Abstract
Zebrafish lost anterior teeth during evolution but retain a posterior pharyngeal dentition that requires retinoic acid (RA) cell-cell signaling for its development. The purposes of this study were to test the sufficiency of RA to induce tooth development and to assess its role in evolution. We found that exposure of embryos to exogenous RA induces a dramatic anterior expansion of the number of pharyngeal teeth that later form and shifts anteriorly the expression patterns of genes normally expressed in the posterior tooth-forming region, such as pitx2 and dlx2b. After RA exposure, we also observed a correlation between cartilage malformations and ectopic tooth induction, as well as abnormal cranial neural crest marker gene expression. Additionally, we observed that the RA-induced zebrafish anterior teeth resemble in pattern and number the dentition of fish species that retain anterior pharyngeal teeth such as medaka but that medaka do not express the aldh1a2 RA-synthesizing enzyme in tooth-forming regions. We conclude that RA is sufficient to induce anterior ectopic tooth development in zebrafish where teeth were lost in evolution, potentially by altering neural crest cell development, and that changes in the location of RA synthesis correlate with evolutionary changes in vertebrate dentitions.—Seritrakul, P., Samarut, E., Lama, T. T. S., Gibert, Y., Laudet, V., Jackman, W. R. Retinoic acid expands the evolutionarily reduced dentition of zebrafish.
Keywords: teeth, neural crest cells, retinaldehyde dehydrogenase
Primitive ray-finned fish possessed numerous teeth in their pharyngeal regions, and the generally reduced dentitions of modern species are thought to be largely the result of evolutionary tooth loss (1, 2). The zebrafish (Danio rerio) lineage, as a member of the order Cypriniformes, lost anterodorsal pharyngeal teeth 65 or more million years ago (3, 4), and only retain part of the original posteroventral pharyngeal dentition. A more extensive dentition has not reevolved in any of the nearly 3000 described cypriniform species, despite diverse feeding modes, including piscivory (5). If there is indeed selective pressure to increase the dentition but it is no longer possible due to developmental constraints, this may represent a modern example of Dollo's law of the irreversibility of evolution (6–8). Such irreversibility may be the result of pleiotropic interactions during development (9, 10).
Retinoic acid (RA), an oxidized form of vitamin A, is a cell-cell signaling molecule with numerous functions in developing vertebrate embryos. Evidence of the crucial roles RA plays in development was established over half a century ago, when the offspring of female rodents fed a vitamin A-deficient diet during pregnancy showed congenital defects in many organ systems (11). Synthesized from carotenoids, the active all-trans form of RA acts as a ligand for nuclear RA receptors, which in turn function as transcription factors to control the expression of numerous targets, including Hox genes (12). The amphiphilic nature of RA, with both polar and nonpolar moieties, allows this molecule to pass relatively easily through cell membranes and act as a positional cue for cells during embryogenesis (13).
In vertebrates, RA is particularly important in anteroposterior (AP) patterning and in the development of cranial neural crest (CNC) cells. During the developmental specification of the AP axis, RA can directly regulate Hox gene expression, and alterations in RA levels result in homeotic changes in AP identity (14–16). Such RA-induced homeotic transformations include the duplication of skeletal elements, such as vertebrae (17, 18). RA is also required for the proper specification of CNC cells, a class of vertebrate pluripotent migratory cells that give rise to cephalic structures, including cartilage, bone, and teeth (19, 20). Disruptions in RA signaling cause malformations of CNC-derived tissues in many vertebrates (21, 22).
Teeth exhibit conservation in their early development across vertebrate species and begin their formation in both mammals and teleost fishes as an epithelial placode (23, 24). Subsequent stages of tooth morphogenesis and differentiation are coordinated by reciprocal cell signaling between this dental epithelium and an underlying mesenchyme, which employs members of several evolutionarily conserved vertebrate gene families, including the fibroblast growth factor and hedgehog signaling pathways (25–27). The mammalian dental mesenchyme is known to be largely derived from CNC cells (28), and the dental mesenchyme of zebrafish pharyngeal teeth also express CNC cell markers (25).
RA signaling has been studied previously in relation to tooth development. In mice, mutants lacking RA receptors develop deformed skulls without teeth (29). Conversely, exposing embryonic mouse mandibles to tissue culture medium containing excess RA alters dental epithelial morphology, increases tooth bud size in the diastema region, and possibly switches tooth identity between molars and incisors (30). These studies provide some clues to the potential roles of RA in mammalian tooth development, although there has been no gene expression data or direct functional tests at a molecular level to explain the mechanisms underlying these phenotypes. In zebrafish, it has been shown that tooth induction depends on an endogenous source of RA from retinaldehyde dehydrogenase-expressing cells and that chemically blocking RA synthesis results in the complete absence of teeth (31). However, effects of RA overexpression on zebrafish tooth development have not yet been described.
Here we report that exposure of zebrafish embryos to exogenous RA induces the formation of an extensive symmetrical pattern of supernumerary teeth. Corresponding with this induction, we find up-regulation of genes normally expressed in developing teeth and in the nearby posterior pharyngeal region such as pitx2, dlx2b, and hoxb5a. After RA treatment, CNC cells are present but with disrupted arrangements and abnormal gene expression, and cartilage development is correspondingly disrupted. We compare the expanded RA-induced zebrafish pharyngeal dentition with the wild-type dentitions of other teleost species and find similarities especially with the teeth of medaka. However, comparisons of retinaldehyde dehydrogenase expression between these species suggest that evolutionary changes in RA signaling were not likely responsible for the reduction of the zebrafish pharyngeal dentition.
MATERIALS AND METHODS
Animal husbandry
Embryos of wild-type zebrafish (Danio rerio, Hamilton 1822) were obtained from in-house stocks derived originally from a commercial supplier (LiveAquaria.com, Rhinelander, WI, USA). Embryos were raised at 28.5°C in an embryo medium consisting of 30% Danieu's medium and 0.003% phenylthiourea to prevent pigmentation (25). Phenylthiourea use is important to note, as it has recently been reported to enhance certain RA-related craniofacial phenotypes in zebrafish (32). Zebrafish staging was done as described previously (33), with developmental time reported in hours postfertilization (hpf) or days postfertilization (dpf). The fli1:GFP reporter fish were of the Tg(fli1:EGFP)y1 line (34), and the dlx2b:GFP reporters were from the Tg(dlx2b:EGFP)cs1 line (35). Astyanax mexicanus (De Filippi 1853) stages are reported in dpf, and medaka (Oryzias latipes, Temminck and Schlegel 1846) stages are reported as dpf or stage numbers (36). Animal use was in accordance with protocols approved by the Institutional Animal Care and Use Committee at Bowdoin College and the Université de Lyon.
Exogenous RA treatment and bead implantation
Embryos were manually dechorionated and added to embryo medium containing either 0.05% DMSO alone as a control or DMSO plus 6 × 10−7 M all-trans RA (Biomol International, Plymouth, PA, USA). RA treatments were from 24 hpf onward unless indicated otherwise. Embryos were kept in the dark or with minimal lighting to avoid RA degradation (31). To inactivate RA, embryos were washed into new medium and exposed to bright light for 5 min. AG 1-X8 beads (Bio-Rad, Hercules, CA, USA) were incubated in fresh solution of 6 × 10−4 M RA and implanted as described previously (37).
Histology and in situ hybridization
Whole-mount mRNA in situ hybridization was performed as described previously (25). Riboprobe sources: zebrafish dlx2b, dlx2a, and pitx2 (25); crestin (38); hoxb5a (39); sox9a (40); foxd3 (41); mitfa (42), lef1 (43); prdm1a (44); aldh1a2 (31); and medaka aldh1a2 (sequences 1367–1830 of Genbank accession number NM_001104821). Optical confocal microscopy sections of in situ hybridization specimens were obtained using 633-nm fluorescence of the BM Purple (Roche, Indianapolis, IN, USA) staining product (45). Double fluorescence in situ hybridization was performed as described previously (46). Alizarin red and alcian blue skeletal single and double staining were performed as described previously (47).
Microscopy and statistical analysis
Embryos were visualized and photographed on a Leica DMI3000B inverted microscope with DFC420C camera (Leica Microsystems, Solms, Germany), a Leica MZ16F stereomicroscope with DFC300FX camera (Leica Microsystems), or a Zeiss 510 Meta confocal laser-scanning microscope (Carl Zeiss, Jena, Germany). Three-dimensional images of teeth were generated from confocal data using Volocity (Perkin Elmer, San Jose, CA, USA). Photograph files were processed with Adobe Photoshop (Adobe Systems, San Jose, CA, USA) and minimally adjusted as a whole for brightness, contrast, and/or color balance. Statistical significance of collected data was calculated using a 2-tailed Fisher's exact test of independence (48).
RESULTS
Exogenous RA expands zebrafish tooth development anteriorly and dorsally
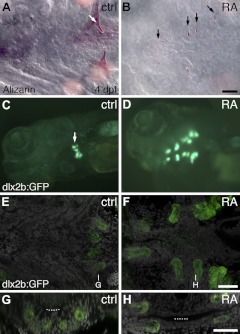
Wild-type zebrafish larvae normally develop a single pair of fully formed teeth on each side of the ventral, posterior pharynx by 4 dpf (Fig. 1A), with 2–3 younger pairs of tooth germs also present nearby but not as easily visualized (49). We found that embryos treated with 6 × 10−7 M exogenous RA from 24 hpf onward or in a window from 24 to 36 hpf exhibited a dramatic supernumerary tooth phenotype by 4 dpf, with tooth formation extending into ectopic anterior and dorsal regions (Fig. 1B; n=119/119 relative to 0/66 controls; P<0.01, 2-tailed Fisher's exact test). These RA-treated larvae possessed ∼10–16 supernumerary teeth arranged in a bilaterally symmetrical pattern. Most of the ectopic teeth were oriented with the tip toward the midline; however, we often also observed 2–4 teeth at the anterior end of the pattern with tips pointing caudally. The apparent fusion of adjacent teeth was also occasionally observed (n=23). We also employed a dlx2b:GFP reporter transgenic line that marks developing dental epithelium and mesenchyme (25, 35). DMSO-treated control fish showed 2–3 closely associated spots of bright GFP expression on each side of the posterior pharynx (Fig. 1C; n=20), while RA-treated embryos exhibited 8–12 supernumerary spots of GFP expression, correlating to the number and location of developing supernumerary teeth (Fig. 1D; n=20; P<0.01, 2-tailed Fisher's exact test). Confocal microscopy additionally revealed the presence of teeth forming in positions dorsal to the pharynx (Fig. 1E–H). Embryos treated with lower RA concentrations or beginning at later stages did not exhibit this supernumerary tooth phenotype as has previously been reported (50), and those treated with higher concentrations or at earlier time points did not survive long enough to develop teeth.
Figure 1.
Exogenous RA treatment expands tooth development. A, B) Ventral view of the pharyngeal region of 4-dpf alizarin red-stained zebrafish larvae, anterior to the left. A) DMSO control exhibiting a pair of well-formed teeth on each side of the ventral, posterior pharynx. B) Specimen treated exogenously with 6 × 10−7 M RA between 24 and 36 hpf, with ectopic teeth positioned more anteriorly than in wild type. Additional teeth located more dorsally are not visible in this focal plane. C, D) Oblique ventrolateral view of the head of 4-dpf dlx2b:GFP reporter larvae. C) Control embryo with GFP expression in developing tooth germs. D) RA-treated larva with anteriorly expanded supernumerary foci of GFP expression. E, F) Confocal micrographs oriented as in A and B, with a single plane of labeled cell nuclei (gray) in the ventral pharynx superimposed on an extended-focus composite of dlx2b:GFP reporter expression (green). G, H) Transverse sections in planes indicated in E and F. G) Control larva with dlx2b:GFP expression in tooth germs developing normally, ventral to the lumen of the pharynx (dotted line). H) RA-treated specimen with tooth germs located both dorsal and ventral to the pharyngeal lumen. Arrows indicate selected teeth or tooth germs. Scale bars = 50 μm.
RA expands expression of tooth-associated genes
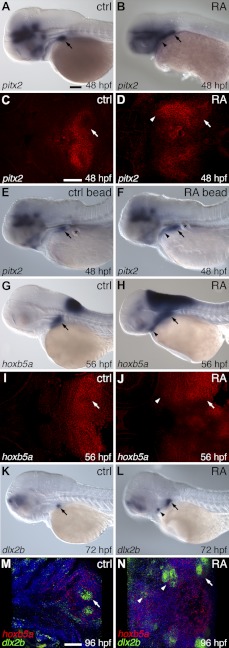
We characterized gene expression changes associated with RA exposure beginning at 24 hpf at several tissue and spatial levels. Expression of the homeodomain transcription factor pitx2 has been reported in both the dental epithelium and in nearby nondental pharyngeal epithelium and represents the earliest marker of tooth germs in both mammals and fish (25, 51). We found that relative to control embryos that express pitx2 mRNA strongly in the posterior pharyngeal region where teeth are developing by 48 hpf (Fig. 2A, C; n=10), in RA-treated embryos pitx2 expression was expanded anteriorly (Fig. 2B, D; n=17; P<0.01, 2-tailed Fisher's exact test). To apply RA in a more localized manner, we implanted RA-coated beads at 24-25 hpf adjacent to the posterior pharynx. In RA bead-implanted larvae, we also observed an expansion of pharyngeal pitx2 expression near the site of implantation by 48 hpf (Fig. 2E; n=5/10), a result significantly different from the normal pitx2 expression observed in control bead-implanted embryos (Fig. 2F; n=0/15; P<0.01, 2-tailed Fisher's exact test).
Figure 2.
RA expands pitx2, dlx2b, and hoxb5a expression. Lateral (A, B, E–H, K, L) or ventral (C, D, I, J, M, N) view of mRNA in situ hybridizations, anterior to the left, with normal site of tooth formation indicated (arrow). A) Control embryo expressing pitx2 in the posterior pharyngeal region at 48 hpf. B) Embryo treated with exogenous RA exhibits an anterior expansion of pitx2 (arrowhead). C) Horizontal confocal fluorescence section of pharyngeal epithelial and tooth germ pitx2 expression in control. D) Identical section after RA treatment with pitx2 expression expanded anteriorly in the pharyngeal epithelium (arrowhead). E) Embryo implanted with a control bead (asterisk) and a wild-type pattern of pitx2 expression. F) RA bead-implanted embryo with pitx2 expression extended more anteriorly (arrowhead). G) Expression of hoxb5a mRNA in the posterior brain and pharyngeal region (arrow) at 56 hpf. H) RA-treated embryo with anterior expansion of pharyngeal hoxb5a expression (arrowhead). I) Horizontal section of hoxb5a expression surrounding control tooth germs (arrow). J) RA-exposed embryo with hoxb5a expression expanded anteriorly (arrowhead). K) dlx2b expression in a control embryo at 72 hpf. L) RA-treated embryo showing dlx2b expression expanded anteriorly (arrowhead). M) 96 hpf larva with dlx2b (green) and hoxb5a (red) expression in the posterior pharynx in and around developing teeth. N) Larva after RA treatment with both dlx2b and hoxb5a expression expanded anteriorly (arrowheads). Scale bars = 100 μm.
The homeobox transcription factor hoxb5a exhibits somewhat broader posterior pharyngeal expression than does pitx2, being strongly expressed in the region where teeth are forming by 56 hpf (Fig. 2G, I; n=9). We found that in RA-treated embryos, pharyngeal hoxb5a expression was expanded both anteriorly and dorsally, correlating to the regions where we find supernumerary teeth (Fig. 2H, J; n=16; P<0.01, 2-tailed Fisher's exact test). We also examined early dlx2b expression as a more restricted marker of the dental epithelium and mesenchyme (25). We found in RA-treated embryos that dlx2b mRNA expression patterns appeared normal at 48 and 60 hpf but at 72 hpf were expanded anteriorly (Fig. 2L; n=11) relative to controls (Fig. 2K; n=8; P<0.01, 2-tailed Fisher's exact test). Simultaneously labeling dlx2b and hoxb5a mRNA at 96 hpf revealed that the ectopic anterior expansion of dlx2b correlated with ectopic anterior hoxb5a expression (Fig. 2N; n=6).
RA tooth expansion correlates with cartilage disruption
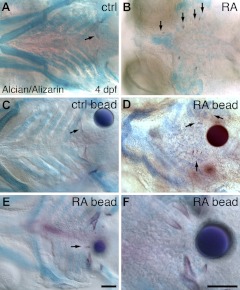
We observed that the occurrence of supernumerary teeth in RA-treated embryos was often associated with cartilage malformation or loss. Zebrafish teeth do not require skeletal support for their initial development (52), but because dental mesenchyme cells and cartilage cells both derive from CNC cell precursors, we further examined this correlation. By 4 dpf, the first pair of pharyngeal teeth has attached to the fifth ceratobranchial cartilage and younger teeth are developing nearby (Fig. 3A). In embryos treated with exogenous RA starting at 24 hpf, cartilage morphology was severely disrupted in the region of supernumerary tooth formation (Fig. 3B). In localized treatments using RA-coated beads implanted at 24 hpf, at 4 dpf we observed cartilage malformation accompanied by supernumerary tooth formation nearby to where the beads were implanted (Fig. 3D–F; n=15/23), relative to controls (Fig. 3C; n=0/13; P<0.01, 2-tailed Fisher's exact test).
Figure 3.
RA tooth expansion correlates with cartilage disruption. Ventral views of the 4-dpf pharyngeal region in alcian blue, alizarin red double-stained larvae, anterior to the left (arrows indicate selected teeth). A) Control zebrafish larva with a pair of well-formed teeth attached to the ossifying fifth ceratobranchial cartilages. B) Larva treated with RA from 24 hpf exhibiting supernumerary teeth and severe cartilage loss. C) Larva showing normal tooth development with a control bead positioned nearby. D–F) Larvae with RA-coated bead implantation at 24 hpf exhibit a range of cartilage deformation and supernumerary tooth phenotypes ranging from relatively severe (D) to relatively mild (E, arrow indicates ectopic midline tooth). F) Closeup view showing supernumerary teeth in proximity to an RA bead. Scale bars = 50 μm.
CNC cells are present but disorganized after RA exposure
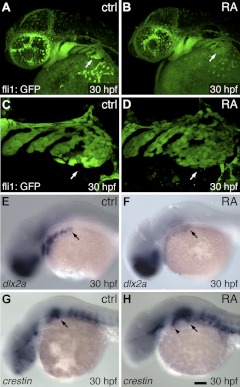
Because CNC cells contribute to both tooth and cartilage development, and because the specification of CNC cells has been shown to be regulated by RA (19), we investigated CNC-cell-associated gene expression after RA exposure. After RA exposure starting at 24 hpf, we surveyed early postmigratory CNC marker expression from 27 to 36 hpf and focused on 30 hpf as having maximal effects. We first employed a fli1:GFP reporter transgenic line that labels CNC cells (34, 53). Relative to controls (Fig. 4A, C; n=12), we found that after 24–30 hpf RA exposure, fli1:GFP reporter expression in CNC cells was present at similar levels but that CNC cell arrangements, especially in the most posterior pharyngeal arch stream near where teeth later arise, appeared less compactly organized (Fig. 4B, D; n=12; P<0.01, 2-tailed Fisher's exact test). dlx2a marks CNC cells during their migration into the pharyngeal arches (54). Similarly to what has previously been reported (55), we found that RA-treated embryos exhibited down-regulation of dlx2a (Fig. 4E; n=25/25) relative to controls (Fig. 4F; n=4/26; P<0.01, 2-tailed Fisher's exact test). crestin marks premigratory and migrating CNC cells (38). Relative to controls (Fig. 4G; n=9), after RA treatment, embryos at 30 hpf showed general up-regulation of crestin (Fig. 4L; n=10; P<0.01, 2-tailed Fisher's exact test). Together, these results suggest that pharyngeal CNC cells are present after RA treatment, but that they are disorganized in arrangement and exhibit abnormal gene expression.
Figure 4.
RA alters late migratory/early postmigratory cranial neural crest organization and gene expression. Lateral views of 30-hpf embryos, anterior to the left (arrows indicate normal tooth-forming region). A) Control fli1:GFP reporter expression. B) RA-treated embryos at with disorganized cell arrangements at 24–30 hpf. C, D) Magnified views from embryos in A and B. E) Control dlx2a mRNA expression. F) RA-treated embryo with down-regulated dlx2a. G) Control crestin mRNA expression. H) RA-treated embryo with crestin expression upregulated in the anterior pharyngeal region (arrowhead). Scale bar = 100 μm.
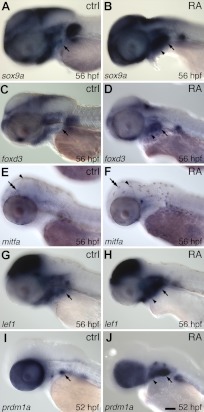
To further assess RA effects on CNC cell fate near the tooth-forming region, we examined later mRNA expression of markers of specific differentiating CNC cell types. sox9a is expressed in differentiating CNC-derived skeleton and has been shown to directly regulate type-II collagen during cartilage development (40, 56). We found that relative to controls (Fig. 5A; n=4), after 24–56 hpf RA exposure sox9a expression was upregulated in the posterior pharyngeal region (Fig. 5B; n=5). Similarly, we examined foxd3, as at later stages its expression pattern includes developing CNC-derived glial cells (57). Similarly we found that compared with controls (Fig. 5C; n=6), foxd3 mRNA was present in a more extensive area of the pharyngeal region after RA exposure (Fig. 5D; n=6). In contrast, the expression of mitfa, a marker of differentiating melanocytes (42), appeared similar between control (Fig. 5E; n=6) and RA-exposed embryos (Fig. 5F; n=6), although the pattern of differentiated melanocytes appeared abnormal. Expression of these markers suggests that 24 hpf RA exposure is affecting the differentiation and/or distribution of both ectomesenchymal and nonectomesenchymal CNC cell types.
Figure 5.
Gene expression analysis of CNC cell subtype markers and potential RA target genes. Lateral views of 52- to 56-hpf embryos, anterior to the left (arrows indicate normal tooth-forming region). A) Control sox9a mRNA expression at 56 hpf. B) RA-treated embryo with up-regulation of sox9a expression in the pharyngeal region (arrowhead) at 24–56 hpf. C) Control foxd3 mRNA expression. D) RA-treated embryo with more widespread pharyngeal foxd3 expression (arrowhead). E) Control mitfa mRNA expression marking developing melanocytes (double arrow). Differentiated melanocytes are also visible (arrowhead). F) RA-treated embryo with similar levels of mitfa expression but a disorganized-appearing melanocyte pattern (arrowhead). G) Control lef1 mRNA expression. H) RA-treated embryo with up-regulation of pharyngeal lef1 expression (arrowhead). I) Control prdm1a mRNA expression, restricted primarily to the developing tooth germ. J) RA-treated embryo showing strong prdm1a up-regulation (arrowhead). Scale bar = 100 μm.
We also examined RA effects on the mRNA expression of the canonical wingless pathway transcription factor lef1 and the zinc-finger protein prdm1a, as both of these genes are required for zebrafish tooth development (58, 59) and both have been characterized to lie downstream of an RA signal during fin development (60). We found lef1 mRNA to be expressed throughout the pharyngeal region at 56 hpf (Fig. 5G; n=8) and that after 24–56 hpf RA exposure, expression levels appeared to increase (Fig. 5H; n=6/7). Pharyngeal expression of prdm1a mRNA was more limited in control embryos, mostly to the developing tooth germs (Fig. 5I; n=11) but appeared greatly expanded after RA treatment (Fig. 5J; n=22). Taken together, these expression data suggest that RA may regulate tooth development with a similar mechanism to its role in fin formation.
Phylogenetic comparisons
To visualize the overall arrangement of teeth in RA-treated zebrafish embryos, and make comparisons with other species, we generated three-dimensional, confocal microscope images of alizarin-red-stained specimens (Fig. 6). Simultaneously viewing teeth from all focal planes revealed a bilaterally symmetrical pattern of zebrafish RA-induced teeth (Fig. 6C). Teeth at particular anteroposterior levels also typically shared the same relative dorsal/ventral positioning with their contralateral counterparts (Fig. 6D). We next examined similarly staged Mexican tetra (Astyanax mexicanus) and medaka (Oryzias latipes) larvae (Fig. 6E–H). Mexican tetras (order Characiformes) are relatively closely related to zebrafish (order Cypriniformes), with medaka (order Beloniformes) as a more distant relative of both (61). To match the developmental stage in all three species as closely as possible, we chose stages when pharyngeal tooth development had commenced but nearby bones were not extensively calcified. At this stage, Mexican tetra possess 2 bilateral pairs of dorsal pharyngeal teeth located rostrally to a pair of ventral teeth, the latter of which correspond to the teeth in wild-type zebrafish (Fig. 6E, F). Medaka at this stage share a similar posteroventral dentition with 2 pairs of teeth but have a much more extensive dorsal pharyngeal dentition with five bilateral pairs of teeth extending further anteriorly (Fig. 6G, H). The supernumerary RA induced teeth in zebrafish appeared similar to the pharyngeal dentition in medaka in tooth number, anteroposterior location, and dorsoventral arrangement, but the patterns are not identical.
Figure 6.
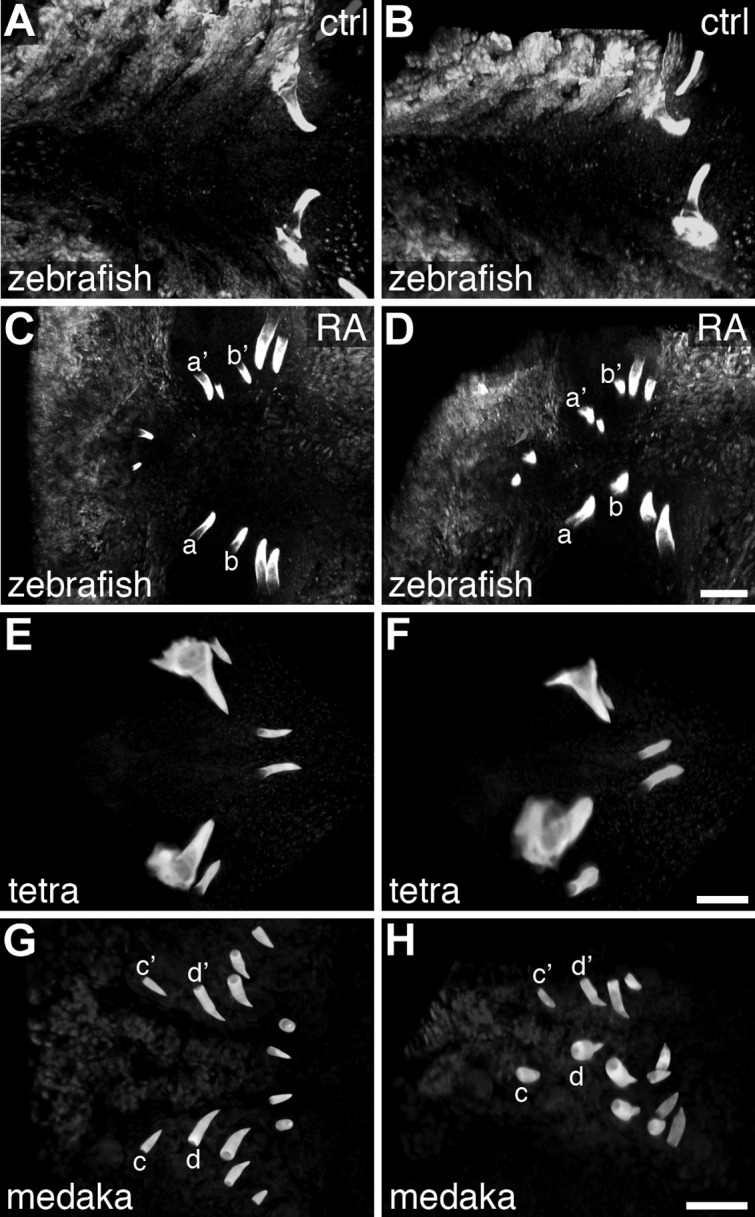
Multispecies comparison of the early larval pharyngeal dentition. Ventral (A, C, E, G) and 45°-rotated, ventrolateral (B, D, F, H) views of alizarin red-stained larva, visualized as 3D projections of confocal z stacks, anterior to the left. A, B) Zebrafish at 4 dpf with a bilateral pair of well-formed teeth located in the ventral, posterior pharynx. C) Zebrafish larva at 4 dpf, after exposure to RA beginning at 24 hpf, exhibiting supernumerary teeth that are anterior to normal positions, yet retaining a pattern of growth that is close to bilaterally symmetrical. For example, one bilateral pair of teeth (a/a′) is located closer to the midline than a nearby pair (b/b′). D) A ventrolateral view of the individual from C reveals dorsoventral bilateral symmetry as well: e.g., the more anterior labeled pair (a/a′) is located more ventrally than the more posterior pair (b/b′). Teeth near the b/b′ position were often found dorsal to the pharyngeal lumen (Fig. 1H). E, F) Mexican tetra larva at 3 dpf with 2 pairs of dorsal pharyngeal teeth located anteriorly to a single pair of ventral teeth. G, H) Medaka larva at 5 dpf with 5 pairs of anterodorsal teeth and 2 pairs of posteroventral teeth. Scale bars = 50 μm.
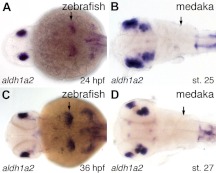
Because of the similarities between the medaka wild-type and zebrafish RA-induced pharyngeal dentitions, we wondered whether the medaka condition may be the result of more extensive RA signaling relative to zebrafish. To address this question, we examined mRNA expression of the retinaldehyde dehydrogenase gene aldh1a2 (formerly raldh2) between zebrafish and medaka, as this gene has been implicated in the RA synthesis necessary for zebrafish tooth development (31). However, we found that unlike zebrafish which express aldh1a2 in the posterior pharyngeal region adjacent to the position where teeth will later form (Fig. 7A; n=66; Fig. 7C; n=28), we were unable to detect aldh1a2 expression at all in the pharyngeal region of comparably staged medaka embryos (Fig. 7B, n=21; Fig. 7D, n=20). This result suggests that RA is not directly involved with medaka pharyngeal tooth specification and is consistent with similar, previously-reported aldh1a2 expression comparisons between zebrafish and Mexican tetras (31).
Figure 7.
Comparison of retinaldehyde dehydrogenase expression between zebrafish and medaka. Dorsal views of approximately stage-matched zebrafish and medaka embryos, anterior to the left, labeled for aldh1a2 mRNA expression by in situ hybridization. A, C) Zebrafish aldh1a2 mRNA expression visualized at 24 hpf (A) and 36 hpf (C) in the posterior pharyngeal region near the location where pharyngeal tooth germs will later form (arrows). B, D) Stage 25 (B) and 27 (D) medaka embryos with no detectable aldh1a2 mRNA expression in any part of the pharyngeal region (arrows).
DISCUSSION
Mechanisms of RA-mediated supernumerary tooth induction
We have considered several potential mechanisms to address how exogenous RA exposure is able to induce supernumerary tooth development in zebrafish. One possible explanation is that RA may be initiating a homeotic transformation of anterior and dorsal pharyngeal tissues to a more posterior and ventral identity and thus indirectly inducing tooth development. RA is known to regulate the expression of Hox genes (62), and differential Hox expression is a fundamental and evolutionarily conserved mechanism for patterning the vertebrate AP axis (12). Previous studies of zebrafish craniofacial development have shown that altering the expression of Hox genes can result in the homeotic transformation of CNC derived skeletal elements (63, 64). We examined hoxb5a expression in this study because of its strong and specific expression in the normal tooth-forming region (Fig. 2G) and because it has been shown specifically to be positively regulated by RA signaling (65). The anterior and dorsal expansion of hoxb5a expression we observed after RA treatment (Fig. 2H, J, N) is consistent with the idea of RA-induced homeotic transformation of the pharyngeal region. We attempted to phenocopy RA-mediated tooth induction with hoxb5a overexpression by mRNA injection but did not observe a tooth phenotype (unpublished results). However, there are many potential explanations for this lack of result, including that homeotic transformation is not responsible for RA-induced ectopic tooth formation, that RA is acting through more than one Hox gene and overexpressing a single gene has no effect, or that RA is acting through other factors to alter AP identity such as Cdx genes (17).
An alternative, although not necessarily mutually exclusive, way RA could be influencing tooth development is through CNC cell specification. One of the defining features of CNC cells is high developmental plasticity: CNC cell fate is largely determined by molecular signals in the microenvironment and generally not preprogrammed (66). CNC cells have long been known to contribute to the development of both cranial cartilage and teeth (20), raising the possibility that a switch in CNC cell fate could lead to the overproduction of one organ type at the expense of the other. CNC cells in the zebrafish pharyngeal region express the homeodomain transcription factor dlx2a (54), and inhibition of dlx2a function results in reduced and abnormal development of pharyngeal cartilages (67). In addition, in chickens, misexpression of Dlx2 leads to ectopic cranial cartilage formation (68), and exogenous RA exposure in zebrafish suppresses Dlx gene expression, resulting in pharyngeal cartilage malformation and loss (55). Together, these data suggest that RA may be involved in directing CNC cells toward a cartilage fate by influencing the expression of Dlx genes. The presence of fli1:GFP-expressing cells in the posterior pharyngeal region suggests that CNC cells are present at late migration stages after RA exposure (Fig. 4D), but down-regulation of dlx2a and up-regulation of crestin suggest that the remaining CNC cells are already not developing normally (Fig. 4F, H). However, inhibition of dlx2a function alone does not result in supernumerary tooth formation (35), suggesting that if RA is altering CNC cell fate specification, it is doing so by changing the expression of multiple downstream targets. In addition, our analysis of later CNC marker expression suggests that multiple CNC cell types may be overrepresented after 24 hpf RA exposure, including chondrocytes (sox9a; Fig. 5B) and glia (foxd3; Fig. 5D), but examining melanocytes suggest that not all CNC cell types are overrepresented after RA treatment (mitfa; Fig. 5F). Together, these data suggest that RA may be promoting the development of certain CNC cell types, such as both cartilage and tooth odontoblasts, rather than directing a switch in fate between CNC cell subtypes.
It is also possible that RA induction of tooth development may be relatively direct. A study using chromatin immunoprecipitation and gel mobility shift assays in mice has shown that RA nuclear receptor complexes directly bind to cis-regulatory regions of the Pitx2 gene (69), the earliest marker of tooth formation (51). A study using similar methods has, in turn, demonstrated direct regulation of Dlx2 by Pitx2 (70). Our observation of expansion of pitx2 expression after RA exposure (Fig. 2B, D, F) is consistent with the direct up-regulation of pitx2 by RA. We also do not observe ectopic dlx2b expression until many hours after the time of initial RA exposure (Fig. 2L), consistent with RA having an indirect effect on dlx2b expression. However, our data from lef1 and prdm1a (Fig. 5G–J), genes known to be downstream of RA signaling in limb development and required for zebrafish tooth development (58–60), are consistent with RA exhibiting a more indirect control of tooth development than by direct up-regulation of pitx2. Direct tests of pitx2 function during tooth development could help establish whether this gene may indeed be acting as an intermediary between RA signaling and tooth morphogenesis.
RA in the evolution of teleost dentitions
The order Cypriniformes is thought to have greatly reduced its dentition early in its evolution such that teeth remained only associated with the posterior and ventral 5th ceratobranchial element (CB5), possibly as an adaptation for suction feeding (1, 71). It is interesting to consider whether the evolutionary reduction or loss of RA signaling in the anterior and dorsal pharynx may have been part of a developmental mechanism by which the Cypriniform ancestor reduced its dentition. If this were the case, one might expect to find similarities between the zebrafish RA-induced dental patterns with the normal tooth arrangements of other teleost fishes that have retained a less modified dentition, as well as more extensive RA signaling in the anterior pharyngeal regions of such species.
The bowfin Amia clava, a species immediately basal to teleosts phylogenetically (61), possesses an extensive larval dentition associated with both anterior and posterior pharyngeal skeletal elements (72). As the teleost sister group, the dentition of Amia may represent the ancestral condition for teleosts. Pharyngeal tooth reduction is common in other orders of the teleost radiation, including the belaniform medaka (Oryzias latipes) and the characiform Mexican tetra (Astyanax mexicanus), but is typically less severe than what is found in the cypriniformes (2, 73). The medaka and zebrafish lineages diverged early in the teleost radiation (61); thus, the teeth of Amia clava may represent the closest proxy for the ancestral pharyngeal dentition of these model species. By this logic, the more extensive pharyngeal dentition of medaka is more similar to the ancestral condition than is the more reduced dentition of zebrafish.
Of the species we examined, we interpret the RA-induced zebrafish tooth pattern as most resembling the wild-type pharyngeal dentition of medaka (Fig. 6). In medaka, pharyngeal teeth attach to 2 dorsal parabranchial bones and ventrally to CB5 (73). Similarities between RA-induced zebrafish teeth and wild-type medaka dentitions include tooth number, orientation, the anteroposterior extent of tooth formation, and symmetry in the left-right and dorsoventral axes. It is also notable that RA-exposed zebrafish often develop teeth dorsal to the pharyngeal lumen, a position where teeth never form in zebrafish or any other cypriniform species (5). These similarities prompted us to wonder whether RA signaling may have been ancestrally more prevalent in the pharyngeal region, and whether the evolutionary reduction of the cypriniform dentition may have been the consequence of reduction in anterior pharyngeal RA signaling. This scenario would predict that a species with a more extensive pharyngeal dentition, such as medaka, would have more RA signaling associated with the development of the anterior pharynx. To test this idea, we compared the zebrafish and medaka mRNA expression of aldh1a2, as this gene has been implicated as a key regulator of RA levels required for zebrafish tooth and craniofacial development (21, 31). However, in contrast to the above scenario, our data suggest instead that medaka have no retinaldehyde dehydrogenase expression associated with pharyngeal tooth development (Fig. 7). This result is consistent with previous work where RA inhibition had a much lesser effect on tooth development in medaka than it had in zebrafish (31). We therefore speculate that the RA-induced ectopic dentition in zebrafish may not be recapitulating an ancestral condition; however, phylogenetic comparisons of RA expression and function during the tooth development of other species will shed further light on this question.
However, even if raising RA signaling levels in zebrafish does not actually restore a more ancestral-like dentition, the number of teeth and area in which they reside is clearly increased by increasing RA, so why has this not occurred during cypriniform evolution to bring back teeth in species that would benefit from a more extensive dentition? Examples of cypriniforms where adaptive pressure may be present to produce more teeth include piscivorous pikeminnows (74) and Danionella dracula, which has evolved fang-like jawbone projections used in male displays (75). The answer to this question may lie with the corresponding cartilage disruptions we have observed to accompany RA-induced tooth expansion. It is possible to induce a more extensive dentition by gross RA overexpression, but to increase tooth number without adversely affecting nearby skeletal elements may require more sophisticated control. Such fine control over RA levels, distribution pattern, or the simultaneous coexpression of modulatory cofactors may be difficult to evolve; thus constraining how cypriniforms could possibly reacquire a more extensive dentition. Further comparative study of the mechanisms of RA action on tooth and skeletal development in zebrafish and medaka and other noncypriniform species, as well as similar comparative analyses of other developmental signaling pathways, will help to explain this apparent case of evolutionary developmental constraint.
Acknowledgments
The authors are very grateful to Kristin Artinger (University of Colorado, Aurora, CO, USA) for sharing ideas, prepublication data, and reagents; Vicki Prince (University of Chicago, Chicago, IL, USA) and Paul Henion (Ohio State University, Columbus, OH, USA) for reagents; and Mike Palopoli, Andrea Jowdry, and David Rivers for helpful suggestions.
This work was supported by U.S. National Institutes of Health grants 5P20-RR-016463-12 and 8P20-GM-103423-12 (to W.R.J.) and Agence Nationale de la Recherche grant ANR-09-BLAN-0127-01 (to V.L.).
Footnotes
- AP
- anteroposterior
- CB5
- 5th ceratobranchial element
- CNC
- cranial neural crest
- dpf
- days postfertilization
- hpf
- hours postfertilization
- RA
- retinoic acid
REFERENCES
- 1. Gosline W. (1973) Considerations regarding the phylogeny of cypriniform fishes, with special reference to structures associated with feeding. Copeia 1973, 761–776 [Google Scholar]
- 2. Stock D. W. (2001) The genetic basis of modularity in the development and evolution of the vertebrate dentition. Philos. Trans. R Soc. Lond. B Biol. Sci. 356, 1633–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patterson C. (1993) Osteichthyes: teleostei. In The Fossil Record 2 (Benton M., ed.) pp. 621–656, Chapman & Hall, London [Google Scholar]
- 4. Sibbing F. (1991) Food capture and oral processing. In Cyprinid Fishes: Systematics, Biology, and Exploitation (Winfield I., Nelson J., eds.) pp. 377–412, Chapman & Hall, New York [Google Scholar]
- 5. Stock D. W. (2007) Zebrafish dentition in comparative context. J. Exp. Zoolog. B Mol. Dev. Evol. 308B, 523–549 [DOI] [PubMed] [Google Scholar]
- 6. Dollo L. (1893) Les lois de l'évolution. Bull. Soc. Bel. Géol. Pal. Hydr. 7, 164–166 [Google Scholar]
- 7. Goldberg E. E., Igić B. (2008) On phylogenetic tests of irreversible evolution. Evolution 62, 2727–2741 [DOI] [PubMed] [Google Scholar]
- 8. Marshall C. R., Raff E. C., Raff R. A. (1994) Dollo's law and the death and resurrection of genes. Proc. Natl. Acad. Sci. U. S. A. 91, 12283–12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galis F., Arntzen J. W., Lande R. (2010) Dollo's law and the irreversibility of digit loss in Bachia. Evolution 64, 2466–2485 [DOI] [PubMed] [Google Scholar]
- 10. Lande R. (1978) Evolutionary mechanisms of limb loss in tetrapods. Evolution 32, 73–92 [DOI] [PubMed] [Google Scholar]
- 11. Wilson J., Roth C., Warkany J. (1953) An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am. J. Anat. 92, 189–217 [DOI] [PubMed] [Google Scholar]
- 12. Mallo M., Wellik D. M., Deschamps J. (2010) Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 344, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niederreither K., Dollé P. (2008) Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541–553 [DOI] [PubMed] [Google Scholar]
- 14. Allan D., Houle M., Bouchard N., Meyer B. I., Gruss P., Lohnes D. (2001) RARγ and Cdx1 interactions in vertebral patterning. Dev. Biol. 240, 46–60 [DOI] [PubMed] [Google Scholar]
- 15. Manzanares M., Wada H., Itasaki N., Trainor P. A., Krumlauf R., Holland P. W. (2000) Conservation and elaboration of Hox gene regulation during evolution of the vertebrate head. Nature 408, 854–857 [DOI] [PubMed] [Google Scholar]
- 16. Kessel M., Gruss P. (1991) Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67, 89–104 [DOI] [PubMed] [Google Scholar]
- 17. Houle M., Sylvestre J.-R., Lohnes D. (2003) Retinoic acid regulates a subset of Cdx1 function in vivo. Development 130, 6555–6567 [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi M., Nakamoto M., Honda H., Nakagawa T., Fujita H., Nakamura T., Hirai H., Narumiya S., Kakizuka A. (1998) Retardation of skeletal development and cervical abnormalities in transgenic mice expressing a dominant-negative retinoic acid receptor in chondrogenic cells. Proc. Natl. Acad. Sci. U. S. A. 95, 7491–7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minoux M., Rijli F. M. (2010) Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137, 2605–2621 [DOI] [PubMed] [Google Scholar]
- 20. De Beer G. (1947) The differentiation of neural crest cells into visceral cartilages and odontoblasts in Amblystoma, and a re-examination of the germ-layer theory. Proc. R Soc. Lond. B Biol. Sci. 134, 377–398 [PubMed] [Google Scholar]
- 21. Begemann G., Schilling T. F., Rauch G. J., Geisler R., Ingham P. W. (2001) The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 128, 3081–3094 [DOI] [PubMed] [Google Scholar]
- 22. Lohnes D., Mark M., Mendelsohn C., Dollé P., Dierich A., Gorry P., Gansmuller A., Chambon P. (1994) Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development 120, 2723–2748 [DOI] [PubMed] [Google Scholar]
- 23. Huysseune A., Van der heyden C., Sire J.-Y. (1998) Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae). Anat. Embryol. 198, 289–305 [DOI] [PubMed] [Google Scholar]
- 24. Peters H., Balling R. (1999) Teeth. Where and how to make them. Trends Genet. 15, 59–65 [DOI] [PubMed] [Google Scholar]
- 25. Jackman W. R., Draper B. W., Stock D. W. (2004) Fgf signaling is required for zebrafish tooth development. Dev. Biol. 274, 139–157 [DOI] [PubMed] [Google Scholar]
- 26. Jackman W. R., Yoo J. J., Stock D. W. (2010) Hedgehog signaling is required at multiple stages of zebrafish tooth development. BMC Dev. Biol. 10, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klein O. D., Lyons D. B., Balooch G., Marshall G. W., Basson M. A., Peterka M., Boran T., Peterkova R., Martin G. R. (2008) An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development 135, 377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chai Y., Jiang X., Ito Y., Bringas P., Han J., Rowitch D. H., Soriano P., McMahon A. P., Sucov H. M. (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679 [DOI] [PubMed] [Google Scholar]
- 29. Mark M., Lohnes D., Mendelsohn C., Dupé V., Vonesch J. L., Kastner P., Rijli F., Bloch-Zupan A., Chambon P. (1995) Roles of retinoic acid receptors and of Hox genes in the patterning of the teeth and of the jaw skeleton. Int. J. Dev. Biol. 39, 111–121 [PubMed] [Google Scholar]
- 30. Kronmiller J. E., Nguyen T., Berndt W. (1995) Instruction by retinoic acid of incisor morphology in the mouse embryonic mandible. Arch. Oral Biol. 40, 589–595 [DOI] [PubMed] [Google Scholar]
- 31. Gibert Y., Bernard L., Debiais-Thibaud M., Bourrat F., Joly J.-S., Pottin K., Meyer A., Rétaux S., Stock D. W., Jackman W. R., Seritrakul P., Begemann G., Laudet V. (2010) Formation of oral and pharyngeal dentition in teleosts depends on differential recruitment of retinoic acid signaling. FASEB J. 24, 3298–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bohnsack B. L., Gallina D., Kahana A. (2011) Phenothiourea sensitizes zebrafish cranial neural crest and extraocular muscle development to changes in retinoic acid and IGF signaling. PLoS ONE 6, e22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- 34. Lawson N. D., Weinstein B. M. (2002) In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 [DOI] [PubMed] [Google Scholar]
- 35. Jackman W. R., Stock D. W. (2006) Transgenic analysis of Dlx regulation in fish tooth development reveals evolutionary retention of enhancer function despite organ loss. Proc. Natl. Acad. Sci. U. S. A. 103, 19390–19395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iwamatsu T. (2004) Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 121, 605–618 [DOI] [PubMed] [Google Scholar]
- 37. White R. J., Nie Q., Lander A. D., Schilling T. F. (2007) Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 5, e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luo R., An M., Arduini B. L., Henion P. D. (2001) Specific pan-neural crest expression of zebrafish Crestin throughout embryonic development. Dev. Dyn. 220, 169–174 [DOI] [PubMed] [Google Scholar]
- 39. Prince V. E., Joly L., Ekker M., Ho R. K. (1998) Zebrafish hox genes: genomic organization and modified colinear expression patterns in the trunk. Development 125, 407–420 [DOI] [PubMed] [Google Scholar]
- 40. Chiang E. F., Pai C. I., Wyatt M., Yan Y. L., Postlethwait J., Chung B. (2001) Two sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Dev. Biol. 231, 149–163 [DOI] [PubMed] [Google Scholar]
- 41. Odenthal J., Nüsslein-Volhard C. (1998) Fork head domain genes in zebrafish. Dev. Genes Evol. 208, 245–258 [DOI] [PubMed] [Google Scholar]
- 42. Lister J. A., Robertson C. P., Lepage T., Johnson S. L., Raible D. W. (1999) nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126, 3757–3767 [DOI] [PubMed] [Google Scholar]
- 43. Dorsky R. I., Snyder A., Cretekos C. J., Grunwald D. J., Geisler R., Haffter P., Moon R. T., Raible D. W. (1999) Maternal and embryonic expression of zebrafish lef1. Mech. Dev. 86, 147–150 [DOI] [PubMed] [Google Scholar]
- 44. Hernandez-Lagunas L., Choi I. F., Kaji T., Simpson P., Hershey C., Zhou Y., Zon L., Mercola M., Artinger K. B. (2005) Zebrafish narrowminded disrupts the transcription factor prdm1 and is required for neural crest and sensory neuron specification. Dev. Biol. 278, 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trinh L. A., McCutchen M. D., Bonner-Fraser M., Fraser S. E., Bumm L. A., McCauley D. W. (2007) Fluorescent in situ hybridization employing the conventional NBT/BCIP chromogenic stain. Biotechniques 42, 756–759 [DOI] [PubMed] [Google Scholar]
- 46. Talbot J. C., Johnson S. L., Kimmel C. B. (2010) Hand and Dlx genes specify dorsal, intermediate and ventral domains within zebrafish pharyngeal arches. Development 137, 2507–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walker M. B., Kimmel C. B. (2007) A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 82, 23–28 [DOI] [PubMed] [Google Scholar]
- 48. McDonald J. (2009) Fisher's exact test of independence. In Handbook of Biological Statistics, pp. 70–75, Sparky House, Baltimore [Google Scholar]
- 49. Van der Heyden C., Huysseune A. (2000) Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae). Dev. Dyn. 219, 486–496 [DOI] [PubMed] [Google Scholar]
- 50. Laue K., Jänicke M., Plaster N., Sonntag C., Hammerschmidt M. (2008) Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development. Development 135, 3775–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mucchielli M. L., Mitsiadis T. A., Raffo S., Brunet J. F., Proust J. P., Goridis C. (1997) Mouse Otlx2/RIEG expression in the odontogenic epithelium precedes tooth initiation and requires mesenchyme-derived signals for its maintenance. Dev. Biol. 189, 275–284 [DOI] [PubMed] [Google Scholar]
- 52. Schilling T. F., Piotrowski T., Grandel H., Brand M., Heisenberg C. P., Jiang Y. J., Beuchle D., Hammerschmidt M., Kane D. A., Mullins M. C., van Eeden F. J., Kelsh R. N., Furutani-Seiki M., Granato M., Haffter P., Odenthal J., Warga R. M., Trowe T., Nusslein-Volhard C. (1996) Jaw and branchial arch mutants in zebrafish I: branchial arches. Development 123, 329–344 [DOI] [PubMed] [Google Scholar]
- 53. Crump J. G., Maves L., Lawson N. D., Weinstein B. M., Kimmel C. B. (2004) An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development 131, 5703–5716 [DOI] [PubMed] [Google Scholar]
- 54. Akimenko M. A., Ekker M., Wegner J., Lin W., Westerfield M. (1994) Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J. Neurosci. 14, 3475–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ellies D. L., Langille R. M., Martin C. C., Akimenko M. A., Ekker M. (1997) Specific craniofacial cartilage dysmorphogenesis coincides with a loss of dlx gene expression in retinoic acid-treated zebrafish embryos. Mech. Dev. 61, 23–36 [DOI] [PubMed] [Google Scholar]
- 56. Bell D. M., Leung K. K., Wheatley S. C., Ng L. J., Zhou S., Ling K. W., Sham M. H., Koopman P., Tam P. P., Cheah K. S. (1997) SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16, 174–178 [DOI] [PubMed] [Google Scholar]
- 57. Ignatius M.S., Moose H. E., El-Hodiri H. M., Henion P. D. (2008) Colgate/hdac1 repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev. Biol. 313, 568–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Birkholz D. A., Olesnicky Killian E. C., George K. M., Artinger K. B. (2009) Prdm1a is necessary for posterior pharyngeal arch development in zebrafish. Dev. Dyn. 238, 2575–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McGraw H. F., Drerup C. M., Culbertson M. D., Linbo T., Raible D. W., Nechiporuk A. V. (2011) Lef1 is required for progenitor cell identity in the zebrafish lateral line primordium. Development 138, 3921–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mercader N., Fischer S., Neumann C. J. (2006) Prdm1 acts downstream of a sequential RA, Wnt and Fgf signaling cascade during zebrafish forelimb induction. Development 133, 2805–2815 [DOI] [PubMed] [Google Scholar]
- 61. Nelson J. (2006). Fishes of the World, 4th Ed., John Wiley & Sons, Hoboken, NJ, USA [Google Scholar]
- 62. Marshall H., Morrison A., Studer M., Pöpperl H., Krumlauf R. (1996) Retinoids and Hox genes. FASEB J. 10, 969–978 [PubMed] [Google Scholar]
- 63. Hunter M. P., Prince V. E. (2002) Zebrafish hox paralogue group 2 genes function redundantly as selector genes to pattern the second pharyngeal arch. Dev. Biol. 247, 367–389 [DOI] [PubMed] [Google Scholar]
- 64. Alexandre D., Clarke J. D., Oxtoby E., Yan Y. L., Jowett T., Holder N. (1996) Ectopic expression of Hoxa-1 in the zebrafish alters the fate of the mandibular arch neural crest and phenocopies a retinoic acid-induced phenotype. Development 122, 735–746 [DOI] [PubMed] [Google Scholar]
- 65. Feng L., Hernandez R. E., Waxman J. S., Yelon D., Moens C. B. (2010) Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev. Biol. 338, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baker C. V., Bronner-Fraser M. (1997) The origins of the neural crest. Part II: an evolutionary perspective. Mech. Dev. 69, 13–29 [DOI] [PubMed] [Google Scholar]
- 67. Sperber S. M., Saxena V., Hatch G., Ekker M. (2008) Zebrafish dlx2a contributes to hindbrain neural crest survival, is necessary for differentiation of sensory ganglia and functions with dlx1a in maturation of the arch cartilage elements. Dev. Biol. 314, 59–70 [DOI] [PubMed] [Google Scholar]
- 68. Gordon C. T., Brinas I. M. L., Rodda F. A., Bendall A. J., Farlie P. G. (2010) Role of Dlx genes in craniofacial morphogenesis: Dlx2 influences skeletal patterning by inducing ectomesenchymal aggregation in ovo. Evol. Dev. 12, 459–473 [DOI] [PubMed] [Google Scholar]
- 69. Kumar S., Duester G. (2010) Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev. Biol. 340, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Venugopalan S. R., Li X., Amen M. A., Florez S., Gutierrez D., Cao H., Wang J., Amendt B. A. (2011) Hierarchical interactions of homeodomain and forkhead transcription factors in regulating odontogenic gene expression. J. Biol. Chem. 286, 21372–21383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nichols J. T. (1930) Speculation on the history of the ostariophysi. Copeia 1930, 148–151 [Google Scholar]
- 72. Grande L., Bemis W. E. (1998) A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. J. Vertebr. Paleontol. Mem. 4, 1–690 [Google Scholar]
- 73. Debiais-Thibaud M., Borday-Birraux V., Germon I., Bourrat F., Metcalfe C. J., Casane D., Laurenti P. (2007) Development of oral and pharyngeal teeth in the medaka (Oryzias latipes): comparison of morphology and expression of eve1 gene. J. Exp. Zool. 308, 693–708 [DOI] [PubMed] [Google Scholar]
- 74. Portz D., Tyus H. (2004) Fish humps in two Colorado River fishes: a morphological response to cyprinid predation? Env. Biol. Fishes 71, 233–245 [Google Scholar]
- 75. Britz R., Conway K. W., Rüber L. (2009) Spectacular morphological novelty in a miniature cyprinid fish, Danionella dracula n. sp. Proc. R. Soc. Lond. B Biol. Sci. 276, 2179–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]