIF pathways in 1% O2 conditions. Collagen IV and fibronectin expression under 1% O2 conditions in ECs transfected with siRNA of luciferase, HIF1α, or HIF2α. Scale bar = 100 μm.
Under 5% O2 conditions, we observed opposite trends between HUAECs and HUVECs. HIF1α-knockdown HUAECs did not produce collagen IV, whereas HIF2α-knockdown HUVECs did not produce collagen IV (Fig. 7). In both cell types, fibronectin production followed the trend of collagen IV production. As previously discussed, collagen I was only produced and deposited under 5% O2 conditions. When all three cell types were cultured under 5% O2 conditions, HIF2α-knockdown cells lost the ability to deposit collagen I (Fig. 8). HIF1α-knockdown cells retained a diminished capacity to produce collagen I under these conditions.
Figure 7.
HIF pathways in 5% O2 conditions. Collagen IV and fibronectin expression under 5% O2 conditions in ECs transfected with siRNA of luciferase, HIF1α, or HIF2α. Scale bar = 100 μm.
Figure 8.
HIF pathways in collagen I production under 5% O2 conditions. Collagen I expression under 5% O2 conditions in cells transfected with siRNA of luciferase, HIF1α, or HIF2α. Scale bar = 100 μm.
DISCUSSION
Engineering blood vessel substitutes remains an imposing challenge in vascular tissue engineering (9, 23). Because ECM production is critical to preserve the function and integrity of both native blood vessels and engineered blood vessels (55), understanding the propensity of ECs to deposit ECM could greatly advance this body of work.
We sought to uncover the endothelial contribution to blood vessel ECM. We tested our hypothesis that ECM production is dependent on the maturation stage of the cells by studying mature ECs (i.e., HUAECs and HUVECs) and progenitor ECs (i.e., ECFCs). When we examined whether inherent differences existed in the EC marker expression, we found that HUAECs and HUVECs express higher levels of CD31, VEcad, and CD146 compared to ECFCs, which is indicative of their maturation state, as these markers are typically associated with a more mature endothelial phenotype. KDR, which is expressed widely among EC types, especially early ECs (56), was found to be expressed at similar levels between all cell types. EphrinB2 and Nrp1 are widely regarded as arterial markers (50–52); accordingly, HUAECs exhibited greater expression of these markers compared to HUVECs and ECFCs. EphB4 and Nrp2 are characterized as venous markers; as such, our findings confirmed that these are expressed more greatly in HUVECs compared to HUAECs and ECFCs.
When ECM protein expression and deposition were examined, we observed striking differences between progenitor ECs and mature ECs. Ours is the first study to correlate ECM deposition and assembly with EC maturation stage. Although collagen I was not observed throughout our normoxic studies, collagen IV, fibronectin, and laminin were abundantly expressed as deposited matrix in ECFCs.
Laminin is known to be a primary component of the endothelial basement membrane and maintains the integrity of the endothelium (57). It has been credited as the main biologically active component of the vascular basement membrane. In our studies, laminin was observed as deposited matrix in ECFC cultures under normoxic conditions, suggesting that progenitor ECs contribute to the laminin composition of the basement membrane. Moreover, it has been shown that laminin-modified vascular grafts enhance endothelialization after transplantation (58); thus, the use of autologous progenitor ECs could confer similar benefits owing to their ability to deposit laminin. Also prominent in the vascular basement membrane, collagen IV is known for its role in stabilizing the basement membrane, especially under conditions of mechanical stress (57, 59). Previous studies have cited the ability of microvascular pericytes to produce collagen IV, in addition to laminin, in the vascular ECM (32). Our studies suggest that ECFCs are able to contribute to collagen IV in the endothelial basement membrane, thereby contributing to basement membrane stabilization. Collagen IV is not required for the initial assembly of ECM components (54) and spatial organization analysis of the ECFC-derived ECM revealed that, in fact, collagen IV follows fibronectin deposition.
Though collagen I has been implicated in blood vessel ECM, we could not detect this ECM protein in our normoxic studies. One possibility for this finding is that collagen I has been observed to be produced by ECs undergoing angiogenesis (60), which was not provoked by our normoxic conditions. Another possibility is that vascular collagen I may primarily be produced by vascular SMCs and fibroblasts (35, 36). Another study suggested that vascular ECs from the bovine aortic arch produce collagen I only in the absence of fibroblast growth factor (61). Overall, these studies suggest that collagen I production may require alternate culture conditions, such as additional cell types, different growth factors, or, as our hypoxia studies demonstrated, a lower oxygen tension.
We note that our findings center on the deposition and structural organization of ECM proteins as they relate to vascular tissue engineering. As such, our primary means of analysis was immunofluorescence as it is the most reliable method to document, quantify, and distinguish extracellular vs. intracellular observations. This approach has been widely utilized in the analysis of ECM deposition (53, 62, 63).
We found that ECM production by ECFCs could be altered via biomolecular mediators. TGFβ is a known potent regulator of ECM deposition in a variety of cell types (16); for example, vascular SMCs significantly increase ECM production when cultured on tethered TGFβ (64). In our ECFC cultures, inhibition of TGFβ signaling markedly reduced collagen IV and fibronectin organization, although both ECM proteins were still produced.
The cytoskeleton of cells is linked to the ECM through integrin adhesion receptors (65). Disruption of the actin cytoskeleton architecture via Rho kinase inhibition yielded only unorganized fibronectin and collagen IV, although small amounts of these proteins were still deposited. Previous literature confirms that Rho kinase signaling mediates fibronectin assembly from fibroblasts (66) and matrix production from vascular SMCs (67). Concordant with these findings, our studies reveal that Rho kinase signaling is also critical for ECM production, deposition, and organization of fibronectin and collagen IV in ECFCs.
Hypoxia is among many factors that drives angiogenesis and has been studied for its ability to amplify ECM production (17). By regulating blood vessel growth in ischemic, injured, or cancerous tissue, hypoxia-driven angiogenesis is vital for ECM formation in tissue repair (14). We found that when mature ECs were exposed to 1% or 5% O2, their production and deposition of ECM proteins collagen IV, fibronectin, and laminin were dramatically increased compared to normoxic conditions. Though hypoxic conditions increased cell growth of mature ECs, increased ECM deposition was not affected by increased cell densities alone. Interestingly, although we could not detect any change in the deposition of other ECM proteins from ECFCs cultured under various oxygen conditions, fibrous collagen I was found to be produced when ECFCs were exposed 5% O2. Collagen I was also deposited by HUAECs and HUVECs only under 5% O2. As previously discussed, ECs have been found to produce collagen I when stimulated to undergo angiogenesis (60); analogously, we speculate that our low oxygen conditions triggered an angiogenic response, thereby eliciting collagen I production.
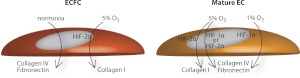
HIFs modulate cellular behavior in response to hypoxia by accumulating and localizing to the nucleus to mediate the angiogenic response (68). HIF1α and HIF2α are stabilized in cells experiencing low O2 tension. HIF1α stabilizes at O2 tension < 1%, whereas HIF2α stabilizes at slightly higher O2 tension, ∼1–5% (44, 69, 70). Concordantly, HIF1α was the primary regulator of collagen IV and fibronectin production in both HUAECs and HUVECs cultured under 1% O2 tension (Fig. 9). At 5% O2, HUAECs and HUVECs displayed contrasting behavior; the production of collagen IV and fibronectin by HUAECs was mediated by HIF1α, whereas collagen IV and fibronectin production by HUVECs was mediated by HIF2α.
Figure 9.
HIF regulation of ECM. Schematic depicting HIF pathways in ECFCs and mature ECs that regulate ECM production under varying oxygen tension.
Knockdown studies at 5% O2 also revealed that collagen I production under low oxygen tension is mediated by HIF2α. HIF2α knockdown cells were unable to deposit collagen I under 5% O2, while HIF1α knockdown cells retained a diminished capacity to produce collagen I under these conditions. HIF2α, in particular, has been vastly implicated in vascular development (21, 22). Embryonic vascular ECs exhibit greater HIF2α mRNA expression compared to most other cell types. It has been previously determined that when HIF2α is deleted from ECs in a murine model, vessel integrity, as well as fibronectin expression, is compromised (21). Our in vitro studies demonstrate a similar fibronectin dependence on HIF2α.
CONCLUSIONS
ECM production is critical to preserve the structure and integrity of engineered tissue. Here, we have defined the parameters and pathways that govern robust ECM production, deposition, and assembly by ECs and reveal that progenitor ECs and mature ECs exhibit disparate behavior with respect to ECM deposition and assembly. Consequently, this work suggests that ECFCs should be considered for the engineering of blood vessels via their deposited ECM.
This work also sheds light on the effect of hypoxia on vasculature ECM. Our studies reveal that when mature cells are stimulated with lower oxygen tensions, they, too, deposit an assembled ECM. Low oxygen-stimulated ECM production by mature ECs resembles normoxic ECM production by ECFCs. Hypoxia is prevalent in injury and tumors, implying that continued studies on deposited ECM of vascular cells may provide much insight into injury recovery or tumor progression. Altogether, these studies can be used as a platform to examine the role of vascular cell-derived ECM in development and disease.
Supplementary Material
Acknowledgments
The authors thank Abigail Hielscher and Hasan Erbil Abaci for their assistance and productive discussions and Bradley Isaacs for technical assistance.
The research was funded by predoctoral awards from the American Heart Association and the U.S. National Institutes of Health (NIH; grant F31HL112644) to S.K., and by NIH grant R01HL107938, an American Heart Association Scientist Development grant, and National Science Foundation grant 1054415 to S.G.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BSA
- bovine serum albumin
- CD
- cluster of differentiation
- CV
- coefficient of variation
- EC
- endothelial cell
- ECFC
- endothelial colony forming cell
- ECM
- extracellular matrix
- EPC
- endothelial progenitor cell
- HIF
- hypoxia-inducible factor
- HUAEC
- human umbilical artery endothelial cell
- HUVEC
- human umbilical vein endothelial cell
- KDR
- kinase domain receptor
- PDGFRβ
- platelet-derived growth factor receptor β
- PE
- phycoerythrin
- SMC
- smooth muscle cell
- TGFβ
- transforming growth factor-β
- VEcad
- vascular endothelial cadherin
REFERENCES
- 1. Takahashi T., Kalka C., Masuda H., Chen D., Silver M., Kearney M., Magner M., Isner J. M., Asahara T. (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434–438 [DOI] [PubMed] [Google Scholar]
- 2. Yoder M. C., Mead L. E., Prater D., Krier T. R., Mroueh K. N., Li F., Krasich R., Temm C. J., Prchal J. T., Ingram D. A. (2007) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109, 1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoder M. C. (2009) Defining human endothelial progenitor cells. J. Thromb. Haemost. 7, 49–52 [DOI] [PubMed] [Google Scholar]
- 4. Hirschi K. K., Ingram D. A., Yoder M. C. (2008) Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 28, 1584–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asahara T., Kawamoto A., Masuda H. (2011) Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells 29, 1650–1655 [DOI] [PubMed] [Google Scholar]
- 6. Silva E. A., Kim E.-S., Kong H. J., Mooney D. J. (2008) Material-based deployment enhances efficacy of endothelial progenitor cells. Proc. Natl. Acad. Sci. 105, 14347–14352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rafii S., Lyden D. (2003) Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 9, 702–712 [DOI] [PubMed] [Google Scholar]
- 8. Melero-Martin J. M., De Obaldia M. E., Kang S.-Y., Khan Z. A., Yuan L., Oettgen P., Bischoff J. (2008) Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 103, 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanjaya-Putra D., Bose V., Shen Y.-I., Yee J., Khetan S., Fox-Talbot K., Steenbergen C., Burdick J. A., Gerecht S. (2011) Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood 118, 804–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis G. E., Senger D. R. (2008) Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr. Opin. Hematol. 15, 197–203 [DOI] [PubMed] [Google Scholar]
- 11. Dickinson L. E., Kusuma S., Gerecht S. (2011) Reconstructing the differentiation niche of embryonic stem cells using biomaterials. Macromol. Biosci. 11, 36–49 [DOI] [PubMed] [Google Scholar]
- 12. Rupert T. (1996) Macromolecular organization of basement membranes. Curr. Opin. Cell Biol. 8, 618–624 [DOI] [PubMed] [Google Scholar]
- 13. Madri J. A. (1997) Extracellular matrix modulation of vascular cell behaviour. Transpl. Immunol. 5, 179–183 [DOI] [PubMed] [Google Scholar]
- 14. Jain R. K. (2003) Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 [DOI] [PubMed] [Google Scholar]
- 15. Pepper M. S. (1997) Transforming growth factor-beta: Vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 8, 21–43 [DOI] [PubMed] [Google Scholar]
- 16. Laping N. J., Grygielko E., Mathur A., Butter S., Bomberger J., Tweed C., Martin W., Fornwald J., Lehr R., Harling J., Gaster L., Callahan J. F., Olson B. A. (2002) Inhibition of transforming growth factor (TGF)-beta induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: SB-431542. Mol. Pharmacol. 62, 58–64 [DOI] [PubMed] [Google Scholar]
- 17. Li L., Liu F., Welser-Alves J. V., McCullough L. D., Milner R. (2011) Upregulation of fibronectin and the a5b1 and avb3 integrins on blood vessels within the cerebral ischemic penumbra. Exp. Neurol. 233, 283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Semenza G. L. (2011) Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 365, 537–547 [DOI] [PubMed] [Google Scholar]
- 19. Rey S., Semenza G. L. (2010) Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 86, 236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Majmundar A. J., Wong W. J., Simon M. C. (2010) Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 40, 294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skuli N., Liu L., Runge A., Wang T., Yuan L., Patel S., Iruela-Arispe L., Simon M. C., Keith B. (2009) Endothelial deletion of hypoxia-inducible factor-2α (HIF-2α) alters vascular function and tumor angiogenesis. Blood 114, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duan L.-J., Zhang-Benoit Y., Fong G.-H. (2005) Endothelium-intrinsic requirement for HIF-2α during vascular development. Circulation 111, 2227–2232 [DOI] [PubMed] [Google Scholar]
- 23. Kusuma S., Gerecht S. (2010) Engineering blood vessels using stem cells: innovative approaches to treat vascular disorders. Expert Rev. Cardiovasc. Ther. 8, 1433–1445 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt C. E., Baier J. M. (2000) Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 21, 2215–2231 [DOI] [PubMed] [Google Scholar]
- 25. Schenke-Layland K., Riemann I., Opitz F., König K., Halbhuber K. J., Stock U. A. (2004) Comparative study of cellular and extracellular matrix composition of native and tissue engineered heart valves. Matrix Biol. 23, 113–125 [DOI] [PubMed] [Google Scholar]
- 26. Ott H. C., Matthiesen T. S., Goh S. K., Black L. D., Kren S. M., Netoff T. I., Taylor D. A. (2008) Perfusion-decellularized matrix: Using nature's platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 [DOI] [PubMed] [Google Scholar]
- 27. Uygun B. E., Soto-Gutierrez A., Yagi H., Izamis M. L., Guzzardi M. A., Shulman C., Milwid J., Kobayashi N., Tilles A., Berthiaume F., Hertl M., Nahmias Y., Yarmush M. L., Uygun K. (2010) Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang B., Zhang Y., Zhou L., Sun Z., Zheng J., Chen Y., Dai Y. (2010) Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng. C Methods 16, 1201–1211 [DOI] [PubMed] [Google Scholar]
- 29. Quint C., Kondo Y., Manson R. J., Lawson J. H., Dardik A., Niklason L. E. (2011) Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. 108, 9214–9219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gui L., Muto A., Chan S. A., Breuer C. K., Niklason L. E. (2009) Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. A 15, 2665–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou M., Liu Z., Wei Z., Liu C., Qiao T., Ran F., Bai Y., Jiang X., Ding Y. (2009) Development and validation of small-diameter vascular tissue from a decellularized scaffold coated with heparin and vascular endothelial growth factor. Artif. Organs 33, 230–239 [DOI] [PubMed] [Google Scholar]
- 32. Jeon H., Ono M., Kumagai C., Miki K., Morita A., Kitagawa Y. (1996) Pericytes from microvessel fragment produce type IV collagen and multiple laminin isoforms. Biosci. Biotechnol. Biochem. 60, 856–861 [DOI] [PubMed] [Google Scholar]
- 33. Stratman A. N., Davis G. E. (2012) Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: Influence on vascular tube remodeling, maturation, and stabilization. Microsc. Microanal. 18, 68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gong Z., Niklason L. E. (2008) Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 22, 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sansilvestri-Morel P., Rupin A., Jaisson S., Fabiani J. N., Verbeuren T. J., Vanhoutte P. M. (2002) Synthesis of collagen is dysregulated in cultured fibroblasts derived from skin of subjects with varicose veins as it is in venous smooth muscle cells. Circulation 106, 479–483 [DOI] [PubMed] [Google Scholar]
- 36. Pickering J. G., Ford C. M., Tang B., Chow L. H. (1997) Coordinated effects of fibroblast growth factor-2 on expression of fibrillar collagens, matrix metalloproteinases, and tissue inhibitors of matrix metalloproteinases by human vascular smooth muscle cells: evidence for repressed collagen production and activated degradative capacity. Arterioscler. Thromb. Vasc. Biol. 17, 475–482 [DOI] [PubMed] [Google Scholar]
- 37. Villanueva A. G., Farber H. W., Rounds S., Goldstein R. H. (1991) Stimulation of fibroblast collagen and total protein formation by an endothelial cell-derived factor. Circ. Res. 69, 134–141 [DOI] [PubMed] [Google Scholar]
- 38. Kuruvilla L., Nair R. R., Umashankar P. R., Lal A. V., Kartha C. C. (2007) Endocardial endothelial cells stimulate proliferation and collagen synthesis of cardiac fibroblasts. Cell Biochem. Biophys. 47, 65–72 [DOI] [PubMed] [Google Scholar]
- 39. Vartanian K. B., Kirkpatrick S. J., McCarty O. J. T., Vu T. Q., Hanson S. R., Hinds M. T. (2009) Distinct extracellular matrix microenvironments of progenitor and carotid endothelial cells. J. Biomed. Mater. Res. A. 91A, 528–539 [DOI] [PubMed] [Google Scholar]
- 40. Cuddy A. C., Qiu C., Gerecht S. (2012) Breast cancer cell-derived matrix supports vascular morphogenesis. Am. J. Physiol. Cell Physiol. 302, C1243–C1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dickinson L. E., Moura M. E., Gerecht S. (2010) Guiding endothelial progenitor cell tube formation using patterned fibronectin surfaces. Soft Matter 6, 5109–5119 [Google Scholar]
- 42. Vo E., Hanjaya-Putra D., Zha Y., Kusuma S., Gerecht S. (2010) Smooth-muscle-like cells derived from human embryonic stem cells support and augment cord-like structures in vitro. Stem Cell Rev. Rep. 6, 237–247 [DOI] [PubMed] [Google Scholar]
- 43. Sun G., Kusuma S., Gerecht S. (2012) Development of a biodegradable, temperature-sensitive dextran-based polymer as a cell-detaching substrate. Macromol. Biosci. 12, 21–28 [DOI] [PubMed] [Google Scholar]
- 44. Abaci H. E., Truitt R., Luong E., Drazer G., Gerecht S. (2010) Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 298, C1527–C1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abaci H. E., Truitt R., Tan S., Gerecht S. (2011) Unforeseen decreases in dissolved oxygen levels affect tube formation kinetics in collagen gels. Am. J. Physiol. Cell Physiol. 301, C431–C440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Critser P. J., Voytik-Harbin S. L., Yoder M. C. (2011) Isolating and defining cells to engineer human blood vessels. Cell. Prolif. 44, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schechner J. S., Crane S. K., Wang F., Szeglin A. M., Tellides G., Lorber M. I., Bothwell A. L. M., Pober J. S. (2003) Engraftment of a vascularized human skin equivalent. FASEB J. 17, 2250–2256 [DOI] [PubMed] [Google Scholar]
- 48. Ito Y., Hasuda H., Terai H., Kitajima T. (2005) Culture of human umbilical vein endothelial cells on immobilized vascular endothelial growth factor. J. Biomed. Mater. Res. A 74, 659–665 [DOI] [PubMed] [Google Scholar]
- 49. Kraehenbuehl T. P., Ferreira L. S., Zammaretti P., Hubbell J. A., Langer R. (2009) Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials 30, 4318–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Torres-Vazquez J., Kamei M., Weinstein B. M. (2003) Molecular distinction between arteries and veins. Cell Tissue Res. 314, 43–59 [DOI] [PubMed] [Google Scholar]
- 51. Wang H. U., Chen Z. F., Anderson D. J. (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741–753 [DOI] [PubMed] [Google Scholar]
- 52. Aitsebaomo J., Portbury A. L., Schisler J. C., Patterson C. (2008) Brothers and sisters: Molecular insights into arterial-venous heterogeneity. Circ. Res. 103, 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Papadimitriou E., Unsworth B. R., Maragoudakis M. E., Lelkes P. I. (1993) Time course and quantification of extracellular matrix maturation in the chick chorioallantoic membrane and in cultured endothelial cells. Endothelium 1, 207–219 [Google Scholar]
- 54. Soucy P. A., Romer L. H. (2009) Endothelial cell adhesion, signaling, and morphogenesis in fibroblast-derived matrix. Matrix Biol. 28, 273–283 [DOI] [PubMed] [Google Scholar]
- 55. Heydarkhan-Hagvall S., Esguerra M., Helenius G., Söderberg R., Johansson B. R., Risberg B. (2006) Tissue Eng. 12, 831–842 [DOI] [PubMed] [Google Scholar]
- 56. Yamaguchi T. P., Dumont D. J., Conlon R. A., Breitman M. L., Rossant J. (1993) Flk-1, an fit-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118, 489–498 [DOI] [PubMed] [Google Scholar]
- 57. Davis G. E., Senger D. R. (2005) Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093–1107 [DOI] [PubMed] [Google Scholar]
- 58. Williams S. K., Kleinert L. B., Patula-Steinbrenner V. (2011) Accelerated neovascularization and endothelialization of vascular grafts promoted by covalently bound laminin type 1. J. Biomed. Mater. Res. A 99A, 67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Poschl E., Schlotzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. (2004) Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131, 1619–1628 [DOI] [PubMed] [Google Scholar]
- 60. Iruela-Arispe M. L., Diglio C. A., Sage E. H. (1991) Modulation of extracellular matrix proteins by endothelial cells undergoing angiogenesis in vitro. Arterioscler. Thromb. Vasc. Biol. 11, 805–815 [DOI] [PubMed] [Google Scholar]
- 61. Tseng S. C. G., Savion N., Stern R., Gospodarowicz D. (1982) Fibroblast growth factor modulates synthesis of collagen in cultured vascular endothelial cells. Eur. J. Biochem. 122, 355–360 [DOI] [PubMed] [Google Scholar]
- 62. Mathews A., Colombus S., Krishnan V. K., Krishnan L. K. (2011) Vascular tissue construction on poly(ε-caprolactone) scaffolds by dynamic endothelial cell seeding: effect of pore size. J. Tissue Eng. Regen. Med. 6, 451–461 [DOI] [PubMed] [Google Scholar]
- 63. Zeiger A. S., Loe F. C., Li R., Raghunath M., Van Vliet K. J. (2012) Macromolecular crowding directs extracellular matrix organization and mesenchymal stem cell behavior. PLoS ONE 7, e37904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mann B. K., Schmedlen R. H., West J. L. (2001) Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 22, 439–444 [DOI] [PubMed] [Google Scholar]
- 65. Hotchin N. A., Hall A. (1995) The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J. Cell Biol. 131, 1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yoneda A., Ushakov D., Multhaupt H. A. B., Couchman J. R. (2007) Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol. Biol. Cell 18, 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chapados R., Abe K., Ihida-Stansbury K., McKean D., Gates A. T., Kern M., Merklinger S., Elliott J., Plant A., Shimokawa H., Jones P. L. (2006) ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ. Res. 99, 837–844 [DOI] [PubMed] [Google Scholar]
- 68. Nilsson I., Shibuya M., Wennström S. (2004) Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp. Cell Res. 299, 476–485 [DOI] [PubMed] [Google Scholar]
- 69. Takahashi R., Kobayashi C., Kondo Y., Nakatani Y., Kudo I., Kunimoto M., Imura N., Hara S. (2004) Subcellular localization and regulation of hypoxia-inducible factor-2α in vascular endothelial cells. Biochem. Biophys. Res. Commun. 317, 84–91 [DOI] [PubMed] [Google Scholar]
- 70. Holmquist-Mengelbier L., Fredlund E., Löfstedt T., Noguera R., Navarro S., Nilsson H., Pietras A., Vallon-Christersson J., Borg A., Gradin K., Poellinger L., Påhlman S. (2006) Recruitment of HIF-1a and HIF-2a to common target genes is differentially regulated in neuroblastoma: HIF-2a promotes an aggressive phenotype. Cancer Cell 10, 413–423 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.