Abstract
When it is difficult to develop selective ligands within a family of related G-protein-coupled receptors (GPCRs), chemically engineered receptors activated solely by synthetic ligands (RASSLs) are useful alternatives for probing receptor function. In the present work, we explored whether a RASSL of the free fatty acid receptor 2 (FFA2) could be developed on the basis of pharmacological variation between species orthologs. For this, bovine FFA2 was characterized, revealing distinct ligand selectivity compared with human FFA2. Homology modeling and mutational analysis demonstrated a single mutation in human FFA2 of C4.57G resulted in a human FFA2 receptor with ligand selectivity similar to the bovine receptor. This was exploited to generate human FFA2-RASSL by the addition of a second mutation at a known orthosteric ligand interaction site, H6.55Q. The resulting FFA2-RASSL displayed a >100-fold loss of activity to endogenous ligands, while responding to the distinct ligand sorbic acid with pEC50 values for inhibition of cAMP, 5.83 ± 0.11; Ca2+ mobilization, 4.63 ± 0.05; ERK phosphorylation, 5.61 ± 0.06; and dynamic mass redistribution, 5.35 ± 0.06. This FFA2-RASSL will be useful in future studies on this receptor and demonstrates that exploitation of pharmacological variation between species orthologs is a powerful method to generate novel chemically engineered GPCRs.—Hudson, B. D., Christiansen, E., Tikhonova, I. G., Grundmann, M., Kostenis, E., Adams, D. R., Ulven, T., Milligan, G. Chemically engineering ligand selectivity at the free fatty acid receptor 2 based on pharmacological variation between species orthologs.
Keywords: GPCR, RASSL
In recent times, it has become apparent that a series of molecules previously considered only as metabolic intermediates are actually able to mediate at least a number of their functions via activation of members of the G-protein-coupled receptor (GPCR) superfamily. These include intermediates of the tricarboxylic acid cycle, such as succinate (1), and molecules such as lactate (2) and β-hydroxybutyrate (3), involved in glucogenic and ketogenic control of metabolism. A further group of such intermediates are the free fatty acids. It is now known that three related GPCRs, free fatty acid receptors 1–3 (FFA1, FFA2, and FFA3; previously designated GPR40, GPR43, and GPR41, respectively; ref. 4), respond to either medium- and longer-chain (FFA1) or short-chain, C1-C5, (FFA2 and FFA3) free fatty acids. There is considerable interest in the physiological roles of FFA2, in particular (5, 6), and whether this receptor might be useful as a novel therapeutic target in areas ranging from diabetes and adiposity to satiety and inflammation (7, 8). In the absence of synthetic ligands that bind to the same region of the receptor as the short-chain fatty acids (SCFAs) and that have reasonable potency and substantial selectivity between FFA2 and FFA3, the marked overlap of potency of C1–C5 fatty acids at FFA2 and FFA3 makes efforts to interpret selective activation of FFA2 vs. FFA3 impractical without more detailed analyses involving knockout or knockdown studies (9–11). It would, therefore, be of considerable value to develop chemically engineered forms of these receptors with unique ligand responsiveness.
Such modified GPCRs have been developed for several other receptors and are often described as either designer receptors exclusively activated by designed drugs (DREADDs) or as receptors activated solely by synthetic ligands (RASSLs) (12–15). To date, two general approaches have been employed to generate these chemically engineered GPCRs (16). The first involves site-directed mutagenesis of known ligand interaction sites in the GPCR followed by screening ligands for activity at the resulting mutant receptors (17), while the second method takes the opposite approach, generating thousands of randomly mutated forms of the receptor and screening these against a candidate synthetic ligand (13). Although each of these approaches has found some success, both rely largely on random screening. Considering this, in the present work, we have explored whether a more direct approach could be taken to develop chemically engineered forms of human FFA2 (hFFA2), based on the variation between species orthologs of this receptor.
Mammalian species orthologs of GPCRs are anticipated to respond to the same endogenously produced agonists. However, the potency and affinity of such agonist ligands may vary depending on the physiology of individual species, and where such receptors respond to a number of related ligands (as in the case of FFA2 and the SCFAs; refs. 4, 18), the rank order of function may differ. Although such variation is likely to be limited for GPCRs that coordinate the responses of ancient hormone and transmitter systems, such as the catecholamines, that underpin key physiological processes, including heart rate and intraneural communication, such differences may be substantially greater for GPCRs that play more modulatory roles, or in cases where the receptor is likely to be exposed to vastly different concentrations of ligand between different species. Because SCFAs are primarily produced by the fermentation of nondigestible carbohydrates by the microflora in the gut (6), it could be hypothesized that FFA2 will show significant ortholog variation between species that have greatly different dietary levels of nondigestible carbohydrates and, therefore, different levels of endogenous SCFA ligands. Considering this, in the present work, the bovine ortholog was chosen as the basis for producing chemically engineered forms of hFFA2, since ruminants, such as bovines, are well known to rely heavily on nondigestible carbohydrates.
MATERIALS AND METHODS
Cell culture, transfection, and production of stably expressing cell lines
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS at 37°C and 5% CO2. For experiments utilizing transiently transfected HEK293 cells, transfections were carried out using polyethylenimine, and experiments were conducted 48 h post-transfection. For experiments in which stable cell lines were produced and used, the Flp-In T-REx system (Life Technologies, Paisley, UK) was used to generate HEK293 cells with tetracycline-inducible expression of the receptor of interest. To generate these cell lines, Flp-In T-REx HEK293 cells were cotransfected with the pOG44 vector and the receptor of interest in pcDNA5/FRT/TO. Transfection with pOG44 drives expression of Flp recombinase, which, in turn, allows for recombination between FRT sites in pcDNA5/FRT/TO and in the genome of the Flp-In T-REx HEK293 cells, thus allowing stable inducible cells for the receptor of interest to be generated by appropriate antibiotic selection.
DNA constructs
Constructs for the human orthologs of FFA2 and FFA3 fused at their C-terminal with enhanced yellow fluorescent protein (eYFP) were as reported previously (19). To clone the bovine orthologs, the coding sequences of bovine FFA2 and FFA3 (bFFA2 and bFFA3) were amplified without their stop codons from commercially available bovine genomic DNA by PCR. The PCR product was then ligated upstream and in-frame with the eYFP sequence present in a pcDNA5/FRT/TO expression vector (Life Technologies, Carlsbad, CA, USA). Mutagenesis of human and bovine orthologs of FFA2 was carried out according to the QuickChange method (Stratagene, Santa Clara, CA, USA).
Compounds
Formic acid (compound C1), acetic acid (compound C2), propionic acid (compound C3), butyric acid (compound C4), valeric acid (compound C5), caproic acid (compound C6), heptanoic acid (compound C7), caprylic acid (compound C8), pelargonic acid (compound C9), methylthioacetic acid (compound 1), 3-methylbutyric acid (compound 2), pivalic acid (compound 3), 2-methylbutyric acid (compound 4), cyclopropylcarboxylic acid (compound 5), cyclobutylcarboxylic acid (compound 6), 1-methylcyclopropanecarboxylic acid (compound 7), vinylacetic acid (compound 9), 3-pentenoic acid (compound 12), acrylic acid (compound 13), propiolic acid (compound 14), 2-butynoic acid (compound 15), trans-crotonic acid (compound 16), 2-methylacrylic acid (compound 18), 3-methylcrotonic acid (compound 19), trans-2-methylcrotonic acid (compound 20), trans-2-pentenoic acid (compound 22), trans-2-hexenoic acid (compound 23), 2,4-pentadienoic acid (compound 24), sorbic acid (compound 25), 4,4,4-trifluoro-3-methyl-2-butenoic acid (compound 26), 1-cyclopentenecarboxylic acid (compound 27), trans-cinnamic acid (compound 28), and 1-cyclohexene-1-carboxylic acid (compound 29) were all purchased from Sigma-Aldrich (Dorset, UK). Angelic acid (compound 21) was obtained from ABCR (Karlsruhe, Germany). Cyclopropylacetic acid (compound 8) was purchased from Alfa Aesar (Heysham, UK). 3-Butynoic acid (compound 10) was synthesized as described previously (20). The identity and purity of all compounds were confirmed by 1H and 13C NMR.
β-Arrestin-2 interaction assay
A bioluminescence resonance energy transfer (BRET)-based approach was used to measure β-arrestin-2 recruitment to human and bovine forms of FFA2. Briefly, a plasmid encoding an eYFP-tagged form of the receptor to be assayed was cotransfected in a 4:1 ratio with a β-arrestin-2 Renilla luciferase (Rluc) plasmid. Cells were transferred into white 96-well plates at 24 h post-transfection. Then, at 48 h post-transfection, cells were washed, and the culture medium was replaced with Hanks' balanced salt solution (HBSS) immediately prior to conducting the assay. To measure β-arrestin-2 recruitment, the Rluc substrate coelenterazine h was added to a final concentration of 5 μM; then, cells were incubated for 10 min at 37°C, test compounds were next added, and cells were incubated for a further 5 min at 37°C. BRET, resulting from FFA2 receptor-β-arrestin-2 interaction, was then assessed by measuring the ratio of luminescence at 535 and 475 nm using a Pherastar FS fitted with the BRET1 optic module (BMG Labtech, Aylesbury, UK).
Extracellular signal-regulated kinase (ERK) 1/2 phosphorylation assay
All ERK phosphorylation experiments were carried out using Flp-In T-REx stable-inducible cell lines for the human or bovine forms of FFA2 to be assayed. Briefly, 80,000 cells/well were seeded in a 96-well plate and then allowed to attach for 3–6 h before the addition of doxycycline (100 ng/ml) to induce expression of the receptor. After incubating overnight, the culture medium was replaced with serum-free DMEM-containing doxycycline (100 ng/ml), and cells were then incubated for a further 5 or 6 h prior to the assay. For the assay, test compounds were added to the cells and incubated at 37°C for 5 min before the cells were lysed and assayed for phospho-ERK using an Alphascreen-based detection kit (Perkin Elmer, Waltham, MA, USA), according to the manufacturer's protocol.
Ca2+ mobilization assay
All Ca2+ experiments were carried out using Flp-In T-REx stable-inducible cell lines for the human or bovine forms of FFA2 to be studied. Cells were plated at 80,000 cells/well in black 96-well plates with clear bottoms and then allowed to adhere for 3–6 h. Doxycycline was then added (100 ng/ml) to induce expression of the receptor of interest, and cells were maintained in culture overnight. Prior to the assay, cells were labeled for 45 min with the calcium-sensitive dye Fura-2 AM; then they were washed and maintained in HBSS. Fura-2 fluorescent emission at 510 nm resulting from 340- or 380-nm excitation was then monitored using a Flexstation plate reader (Molecular Devices, Sunnyvale, CA, USA). Basal fluorescence was measured for 16 s, test compounds were then added, and fluorescence was measured for an additional 74 s. The maximum difference in 340/380 ratios obtained before and after compound addition was then used to plot concentration-response data.
cAMP assay
All cAMP experiments were carried out using Flp-In T-REx stable-inducible cell lines for the forms of FFA2 to be studied. These experiments were carried out using a homogenous time-resolved FRET-based detection kit (CisBio Bioassays; CisBio, Codolet, France) according to the manufacturer's protocol. Cells were plated at 2000 cells/well in low-volume 384-well plates, and the inhibition of 1 μM forskolin-stimulated cAMP production was assessed following a 30-min coincubation with test compounds.
[35S]GTPγS incorporation assay
Total cell membranes were prepared from stable, doxycyline-inducible Flp-In T-REx HEK293 cell lines. [35S]GTPγS binding assays were then carried out in reactions with 5 μg of cell membrane protein preincubated for 15 min at 25°C in assay buffer (50 mM Tris-HCl, pH 7.4; 10 mM MgCl2; 100 mM NaCl; 1 mM EDTA; 1 μM GDP; and 0.5% fatty acid-free BSA) containing the indicated concentrations of ligands. The reaction was initiated with the addition of 50 nCi of [35S]GTPγS to each tube, and the reaction was terminated after 1 h incubation by rapid filtration through GF/C glass filters. Unbound radioligand was washed from filters by 3 washes with ice-cold wash buffer (50 mM Tris-HCl, pH 7.4, and 10 mM MgCl2), and [35S]GTPγS binding was determined by liquid scintillation spectrometry.
Homology modeling
Modeling of hFFA2 was carried out using the β2-adrenergic receptor structure as a template (20, 21). The bFFA2 homology model was constructed on the basis of this hFFA2 model using the Prime module of Schrödinger software with default options (Schrödinger LLC, New York, NY, USA).
Dynamic mass redistribution (DMR) assays
DMR assays were performed on a beta version of the Corning Epic biosensor (Corning Life Sciences, Corning, NY, USA), as described previously in detail (22, 23). Briefly, stable inducible cell lines expressing the hFFA2 or hFFA2-C4.57G/H6.55Q mutant were grown to confluence for 20–24 h on fibronectin-coated Epic biosensor 384-well microplates. Cells were then washed twice with HBSS containing 20 mM HEPES and kept for 1 h in the Epic reader at 28°C. DMR was monitored before (baseline read) and after the addition of compound solutions for ≥6000 s. Concentration-effect curves were generated from the real-time optical traces using the area under the curve between 0 and 6000 s after ligand addition.
Curve fitting and statistical analysis
All data presented represent means ± se of ≥3 independent experiments. Data analysis and curve fitting were carried out using the GraphPad Prism 5.0b software package (GraphPad, San Diego, CA, USA). Concentration-response data were fit to 3-parameter sigmoidal concentration-response curves. Statistical analysis of curve fit parameters was carried out by independently fitting the data from triplicate experiments and comparing the resulting curve fit values by t test or 1-way ANOVA, as appropriate.
RESULTS
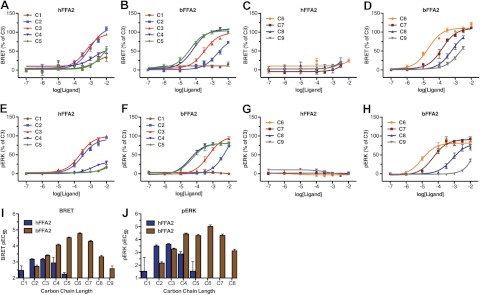
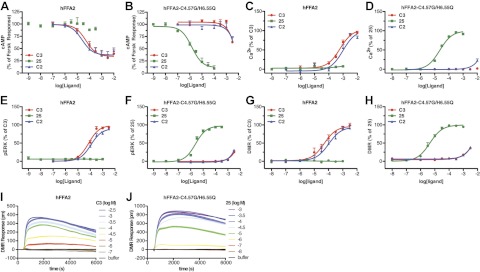
Postactivation assays were established for both the human and bovine orthologs of FFA2. In the first of these, an ortholog of FFA2 to which eYFP had been attached in-frame to the C terminus was cotransfected into HEK 293 cells along with β-arrestin-2 C-terminally modified by the in-frame addition of Rluc. BRET generated between Rluc and eYFP in response to an agonist ligand reflects induced interactions between FFA2 and β-arrestin-2. As anticipated from previous studies using a number of different endpoints (19–21), the SCFAs acetic acid (chain length C2) and propionic acid (C3) displayed modest and similar potency at hFFA2 in this assay (Fig. 1A). Although active at hFFA2, n-butanoic acid (C4) displayed lower potency (Fig. 1A), while both formic acid (C1) and valeric acid (C5) had little effect at concentrations below 10 mM (Fig. 1A). When equivalent studies were performed using bFFA2-eYFP, a substantially different structure-activity relationship (SAR) was observed. C1 was without effect, while C2 was significantly (P<0.05) less potent than compound C3 (Fig. 1B). Furthermore, when exploring the activity of fatty acids of longer chain length than C3, potency significantly increased (P<0.05), such that C3 < C4 < C5 (Fig. 1B). Because of the increasing potency of n-fatty acids with longer chain lengths observed at bFFA2, we examined extended chain lengths from C6 to C9 at both the human and bovine forms of the receptor. While none of the compounds C6–C9 showed appreciable activity at hFFA2 (Fig. 1C), all were full agonists at bFFA2, although potency in this case significantly decreased with each additional carbon (P<0.05), as chain length increased beyond C6 (Fig. 1D). Analysis of the potencies across the complete series of n-fatty acids at hFFA2 and bFFA2 (Table 1) reveals clearly distinct rank orders of potency, such that for hFFA2, C3 = C2 > C1 = C5, and all other compounds were without effect, while for bFFA2, C6 > C5 > C4 = C7 > C3 = C8 > C2 = C9, and only C1 was without effect.
Figure 1.
bFFA2 is activated by longer-chain length n-fatty acids than the human ortholog. A–H) The ability of fatty acids of chain length C1–C5 or C6–C9 to activate hFFA2 (A, C, E, G) or bFFA2 (B, D, F, H) was studied in either receptor-β-arrestin-2 interaction assays (A–D) or phospho-ERK 1/2 assays (E–H). Data are presented as a percentage of the maximal effect of C3. I, J) Comparison of pEC50 values for each fatty acid at hFFA2 and bFFA2 in β-arrestin-2 recruitment (I) and phospho-ERK 1/2 assays (J).
Table 1.
Potency values for short-chain fatty acids in various assays at human and bovine orthologs of FFA2 and FFA3
| Chain length | FFA2 β-arrestin-2 BRET |
FFA2 pERK |
FFA3 [35S]GTPγS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Human | Bovine | Selectivity | Human | Bovine | Selectivity | Human | Bovine | Selectivity | |
| C1 | 2.60 ± 0.40 | <2 | >0.6 | <2 | <2 | <2 | <2 | ||
| C2 | 3.00 ± 0.12 | 2.69 ± 0.11 | 0.31 | 3.50 ± 0.08 | 2.16 ± 0.10 | 1.34 | 2.12 ± 0.34 | 2.36 ± 0.34 | −0.24 |
| C3 | 3.29 ± 0.10 | 3.46 ± 0.07 | −0.17 | 3.64 ± 0.06 | 3.26 ± 0.07 | 0.38 | 3.70 ± 0.13 | 3.81 ± 0.18 | −0.11 |
| C4 | 2.83 ± 0.26 | 4.16 ± 0.06 | −1.33 | 2.87 ± 0.18 | 4.43 ± 0.07 | −1.56 | 3.60 ± 0.21 | 3.58 ± 0.14 | 0.02 |
| C5 | 2.15 ± 0.33 | 4.37 ± 0.09 | −2.22 | <2 | 4.30 ± 0.10 | <−2.30 | 4.44 ± 0.24 | 3.91 ± 0.18 | 0.53 |
| C6 | <2 | 4.68 ± 0.09 | <−2.68 | <2 | 5.01 ± 0.13 | <−3.01 | 3.69 ± 0.33 | 3.90 ± 0.21 | −0.21 |
| C7 | <2 | 3.81 ± 0.09 | <−1.81 | <2 | 4.31 ± 0.12 | <−2.31 | 4.16 ± 0.56 | 3.80 ± 0.61 | 0.36 |
| C8 | <2 | 3.21 ± 0.11 | <−1.21 | <2 | 3.12 ± 0.13 | <−1.12 | <2 | <2 | |
| C9 | <2 | 2.47 ± 0.22 | <−0.47 | <2 | <2 | <2 | <2 | ||
Selectivity is measured as human pEC50 − bovine pEC50 for each assay.
To ensure that these differences were not limited to measurement at only a β-arrestin recruitment endpoint, equivalent studies were performed by measuring phosphorylation of ERK 1/2, and similar results were obtained (Fig. 1E–H). In comparing the potencies for the complete series of n-fatty acids in β-arrestin-2 recruitment (Fig. 1I) and ERK 1/2 phosphorylation (Fig. 1J), it is clear that consistently across assays, bFFA2 preferentially responded to longer chain lengths than did hFFA2. By contrast, human and bovine orthologs of the closely related GPCR FFA3 displayed very similar patterns of responsiveness for each of the C1–C9 n-fatty acids, as assessed in a [35S]GTPγS assay (chosen because either bFFA3 or hFFA3 did not generate consistent responses in the β-arrestin-2 or ERK 1/2 assays). Unlike bFFA2, bFFA3 displayed no preference for longer chain lengths (Table 1).
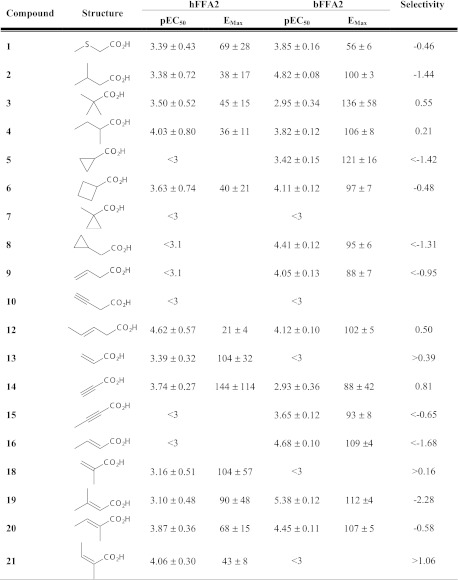
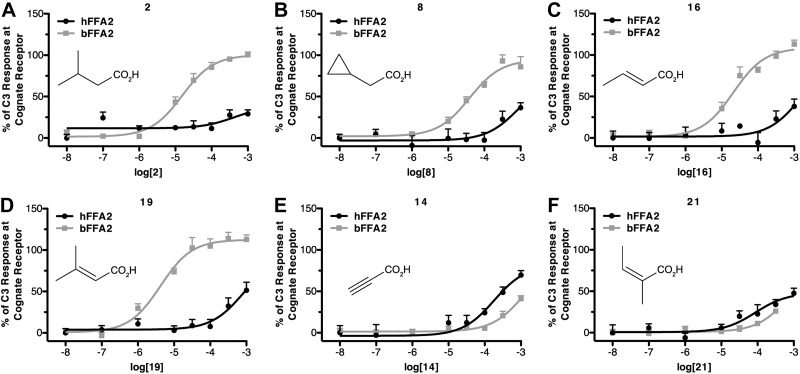
We have recently shown that a series of small carboxylic acids (SCAs) can also act as agonists at hFFA2 (20). Considering the marked differences among the n-fatty acids at hFFA2 and bFFA2, we next screened these SCAs in the β-arrestin-2 interaction assay to determine whether there were also species differences within this series (Fig. 2). A number of the SCAs, including 3-methylbutyric acid (compound 2), cyclopropylacetic acid (compound 8), trans-crotonic acid (compound 16), and 3-methylcrotonic acid (compound 19), each with modest potency and/or efficacy at hFFA2 in the β-arrestin-2 interaction assay were ∼10- to 100-fold more potent at bFFA2 (Fig. 3A–D). This was, however, not a general reflection that all SCAs were more potent at the bovine ortholog, as propiolic acid (compound 14) and angelic acid (compound 21), although displaying modest efficacy, were both more potent at hFFA2 than bFFA2 (Fig. 3E, F). SAR analyses of these results with the SCAs suggest that in addition to its preference for longer-chain fatty acids, bFFA2 also prefers compounds with sp2 hybridization of the α carbon and those with β-carbon substituents.
Figure 2.
SCA concentration-response curve fit parameters in β-arrestin-2 BRET at hFFA2 and bFFA2. Selectivity is expressed as hFFA2 pEC50 − bFFA2 pEC50. Emax values are reported as a percentage of C3 maximum response at the cognate receptor.
Figure 3.
A number of SCAs display marked selectivity for bFFA2. Ability of the noted compounds to promote β-arrestin-2 recruitment at either hFFA2 or bFFA2 is displayed as a percentage of maximally effective concentration of C3 at each species ortholog.
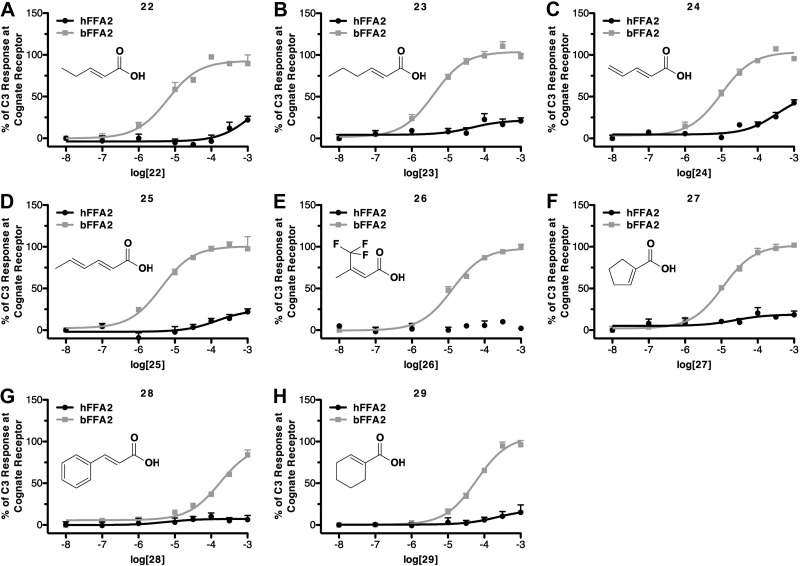
On the basis of these SAR observations, we selected a further set of SCAs predicted to have good selectivity for bFFA2 because they contained longer chain lengths (C5 or C6), sp2 hybridization of the α-carbon, or bulky substitutions at the β-carbon (Table 2). Within this series, all compounds were found to be full agonists at bFFA2, while none possessed substantial activity at the human receptor. In particular, it was the C5 and C6 compounds with conjugated double-bond systems that displayed the highest potency at bFFA2. These compounds included trans-2-pentenoic acid (compound 22; Fig. 4A), trans-2-hexenoic acid (compound 23; Fig. 4B), 2,4-pentadienoic acid (compound 24; Fig. 4C), and 2,4-hexadienoic acid (sorbic acid; compound 25; Fig. 4D). Although some compounds in this series did display weak activity at the human receptor (compounds 22 and 24), all those tested that contained larger β-carbon substituents appeared to be essentially inactive at hFFA2. These included 4,4,4-trifluoro-3-methyl-2-butenoic acid (compound 26; Fig. 4E), 1-cyclopentenecarboxylic acid (compound 27; Fig. 4F), trans-cinnamic acid (compound 28; Fig. 4G), and 1-cyclohexene-1-carboxylic acid (compound 29; Fig. 4H). In addition, each of these bFFA2-selective ligands was also inactive at the closely related hFFA3 receptor (data not shown).
Table 2.
Concentration-response curve-fit parameters for a refined set of SCAs predicted to have high potency and selectivity for bovine FFA2
| Compound | hFFA2 |
bFFA2 |
Selectivity | ||
|---|---|---|---|---|---|
| pEC50 | Emax | pEC50 | Emax | ||
| 22 | <3 | 5.28 ± 0.08 | 91 ± 2 | >2.28 | |
| 23 | <2 | 5.40 ± 0.09 | 104 ± 3 | >3.40 | |
| 24 | <3.5 | 5.00 ± 0.06 | 104 ± 2 | >1.50 | |
| 25 | <2 | 5.37 ± 0.09 | 100 ± 3 | >3.37 | |
| 26 | <2 | 4.89 ± 0.06 | 98 ± 2 | >2.89 | |
| 27 | <2 | 4.96 ± 0.07 | 102 ± 3 | >2.96 | |
| 28 | <2 | 3.76 ± 0.09 | 96 ± 6 | >1.76 | |
| 29 | <2 | 4.22 ± 0.06 | 107 ± 4 | >2.22 | |
Selectivity is expressed as bFFA2 pEC50 − hFFA2 pEC50. Values of pEC50 < 2 indicate that no measureable response was obtained. Emax values are reported as a percentage of the C3 response at bFFA2.
Figure 4.
Selected natural and synthetic compounds display marked selectivity for bovine FFA2. As in Fig. 3, ability of the noted compounds to promote β-arrestin-2 recruitment at either hFFA2 or bFFA2 is displayed as a percentage of maximally effective concentration of C3 at each species ortholog.
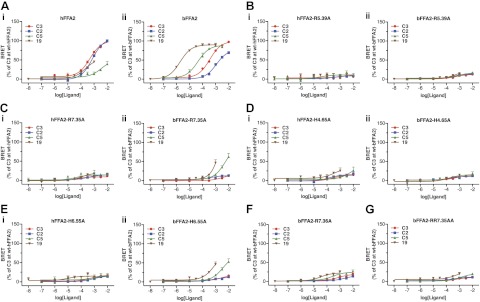
Having now identified several ligands with very good selectivity for bFFA2 over hFFA2, we next set out to define the molecular basis for this selectivity. In previous studies on hFFA2, sequence alignment with the other members of the free fatty acid receptor family, FFA1 and FFA3, identified 4 key positively charged amino acids within the transmembrane domains. These were arginine residues at positions 5.39 and 7.35 and histidine residues at positions 4.56 and 6.55 (numbering according to the system introduced by Ballesteros and Weinstein; ref. 24) and subsequent mutagenesis demonstrated the importance of particularly R5.39, R7.35, and H6.55 in coordination of the carboxylate group of the SCFAs (19, 21). Alignment of hFFA2 and bFFA2 indicated the presence of equivalent residues in the bovine ortholog. However, in addition to R7.35, the bovine ortholog has an additional arginine at position 7.36, (i.e., the next amino acid in the primary sequence). Each of these residues in bFFA2 was mutated to alanine, and the resulting mutants were screened using the β-arrestin-2 interaction assay to determine what role (if any) these residues had in the selectivity among the identified bFFA2 ligands. The four compounds that were selected for this screen were the most prevalent SCFAs C2 and C3, and the bovine-selective compounds C5 and 19, selected as being the most potent bovine-selective ligands that still had appreciable activity at the human receptor (Fig. 5A; see Supplemental Table S1 for complete mutant screen data). Mutation of R5.39 to alanine resulted in virtual complete loss of function for all four compounds at both the human and bovine forms of FFA2 (Fig. 5B). Interestingly, although mutation to generate R7.35A in hFFA2 resulted in complete loss of activity to each ligand, the equivalent mutation in bFFA2 retained some activity to the bovine-selective ligands C5 and 19, although with >100-fold loss of potency (Fig. 5C). Mutation of H4.56 to alanine in either hFFA2 or bFFA2 completely eliminated function (Fig. 5D), while the H6.55 to alanine mutant retained some activity only in bFFA2 to C5 and 19 (Fig. 5E). Mutation of the additional arginine residue present only in bFFA2 to generate R7.36A resulted in large reductions in both efficacy and potency, although the rank-order of potency of 19 > C5 > C3 > C2 was preserved (Fig. 5F). To assess the combined role of the two adjacent arginine residues in bFFA2, a double R7.35A/R7.36A mutant was generated, and this essentially failed to respond to any ligand tested (Fig. 5G).
Figure 5.
Mutational analysis of the orthosteric binding pocket of bFFA2: comparisons with the human ortholog. A–E) Wild-type (A) and X-A mutants of residues 5.39 (B), 7.35 (C), 4.56 (D), and 6.55 (E) were assessed for β-arrestin-2 recruitment at hFFA2 (Ai–Ei) and bFFA2 (Aii–Eii) in response to C2, C3, C5, and compound 19. F, G) Effects of mutating R7.36 that is present only in bFFA2 to alanine (F) and of a double (R7.35A, R7.36A) mutant (G) were also assessed.
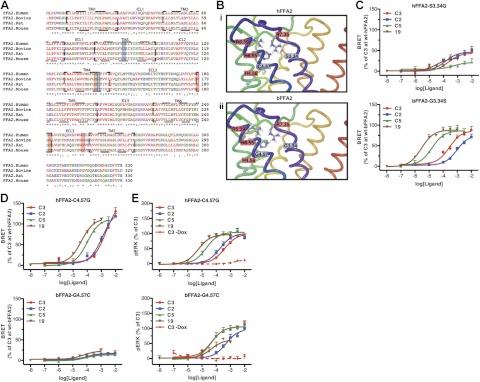
To identify residues in bFFA2 that may provide the basis for its greater chain length acceptance and distinct SCA SAR, we aligned the sequence of human, bovine, rat, and murine forms of FFA2 (Fig. 6A) and generated a homology model of bFFA2 (Fig. 6B) based on our previous models of the human receptor (20, 21). Nonconserved residues predicted to lie within 8 Å of R5.39 and R7.35 of the orthosteric binding site were highlighted (Fig. 6B). Two of these, human S3.34/bovine G3.34 and human C4.57/bovine G4.57, were selected for mutational analysis. Alteration to generate either human S3.34G or the reciprocal bovine G3.34S forms of FFA2 had limited effects on ligand pharmacology, with each mutant exhibiting the same rank order of potency for the four ligands tested as the corresponding wild-type receptor (Fig. 6C). By contrast, mutation to produce hFFA2-C4.57G produced a receptor where although C2 and C3 remained equipotent, the potency of C5 was increased markedly and became significantly (P<0.01) more potent than either C2 or C3 (Fig. 6D). Equally, compound 19 was also now substantially more potent than C2 or C3 at this mutant (P<0.01) and, as at wild-type bFFA2, was also more potent than C5. To confirm that this observation was not restricted to only the β-arrestin-2 interaction assay, hFFA2-C4.57G was also assessed in ERK 1/2 phosphorylation assays, and similar results were obtained (Fig. 6E). The reciprocal mutation G4.57C was also generated in bFFA2 (Fig. 6D), and although it did appear that both C5 and 19 had reduced potency relative to C2 and C3, this was difficult to assess accurately, as this variant receptor displayed substantially reduced efficacy in the β-arrestin-2 interaction assay. Therefore, to confirm this observation, bFFA2-G4.57C was also tested in the ERK 1/2 phosphorylation assay, which demonstrated that it did, indeed, lose potency for the bovine-selective compounds C5 and 19 relative to C2 and C3 (Fig. 6E).
Figure 6.
Homology modeling and mutagenesis of bFFA2 indicate a key role for residue G4.57 in fatty acid chain length and compound selectivity. A, B) Species orthologs of FFA2 were aligned (A), and homology models of hFFA2 (Bi) and bFFA2 (Bii) were generated. Key amino acids known to play roles in the binding of SCFAs are boxed in red; those targeted for mutagenesis are boxed in blue. C, D) Residues at positions 3.34 (C) and 4.57 (D) were exchanged between the orthologs, and the ability of C2, C3, C5, and compound 19 to promote β-arrestin-2 recruitment to these variants was compared to wild type. E) hFFA2 and bFFA2 mutants with exchanged residues at 4.57 were assessed in an ERK 1/2 phosphorylation assay. To confirm responses were receptor mediated, C3 concentration-response studies were also carried out in the absence of doxycycline, to confirm it had no effect when receptor expression was not induced.
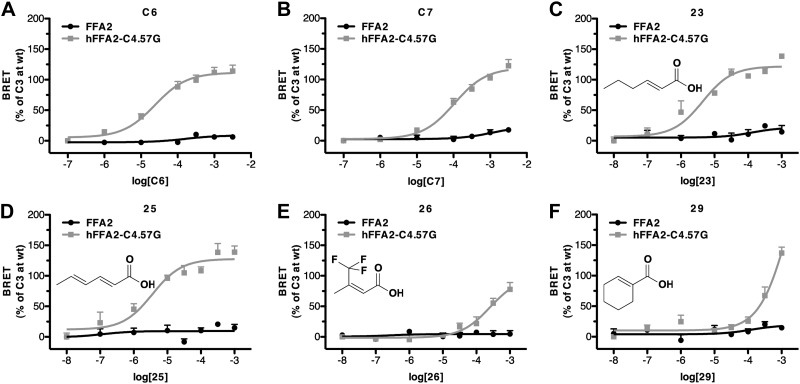
On the basis of the gain in function we observed for bovine-selective ligands C5 and 19 at hFFA2-C4.57G, we next assessed whether the additional SCFAs and bovine-selective SCAs would also activate this mutant receptor using the β-arrestin-2 interaction assay (Fig. 7 and Table 3). Indeed, C6 and C7 were effective agonists at this variant and now displayed potency akin to that at the bovine ortholog (Fig. 7A, B). Among the SCAs, particularly compounds with C6 chain lengths, such as compounds 23 and 25, displayed dramatic gain of function at this mutant (Fig. 7C, D), and indeed, these compounds had equivalent activity at hFFA2-C4.57G, as they do at the bovine receptor. In contrast, although the series of β-carbon-substituted carboxylic acids, including, for example, compounds 26 and 29, also gained function at the C4.57G mutant (Fig. 7E, F), these compounds were substantially less potent at hFFA2-C4.57G than at bFFA2, suggesting that additional residues besides 4.57 are likely to contribute to this aspect of the bFFA2 ligand SAR.
Figure 7.
Substitution of C4.57G in hFFA2 generates responses similar to the bovine ortholog. Pharmacology of hFFA2-C4.57G was assessed in β-arrestin-2 recruitment assays with a number of ligands shown earlier to display marked selectivity for bFFA2: C6 (A), C7 (B), compound 23 (C), compound 25 (D), compound 26 (E), and compound 29 (F).
Table 3.
Concentration-response curve parameters for bovine selective SCFAs and SCAs at wild-type and C4.57G human FFA2
| Compound | FFA2-wt |
FFA2-C4.57G |
Selectivity | |||
|---|---|---|---|---|---|---|
| pEC50 | Emax | pEC50 | Emax | vs. bFFA2a | ||
| C6 | <2 | 4.62 ± 0.14 | 112 ± 4 | NS | >2.62 | |
| C7 | <2 | 3.98 ± 0.10 | 119 ± 5 | NS | >1.98 | |
| C8 | <2 | 2.97 ± 0.13 | 109 ± 13 | NS | >0.97 | |
| 22 | <3 | 4.48 ± 0.15 | 107 ± 7 | P < 0.05 | >1.48 | |
| 23 | <2 | 5.49 ± 0.17 | 109 ± 4 | NS | >3.49 | |
| 24 | <3 | 4.64 ± 0.14 | 113 ± 7 | P < 0.01 | >1.64 | |
| 25 | <2 | 5.34 ± 0.21 | 113 ± 6 | NS | >3.34 | |
| 26 | <2 | 3.59 ± 0.16 | 96 ± 12 | P < 0.01 | >1.59 | |
| 27 | <2 | 4.08 ± 0.09 | 98 ± 5 | P < 0.01 | >2.08 | |
| 28 | <2 | 3.41 ± 0.31 | 93 ± 23 | P < 0.01 | >1.41 | |
| 29 | <2 | 3.80 ± 0.20 | 78 ± 11 | P < 0.05 | >1.80 | |
Selectivity is expressed as FFA2-C4.57G pEC50 − FFA2-wt pEC50. Values of pEC50 < 2 indicate that no measureable response was obtained. Values of pEC50 < 3 indicate that a response was observed; however, the data did not allow for the derivation of accurate curve-fit parameters. Emax values are reported as a percentage of the C3 response at wild-type bFF2A.
Statistical comparison of pEC50 curve-fit data between bFFA2 and hFFA2-C4.57G. NS, not significant.
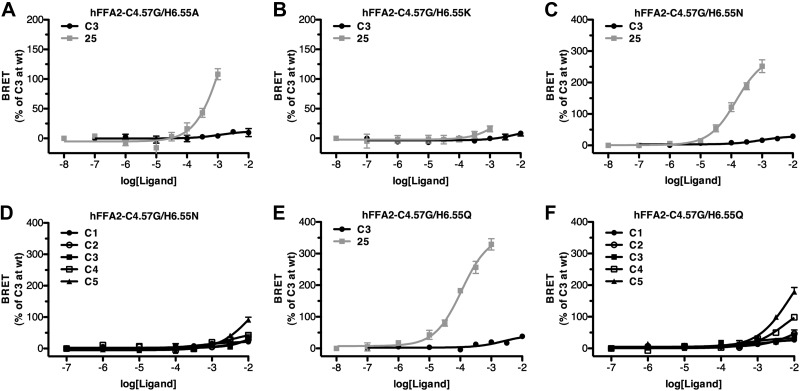
The substantial gain in function at hFFA2-C4.57G for several ligands that are inactive at wild-type hFFA2 and hFFA3 suggested that this mutant could form the basis of a construct to selectively probe the function of FFA2. However, to be useful in practice, a RASSL form of FFA2 that not only gains function to one or more nonendogenously generated ligands but also loses response to the endogenous ligands is required. Therefore, we used hFFA2-C4.57G as a lead in order to generate a true RASSL form of hFFA2. For this, we returned to the four positively charged residues, R5.39, R7.35, H4.56, and H6.55, previously implicated in SCFA binding to hFFA2 (19). Although our initial studies on the bovine ortholog of FFA2 suggested that mutation to alanine of either R5.39 or H4.56 resulted in a complete loss of function (Fig. 5B, D), the alanine mutations of both R7.35 or H6.55 each retained some activity for the most potent bovine-selective ligands (Fig. 5C, E). Because the retained function at R7.35 appeared to result from the presence of an additional arginine at 7.36 in the bovine receptor that is not present in hFFA2, we focused on H6.55. Initially, a H6.55A mutation was incorporated into hFFA2-C4.57G (Fig. 8A). Like bovine H6.55A, this form of hFFA2 also retained some activity to the most potent ligand at the human C4.57G receptor, compound 25, while losing function for the endogenous SCFA C3. However, the potency of compound 25 remained very low at this mutant. Several less extreme mutations were, therefore, also assessed. Replacement by lysine resulted in complete loss of function (Fig. 8B). By contrast, introduction of asparagine, which is the residue present at this position in the related long-chain fatty acid receptor FFA1 (25, 26) to generate hFFA2-C4.57G/H6.55N produced a receptor with reasonable potency for compound 25 (Fig. 8C), while losing nearly all measurable activity to the endogenous SCFA ligands C1–C5 (Fig. 8D). To examine this further, we also generated hFFA2-C4.57G/H6.55Q due to the similarity between asparagine and glutamine. This form of hFFA2 also retained potency for compound 25, which was somewhat improved over hFFA2-C4.57G/H6.55N (Fig. 8E) and also showed very little response to each of C1–C5.
Figure 8.
Alterations at residue 6.55 to hFFA2-C4.57G generate a RASSL form of hFFA2. hFFA2-C4.57G was modified further by substitution of H6.55 to alanine (A), lysine (B), asparagine (C, D) or glutamine (E, F). Action of either C3 or compound 25 was then assessed in β-arrestin-2 recruitment assays. D, F) C1–C5 n-fatty acids were tested for activity.
Considering the better potency observed for compound 25, we further examined the potential of hFFA2-C4.57G/H6.55Q as a true RASSL form of this receptor. For this, it was tested across multiple functional endpoints, reflective of the range of known signaling pathways FFA2 is able to regulate (27). To assess Gαi/o coupling, we compared the effect of C3 at wild-type hFFA2 on inhibition of forskolin-stimulated cAMP production (Fig. 9A) with that of compound 25 at the mutant receptor (Fig. 9B). While C3 and C2 were able to inhibit forskolin-stimulated cAMP production via wild-type FFA2 (pEC50=4.28±0.19 and 4.60±0.30, respectively), they both had ≥100-fold reduced effect on the mutant receptor. Strikingly, compound 25, although completely inactive at the wild-type hFFA2 receptor, strongly and potently inhibited forskolin-stimulated cAMP production via the mutant (pEC50=5.83±0.11). To assess coupling of hFFA2-C4.57G/H6.55Q to Gαq/11 pathways, the ability of C3 and compound 25 to stimulate Ca2+ mobilization was examined in cells expressing wild-type hFFA2 (Fig. 9C) or hFFA2-C4.57G/H6.55Q (Fig. 9D). Although C3 and C2 produced an increase in Ca2+ via wild-type hFFA2 (pEC50=3.21±0.09 and 3.01±0.11, respectively), C3 had no measurable effect at the C4.57G/H6.55Q mutant, while C2 only produced a very small effect at 10 mM. By contrast, compound 25 again had no effect on wild-type hFFA2 but clearly increased Ca2+ via the mutant receptor (pEC50=4.63±0.05). Comparable experiments were conducted measuring ERK 1/2 phosphorylation by C3 and compound 25 via the wild-type (Fig. 9E) and C4.57G/H6.55Q (Fig. 9F) forms of hFFA2. Again, similar results were obtained: C3 and C2 effectively promoted ERK 1/2 phosphorylation via the wild-type receptor (pEC50=4.13±0.09 and 3.94±0.08, respectively) but had little effect on the mutant form, while compound 25 had no effect at the wild type but was active and potent at the mutant form of FFA2 (pEC50=5.61±0.06). Importantly, not only did compound 25 activate hFFA2-C4.57G/H6.55Q in each assay, the rank order of potency for compound 25 at this RASSL form of hFFA2 across the various assays was similar to that for C3 and C2 at the wild-type receptor, such that cAMP > pERK 1/2 > Ca2+ > β-arrestin-2, suggesting that the active conformation(s) of hFFA2-C4.57G/H6.55Q induced by compound 25 are very similar to those of the wild-type receptor occupied by C2 or C3. To more directly test this in an unbiased manner, we employed a DMR assay, a method that has been used in the past to broadly measure cellular responses to GPCR activation (20, 22, 23). As in the other assays assessed, C3 and C2 produced DMR responses at hFFA2 (pEC50=4.20±0.11 and 3.85±0.12, respectively), and compound 25 was without effect (Fig. 9E), while at hFFA2-C4.57G/H6.55Q, compound 25 produced a good response (pEC50=5.35±0.06), and C3 and C2 had only small effects at the highest concentrations tested (Fig. 9F). Finally, DMR time-course experiments were conducted using C3 on hFFA2 (Fig. 9G) or compound 25 on hFFA2-C4.57G/H6.55Q (Fig. 9H). The resulting DMR responses showed very similar profiles between the two different forms of hFFA2, further supporting the conclusion that the cellular responses of hFFA2-C4.57G/H6.55Q to compound 25 are very similar to those of the wild-type hFFA2 to its endogenous ligands.
Figure 9.
RASSL behavior of hFFA2-C4.57G/H6.55Q is observed in a range of signal transduction endpoints. A–H) Activity of C2, C3, and compound 25 was assessed at hFFA2 wild type (A, C, E, G) and hFFA2-C4.57G/H6.55Q (B, D, F, H) for their ability to inhibit forskolin-stimulated cAMP production (A, B), elevate intracellular Ca2+ (C, D), increase phospho-ERK 1/2 (E, F) and affect DMR (G, H). I, J) DMR time course experiments are shown for hFFA2 with compound C3 (I) and for hFFA2-C4.57G/H6.55Q with compound 25 (J).
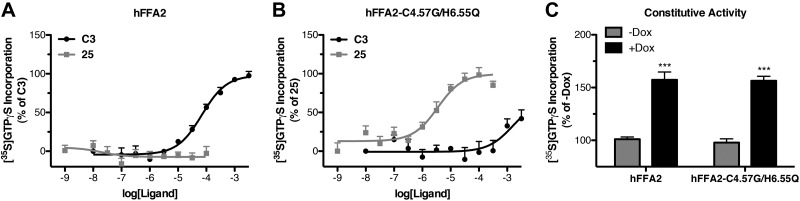
In the past, generation of RASSL GPCRs has often resulted in increased levels of ligand-independent constitutive activity in the RASSL receptor (16). Therefore, in addition to confirming that the cellular response to hFFA2-C4.57G/H6.55Q is similar to wild-type hFFA2, it is also important to demonstrate that hFFA2-C4.57G/H6.55Q does not display altered levels of constitutive activity. The Flp-In T-REx cells provide an optimal experimental system to examine this, as they allow direct comparison of basal signaling within the same cell line, either with or without induced receptor expression. However, the assays used to describe the signaling properties of hFFA2-C4.57G/H6.55Q, including cAMP, Ca2+, pERK, and DMR, are not well suited to measuring ligand-independent constitutive activity; therefore, we extended our studies to measure incorporation of [35S]GTPγS, an assay that has been widely used previously to study GPCR constitutive activity (28–29). Initially, we demonstrated that hFFA2 activity can be measured in this assay, as C3 (pEC50=4.16±0.11) but not compound 25 stimulated [35S]GTPγS incorporation in membranes induced to express wild-type hFFA2 (Fig. 10A). Similarly, in hFFA2-C4.57G/H6.55Q-expressing membranes, compound 25 promoted incorporation of [35S]GTPγS (pEC50=5.46±0.15), while C3 produced very little response and only at high concentrations (Fig. 10B). To examine directly ligand-independent activity, basal [35S]GTPγS incorporation was measured in membranes isolated from either hFFA2 or hFFA2-C4.57G/H6.55Q cells in the absence or presence of doxycycline to induce receptor expression (Fig. 10C). In both cases, doxycycline induction resulted in a statistically significant (P<0.001) increase in [35S]GTPγS incorporation. Specifically, induced expression of hFFA2 resulted in a 157 ± 7% increase in basal [35S]GTPγS incorporation, while induced expression of hFFA2-C4.57G/H6.55Q resulted in a similar 156 ± 4% (P>0.05) increase. These findings demonstrate that wild-type hFFA2 displays constitutive activity and that this is unaltered by the alterations to generate hFFA2-C4.57G/H6.55Q.
Figure 10.
Wild-type hFFA2 and hFFA2-C4.57G/H6.55Q display similar levels of ligand-independent constitutive activity. A, B) Ability of C3 and compound 25 to stimulate [35S]GTPγS incorporation was assessed in membranes expressing either hFFA2 (A) or hFFA2-C4.57G/H6.55Q (B). C) Basal levels of [35S]GTPγS incorporation were measured in membranes harvested from cells that were either untreated or stimulated with doxycycline (Dox; 100 ng/ml) to induce expression of either hFFA2 or hFFA2-C4.57G/H6.55Q.
DISCUSSION
By exploring differences in pharmacology between the human and bovine orthologs of FFA2, the most dissimilar species variants of this receptor that have currently been cloned, then generating homology models and focusing on residues within 8 Å of the predicted ligand-binding site for endogenously produced SCFAs, we have been able to engineer the human ortholog to respond to a novel group of ligands. By so doing, we have provided new insights into the binding pocket of the human ortholog that may allow the design of novel and more potent ligands that target FFA2, a receptor that is a potential therapeutic target in areas including inflammation and metabolic diseases (4, 7, 8). Moreover, the hFFA2-C4.57G/H6.55Q mutant that we generated acts as a RASSL form of this receptor because it has also lost responsiveness to endogenously produced activators. In this regard, the current studies have similarity to the production of RASSL forms of the muscarinic acetylcholine receptors, which no longer respond with significant potency to acetylcholine but do so instead to the synthetic ligand clozapine N-oxide (12, 13). However, unlike the muscarinic receptor RASSLs, where the starting point to evolve the variant forms was random mutagenesis (13), herein, we have taken advantage of the SAR differences in two species orthologs of the FFA2 receptor to the n-fatty acids and SCAs. In combination with our previous analysis of the mode of binding and selectivity of SCAs for FFA2 vs. the closely related receptor FFA3 (19–21), this has allowed rational design of a RASSL based on homology modeling and sequence alignments. By contrast, rationalization of the basis of the selective binding of clozapine N-oxide to a muscarinic RASSL (13) was only possible post hoc (12).
A major challenge to understanding the physiological roles of FFA2 is the similarity of the pharmacology of the closely related receptor FFA3, a problem that is compounded by the fact that these two receptors are often coexpressed (30, 31). Although limited variation in potency of C2 between human FFA2 and FFA3 has resulted in its use as a selective FFA2 agonist (32), because C2 does still have activity at FFA3 and has only low potency at FFA2, this is far from ideal. Furthermore, apart from a series of SCAs described by Schmidt et al. (20), which, because of their small size, also have poor potency, the only FFA2-selective ligands currently described derive from the phenylacetamide 4-CMTB (21, 33). Although this compound has been used to delineate effects of FFA2 (33, 34), it clearly binds to a site distinct from the SCFAs and acts as a positive allosteric modulator of the action of the SCFAs, as well as a direct agonist (21, 33). With increasing appreciation of the ability of different ligands that bind to overlapping sites on GPCRs to generate different signaling profiles, a feature that is described as either functional selectivity (35) or ligand bias (36, 37), there must be the possibility that agonists that bind to different sites of a GPCR will result in such bias. This issue, although important, remains to be explored for the actions of 4-CMTB and related allosteric ligands at FFA2, and thus, we wished to modify the orthosteric binding pocket of FFA2.
An important secondary finding of this work is our demonstration that the pharmacology of bFFA2 is significantly different from that of hFFA2. Although previous work had cloned the bovine SCFA receptors FFA2 and FFA3 (38), little was known about their pharmacology. Our demonstration that bFFA2 responds with a completely different rank order of potency to the SCFAs than does hFFA2 suggests that this receptor may serve different functions in bovines compared with humans, and, clearly, future studies on bFFA2 should take into account its unique ligand selectivity. The bovine ortholog of FFA2 was chosen for this study based on the hypothesis that species, including ruminants, that rely heavily on nondigestible carbohydrates are exposed to significantly higher SCFA concentrations (39) and, therefore, were most likely to have SCFA receptors with distinct pharmacology. Although our findings with bFFA2 appear to support this conclusion, it is also important to consider whether this pharmacology may be preserved in other species with high dietary levels of nondigestible carbohydrates. An alignment of species orthologs of FFA2 (Supplemental Fig. S1) reveals that the only other species with glycine at position 4.57, and, therefore, likely to display similar pharmacology to bFFA2, is the goat, the only other ruminant for which sequencing data are available. Interestingly, the second unique residue that appears to contribute to the pharmacology of bFFA2, R7.36, is also conserved in goat FFA2, indicating that the pharmacology of goat FFA2 is likely to be very similar to bFFA2. Interestingly, the horse ortholog of FFA2 does not appear likely to share pharmacology with bFFA2 on the basis of this sequence alignment (Supplemental Fig. S1), this despite the fact that the horse is also heavily reliant on nondigestible carbohydrates, and, in fact, shows SCFAs concentrations in the digestive tract similar to those observed in ruminants (39). Therefore, it appears that the unique pharmacology of bFFA2 is likely restricted to ruminants and may be related to the fact that FFA2 is expressed in the rumen of these animals (38, 40). Indeed, it is conceivable that the two primary changes that we describe in the pharmacology of bFFA2, namely, a loss of potency to C2 and gain of function to longer chain lengths, may be an evolutionary adaptation designed to maintain dynamic FFA2 function in the high-SCFA-concentration environment of the rumen, where C2, as the predominant SCFA, is present in very high concentrations, but lower levels of longer-chain compounds are also observed (39).
Our initial studies, demonstrating the unique pharmacology of bFFA2, precipitated efforts to understand the molecular basis for this finding. Despite the availability of atomic level structure of a number of GPCRs (41–43), homology modeling remains challenging. This is particularly true for receptors with limited sequence homology to GPCRs of known structure. However, both because the binding sites for fatty acids in FFA1 (25, 26, 44), FFA2 (19), and FFA3 (19) have been explored by mutagenesis and our homology model of FFA1 has been validated by its use in “virtual screening” to identify novel ligands at FFA1 (26), we have been able to model and compare hFFA2 and bFFA2 with some confidence. The models indicated two residues that differ between hFFA2 and bFFA2 that are in proximity to arginine residues, two in the human (5.39 and 7.35), and as we now show, three in the bovine (5.39, 7.35, and 7.36) that coordinate the carboxylate group of the fatty acid ligands. These were, therefore, potential candidates to underpin the species chain-length selectivity. While alteration of position 3.34 in hFFA2 to the bovine sequence had little effect on pharmacology, alteration of the cysteine at position 4.57 in hFFA2 to glycine, which is present in this ¹position of bFFA2, was sufficient to markedly enhance potency to the longer-chain SCFAs C5–C8, as well as to a number of SCAs that we showed to be markedly selective for bFFA2 over hFFA2. To generate a more useful RASSL form of hFFA2, it was also important to develop a receptor that no longer responded to its endogenous SCFA ligands. We achieved this through the addition of a second mutation H6.55Q, one of the positively charged residues previously shown to play a role in SCFA ligand binding (19). Overall, our development of this RASSL form of hFFA2 represents a novel, more rational, approach to chemically engineering ligand selectivity at a GPCR based on pharmacological differences between species. This is in contrast to approaches based on random mutagenesis and directed molecular evolution used to develop RASSL forms of the muscarinic receptors (13). However, a combination of these two approaches, whereby species differences could be used to rationally identify a RASSL “lead,” followed by subsequent molecular evolution to mature an ideal RASSL ligand binding site, may well represent an approach to generate RASSLs with even more favorable properties in the future.
In addition to modifying the RASSL receptor to alter its pharmacology, the selection of an ideal ligand to activate the RASSL is also of critical importance. The ligand chosen to activate the hFFA2-RASSL described here, compound 25, is a natural compound that is widely used as an antifungal preservative in various foodstuffs (45). The compound is essentially nontoxic, with a reported LD50 in rodents of 8–10 g/kg (46), suggesting that it could be used in vivo without significant side effects. At present, little is known about the bioavailability or tissue distribution of compound 25, and future work will need to address these issues. Interestingly, the compound is approved for use as an additive to cattle feed, and although at least one study has suggested that its use does not affect cattle weight gain (47), future work may well consider what biological effects its use in this context might have, given its ability to activate bFFA2.
Similar RASSLs of muscarinic acetylcholine receptor subtypes have been used to produce transgenic animals to selectively study the functions of individual muscarinic subtypes in a variety of both ex vivo and in vivo assays (14, 15). Our demonstration that compound 25 has similar signaling properties at the hFFA2-RASSL to those of C3 at the wild receptor across multiple assays indicates that this hFFA2-RASSL would be ideal for these types of studies using knock-in transgenesis. In addition to their use differentiating the actions of closely related GPCRs, RASSLs have also been used as a means to explore selective activation of individual signaling pathways in cells or tissues that have been engineered to express the RASSL (16). Although there are RASSLs described previously for each of the three primary Gα signaling pathways (Gs, Gi, and Gq), the hFFA2-RASSL described in the present work is the first RASSL to selectively coactivate two of these pathways, and thus may represent a novel means to explore combined Gi/Gq activation after its expression in vitro or in vivo. Furthermore, RASSLs have generated recent interest for their potential use as molecular switches in engineered tissues that could ultimately be used therapeutically in humans (16). The FFA2 RASSL described in the current work may be of particular interest in this respect, given that its ligand is nontoxic and, indeed, already approved for human consumption. Taken together, in the present work, we have described a novel approach to developing chemically engineered GPCRs based on the pharmacological variation between species orthologs, and in doing so, have developed a valuable reagent for the future study of FFA2 function.
Supplementary Material
Acknowledgments
These studies were supported by the Wellcome Trust (grant 089600/Z/09/Z to G.M.), the Danish Council for Strategic Research (grant 11-116196 to T.U., G.M., and E.K.), and a Canadian Institutes of Health Research Fellowship (to B.D.H.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- bFFA2
- bovine free fatty acid receptor 2
- bFFA3
- bovine free fatty acid receptor 3
- BRET
- bioluminescence resonance energy transfer
- DMEM
- Dulbecco's modified Eagle's medium
- DMR
- dynamic mass redistribution
- DREADD
- designer receptor exclusively activated by designed drug
- ERK
- extracellular signal-regulated kinase
- eYFP
- enhanced yellow fluorescent protein
- HBSS
- Hanks' balanced salt solution
- hFFA2
- human free fatty acid receptor 2
- FFA1
- free fatty acid receptor 1
- FFA2
- free fatty acid receptor 2
- FFA3
- free fatty acid receptor 3
- GPCR
- G-protein-coupled receptor
- RASSL
- receptor activated solely by synthetic ligand
- Rluc
- Renilla luciferase
- SAR
- structure-activity relationship
- SCA
- small carboxylic acid
- SCFA
- short-chain fatty acid
REFERENCES
- 1. He W., Miao F. J., Lin D. C., Schwandner R. T., Wang Z., Gao J., Chen J. L., Tian H., Ling L. (2004) Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429, 188–193 [DOI] [PubMed] [Google Scholar]
- 2. Liu C., Wu J., Zhu J., Kuei C., Yu J., Shelton J., Sutton S. W., Li X., Yun S. J., Mirzadegan T., Mazur C., Kamme F., Lovenberg T. W. (2009) Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 284, 2811–2822 [DOI] [PubMed] [Google Scholar]
- 3. Offermanns S., Colletti S. L., Lovenberg T. W., Semple G., Wise A., IJzerman A. P. (2011) International Union of Basic and Clinical Pharmacology. LXXXII. Nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B). Pharmacol. Rev. 63, 269–290 [DOI] [PubMed] [Google Scholar]
- 4. Stoddart L. A., Smith N. J., Milligan G. (2008) International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol. Rev. 60, 405–417 [DOI] [PubMed] [Google Scholar]
- 5. Hudson B. D., Smith N., Milligan G. (2011) Experimental challenges to targeting poorly characterized GPCRs: uncovering the therapeutic potential for free fatty acid receptors. Adv. Pharmacol. 62, 175–218 [DOI] [PubMed] [Google Scholar]
- 6. Sleeth M. L., Thompson E. L., Ford H. E., Zac-Varghese S. E., Frost G. (2010) Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr. Res. Rev. 23, 135–145 [DOI] [PubMed] [Google Scholar]
- 7. Tiwari A. (2010) GPR43: an emerging target for the potential treatment of type 2 diabetes, obesity and insulin resistance. Curr. Opin. Investig. Drugs 11, 385–393 [PubMed] [Google Scholar]
- 8. Swaminath G. (2008) Fatty acid binding receptors and their physiological role in type 2 diabetes. Arch. Pharm. (Weinheim) 341, 753–761 [DOI] [PubMed] [Google Scholar]
- 9. Maslowski K. M., Vieira A. T., Ng A., Kranich J., Sierro F., Yu D., Schilter H. C., Rolph M. S., Mackay F., Artis D., Xavier R. J., Teixeira M. M., Mackay C. R. (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sina C., Gavrilova O., Förster M., Till A., Derer S., Hildebrand F., Raabe B., Chalaris A., Scheller J., Rehmann A., Franke A., Ott S., Häsler R., Nikolaus S., Fölsch U. R., Rose-John S., Jiang H. P., Li J., Schreiber S., Rosenstiel P. (2009) G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 183, 7514–7522 [DOI] [PubMed] [Google Scholar]
- 11. Zaibi M. S., Stocker C. J., O'Dowd J., Davies A., Bellahcene M., Cawthorne M. A., Brown A. J., Smith D. M., Arch J. R. (2010) Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 584, 2381–2386 [DOI] [PubMed] [Google Scholar]
- 12. Alvarez-Curto E., Prihandoko R., Tautermann C. S., Zwier J. M., Pediani J. D., Lohse M. J., Hoffmann C., Tobin A. B., Milligan G. (2011) Developing chemical genetic approaches to explore G protein-coupled receptor function—validation of the use of a receptor activated solely by synthetic ligand (RASSL). Mol. Pharmacol. 80, 1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armbruster B. N., Li X., Pausch M. H., Herlitze S., Roth B. L. (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U. S. A. 104, 5163–5168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guettier J. M., Gautam D., Scarselli M., Ruiz de Azua I., Li J. H., Rosemond E., Ma X., Gonzalez F. J., Armbruster B. N., Lu H., Roth B. L., Wess J. (2009) A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc. Natl. Acad. Sci. U. S. A. 106, 19197–19202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sasaki K., Suzuki M., Mieda M., Tsujino N., Roth B., Sakurai T. (2011) Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One 6, e20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conklin B. R., Hsiao E. C., Claeysen S., Dumuis A., Srinivasan S., Forsayeth J. R., Guettier J. M., Chang W. C., Pei Y., McCarthy K. D., Nissenson R. A., Wess J., Bockaert J., Roth B. L. (2008) Engineering GPCR signalling pathways with RASSLs. Nat. Methods 5, 673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Claeysen S., Joubert L., Sebben M., Bockaert J., Dumuis A. (2003) A single mutation in the 5-HT4 receptor (5-HT4-R D100(3.32)A) generates a Gs-coupled receptor activated exclusively by synthetic ligands (RASSL). J. Biol. Chem. 278, 699–702 [DOI] [PubMed] [Google Scholar]
- 18. Milligan G., Stoddart L. And A., Smith N. J. (2009) Agonism and allosterism: the pharmacology of the free fatty acid receptors FFA2 and FFA3. Br. J. Pharmacol. 158: 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoddart L. A., Smith N. J., Jenkins L., Brown A. J., Milligan G. (2008) Conserved polar residues in transmembrane domains V, VI and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J. Biol. Chem. 283, 32913–32924 [DOI] [PubMed] [Google Scholar]
- 20. Schmidt J., Smith N. J., Christiansen E., Tikhonova I. G., Grundmann M., Hudson B. D., Ward R. J., Drewke C., Milligan G., Kostenis E., Ulven T. (2011) Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: Identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J. Biol. Chem. 286, 10628–10640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith N. J., Ward R. J., Stoddart L. A., Hudson B. D., Kostenis E., Ulven T., Morris J. C., Trankle C., Tikhonova I. G., Adams D. R., Milligan G. (2011) Extracellular loop 2 of the free fatty acid receptor 2 mediates allosterism of a phenylacetamide ago-allosteric modulator. Mol. Pharmacol. 80, 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schröder R., Janssen N., Schmidt J., Kebig A., Merten N., Hennen S., Müller A., Blättermann S., Mohr-Andrä M., Zahn S., Wenzel J., Smith N. J., Gomeza J., Drewke C., Milligan G., Mohr K., Kostenis E. (2010) Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat. Biotechnol. 28, 943–949 [DOI] [PubMed] [Google Scholar]
- 23. Schröder R., Schmidt J., Blättermann S., Peters L., Janssen N., Grundmann M., Seemann W., Kaufel D., Merten N., Drewke C., Gomeza J., Milligan G., Mohr K., Kostenis E. (2011) Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat. Protoc. 6, 1748–1760 [DOI] [PubMed] [Google Scholar]
- 24. Ballesteros J. A., Weinstein H. (1995) Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 [Google Scholar]
- 25. Sum C. S., Tikhonova I. G., Neumann S., Engel S., Raaka B. M., Costanzi S., Gershengorn M. C. (2007) Identification of residues important for agonist recognition and activation in GPR40. J. Biol. Chem. 282, 29248–29255 [DOI] [PubMed] [Google Scholar]
- 26. Tikhonova I. G., Sum C. S., Neumann S., Engel S., Raaka B. M., Costanzi S., Gershengorn M. C. (2008) Discovery of novel agonists and antagonists of the free fatty acid receptor 1 (FFAR1) using virtual screening. J. Med. Chem. 51, 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown A. J., Goldsworthy S. M., Barnes A. A., Eilert M. M., Tcheang L., Daniels D., Muir A. I., Wigglesworth M. J., Kinghorn I., Fraser N. J., Pike N. B., Strum J. C., Steplewski K. M., Murdock P. R., Holder J. C., Marshall F. H., Szekeres P. G., Wilson S., Ignar D. M., Foord S. M., Wise A., Dowell S. J. (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 [DOI] [PubMed] [Google Scholar]
- 28. Harrison C., Traynor J. R. (2003) The [35S]GTPγS binding assay: approaches and applications in pharmacology. Life Sci. 74, 489–508 [DOI] [PubMed] [Google Scholar]
- 29. Stoddart L. A., Milligan G. (2010) Constitutive activity of GPR40/FFA1 intrinsic or assay dependent? Methods Enzymol. 484, 569–590 [DOI] [PubMed] [Google Scholar]
- 30. Le Poul E., Loison C., Struyf J. Y., Lannoy V., Decobecq M. E., Brezillon S., Dupriez V., Vassart G., Van Damme J., Parmentier M., Detheux M. (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 [DOI] [PubMed] [Google Scholar]
- 31. Kebede M. A., Alquier T., Latour M. G., Poitout V. (2009) Lipid receptors and islet function: therapeutic implications? Diabetes Obes. Metab. 11(Suppl. 4), 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ge H., Li X., Weiszmnn J., Wang P., Baribault H., Che J. L., Tian H., Li Y. (2008) Activation of G protein-coupled receptor 43 in adipocytes leads to inhibitions of lipolysis and suppression of plasma free fatty acids. Endocrinology 149, 4519–4526 [DOI] [PubMed] [Google Scholar]
- 33. Lee T., Schwandner R., Swaminath G., Weiszmann J., Cardozo M., Greenberg J., Jaeckel P., Ge H., Wang Y., Jiao X., Liu J., Kayser F., Tian H., Li Y. (2008) Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol. Pharmacol. 74, 1599–1609 [DOI] [PubMed] [Google Scholar]
- 34. Vinolo M. A., Ferguson G. J., Kulkarni S., Damoulakis G., Anderson K., Bohlooly -Y. M., Stephens L., Hawkins P. T., Curi R. (2011) SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One 6, e21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Urban J. D., Clarke W. P., von Zastrow M., Nichols D. E., Kobilka B., Weinstein H., Javitch J. A., Roth B. L., Christopoulos A., Sexton P. M., Miller K. J., Spedding M., Mailman R. B. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 [DOI] [PubMed] [Google Scholar]
- 36. Kenakin T. (2007) Functional selectivity through protean and biased agonism: who steers the ship? Mol. Pharmacol. 72, 1393–1401 [DOI] [PubMed] [Google Scholar]
- 37. Violin J. D., Lefkowitz R. J. (2007) Beta-arrestin-biased ligands at seven transmembrane receptors. Trends Pharmacol. Sci. 28, 416–422 [DOI] [PubMed] [Google Scholar]
- 38. Wang A., Gu Z., Heid B., Akers R. M., Jiang H. (2009) Identification and characterization of the bovine G protein-coupled receptor GPR41 and GPR43 genes. J. Dairy Sci. 92, 2696–2705 [DOI] [PubMed] [Google Scholar]
- 39. Bergman E. N. (1990) Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70, 567–590 [DOI] [PubMed] [Google Scholar]
- 40. Wang A., Akers R. M., Jiang H. (2012) Short communication: Presence of G protein-coupled receptors 43 in rumen epithelium but not in the islets of Langerhans in cattle. J. Dairy Sci. 95, 1371–1375 [DOI] [PubMed] [Google Scholar]
- 41. Congreve M., Langmead C., Marshall F. H. (2011) The use of GPCR structures in drug design. Adv. Pharmacol. 62, 1–36 [DOI] [PubMed] [Google Scholar]
- 42. Rosenbaum D. M., Rasmussen S. G., Kobilka B. K. (2009) The structure and function of G-protein-coupled receptors. Nature 459, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hanson M. A., Stevens R. C. (2009) Discovery of new GPCR biology: one receptor structure at a time. Structure 17, 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith N. J., Stoddart L. A., Devine N. M., Jenkins L., Milligan G. (2009) The action and mode of binding of thiazolidinedione ligands at free fatty acid receptor 1. J. Biol. Chem. 284, 17527–17539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lück E. (1990) Food applications of sorbic acid and its salts. Food Addit. Contam. 7, 711–715 [DOI] [PubMed] [Google Scholar]
- 46. Walker R. (1990) Toxicology of sorbic acid and sorbates. Food Addit. Contam. 7, 671–676 [DOI] [PubMed] [Google Scholar]
- 47. Knický M., Spörndly R. (2009) Sodium benzoate, potassium sorbate and sodium nitrite as silage additives. J. Sci. Food Agric. 89, 2659–2667 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.