Abstract
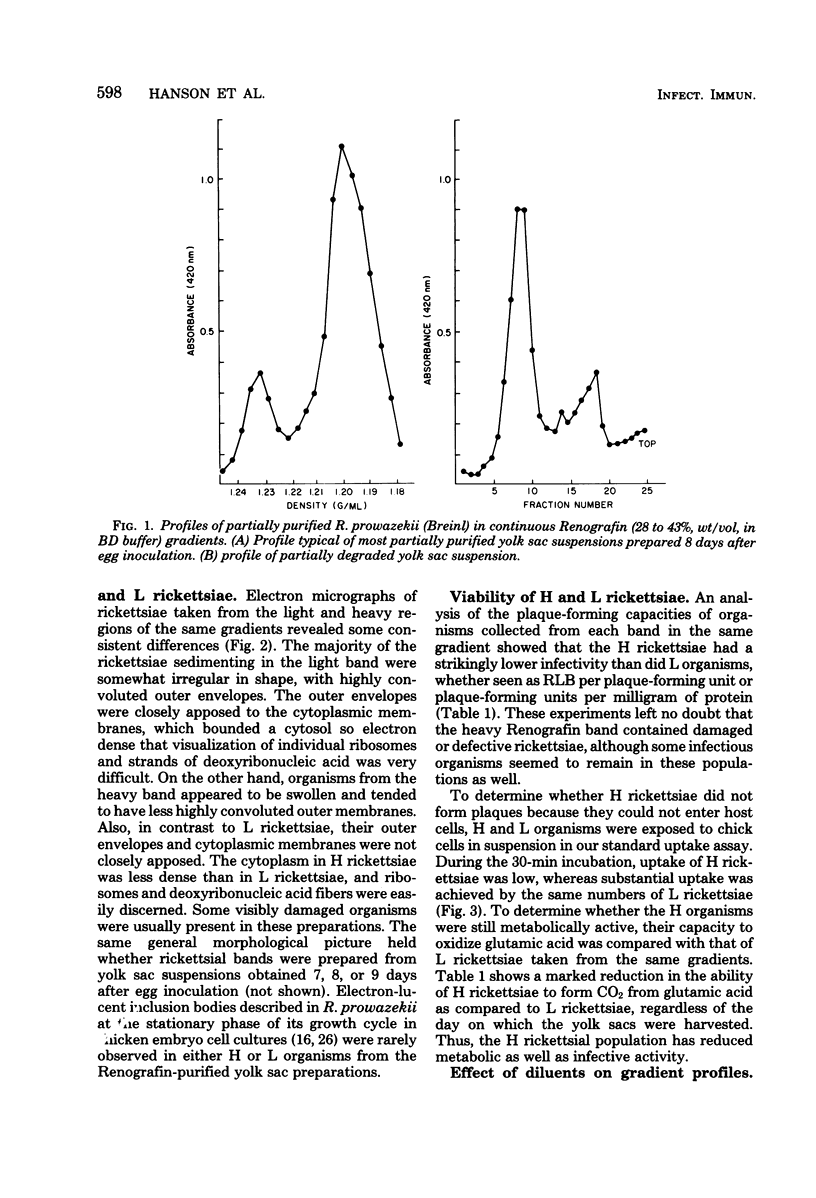
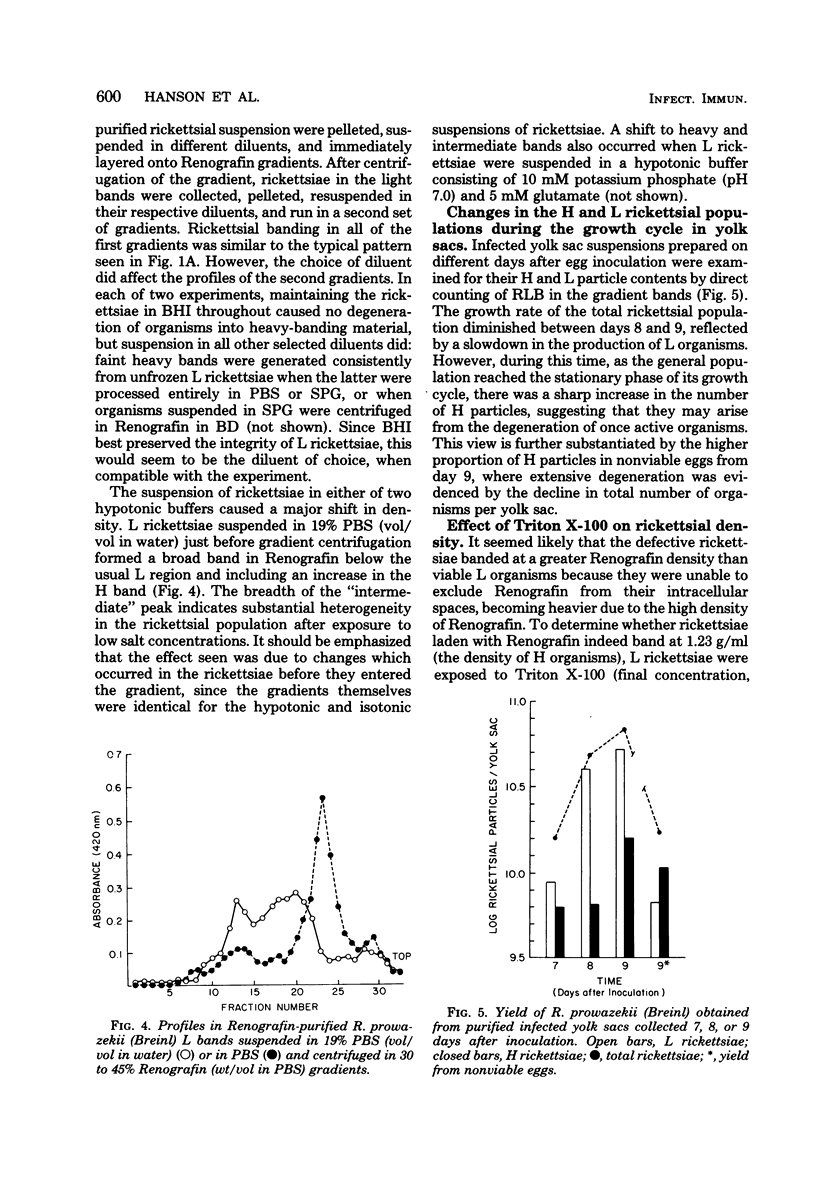
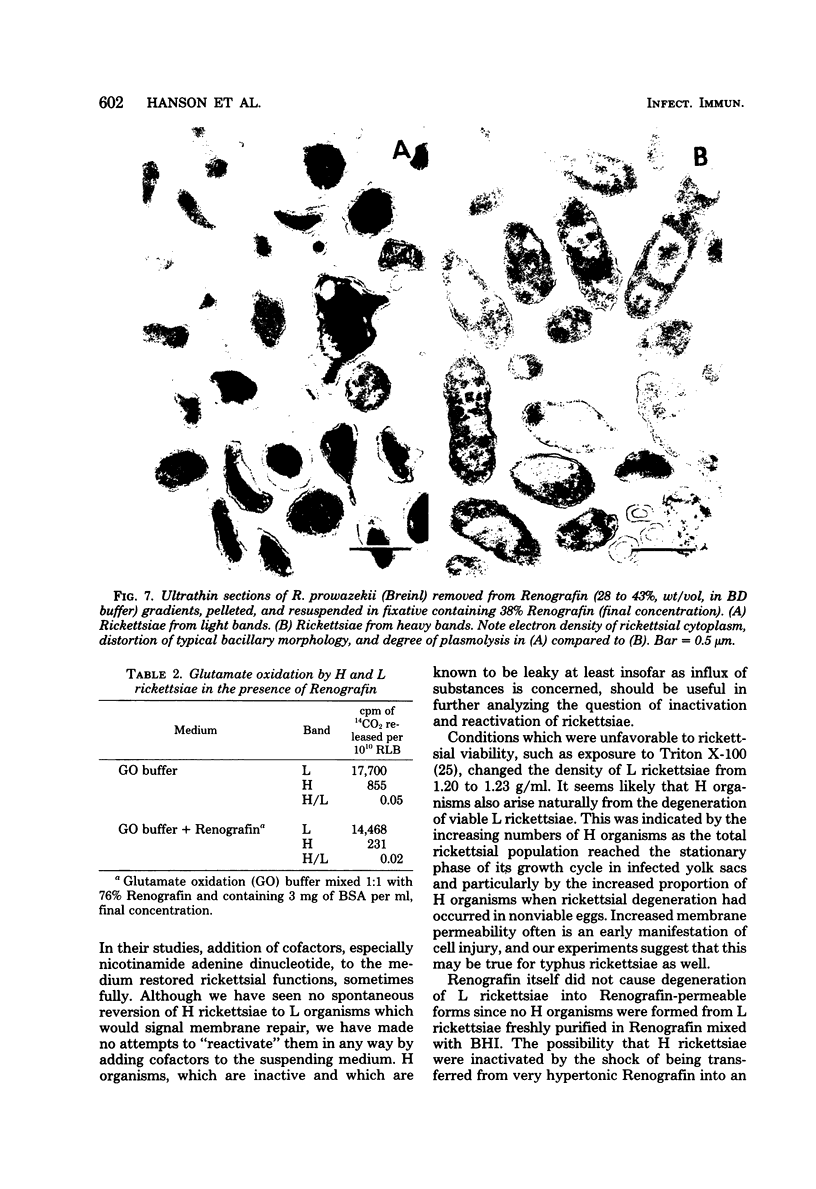
Suspensions of partially purified Rickettsia prowazekii yielded two bands of organisms when centrifuged to equilibrium in Renografin density gradients. Rickettsiae from the lower, heavy band were defective in their infective and metabolic activities, as compared to organisms from the light band. The greater density in Renografin of heavy-banding organisms was due to their lack of permeability barrier to it, as evidenced by the absence of plasmolysis in hypertonic Renografin. In contrast, light-banding rickettsiae were able to exclude Renografin, since they were plasmolyzed in it. The proportion of heavy-banding organisms in a rickettsial suspension was influenced by the growth phase they were in when harvested from infected yolk sacs, as well as by the conditions and media to which they subsequently were exposed. We have concluded that these defective forms arise from the degeneration of light-banding rickettsiae. This separation of two functional classes of rickettsiae in Renografin density gradients has been exploited (i) to increase the uniformity of the suspensions by removing many noninfectious particles and (ii) to determine rapidly the integrity of certain properties of the cytoplasmic membrane of organisms exposed to a variety of conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus Rickettsiae. I. Inactivation by freezing. J Gen Physiol. 1954 Nov 20;38(2):169–179. doi: 10.1085/jgp.38.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C. Oxidation and transamination of glutamate by typhus rickettsiae. J Biol Chem. 1950 Jun;184(2):661–676. [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasch G. A., Weiss E. Characterization of the Madrid E strain of Rickettsia prowazekii purified by renografin density gradient centrifugation. Infect Immun. 1977 Jan;15(1):280–286. doi: 10.1128/iai.15.1.280-286.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S., Tyrrell D. A., Head B., Rees R. J. Inhibition of haemaggregation by lepromin and other mycobacterial substances. Nature. 1967 Dec 9;216(5119):1019–1020. doi: 10.1038/2161019a0. [DOI] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARP A. An immunological purification of typhus Rickettsiae. J Bacteriol. 1954 Apr;67(4):450–455. doi: 10.1128/jb.67.4.450-455.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Myers W. F., Provost P. J., Wisseman C. L., Jr Permeability properties of Rickettsia mooseri. J Bacteriol. 1967 Mar;93(3):950–960. doi: 10.1128/jb.93.3.950-960.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormsbee R., Peacock M., Philip R., Casper E., Plorde J., Gabre-Kidan T., Wright L. Serologic diagnosis of epidemic typhus fever. Am J Epidemiol. 1977 Mar;105(3):261–271. doi: 10.1093/oxfordjournals.aje.a112382. [DOI] [PubMed] [Google Scholar]
- Rees H. B., Jr, Weiss E. Glutamate catabolism of Rickettsia rickettsi and factors affecting retention of metabolic activity. J Bacteriol. 1968 Feb;95(2):389–396. doi: 10.1128/jb.95.2.389-396.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Fiset P., Wisseman C. L., Jr Simple, differential staining technique for enumerating rickettsiae in yolk sac, tissue culture extracts, or purified suspensions. J Clin Microbiol. 1979 Mar;9(3):437–440. doi: 10.1128/jcm.9.3.437-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. D., Jones M. External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun. 1978 Oct;22(1):233–246. doi: 10.1128/iai.22.1.233-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. In vitro studies of Rickettsia-host cell interactions: ultrastructural study of Rickettsia prowazekii-infected chicken embryo fibroblasts. Infect Immun. 1980 Aug;29(2):778–790. doi: 10.1128/iai.29.2.778-790.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter R. F., Briggs G. P., Gangemi J. D., Pedersen C. E., Jr Changes in buoyant density relationships of two cell types of Coxiella burneti phase I. Infect Immun. 1975 Aug;12(2):433–436. doi: 10.1128/iai.12.2.433-436.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter R. F., Briggs G. P., Pedersen C. E., Jr Differences in buoyant-density properties of Coxiella burnetii and Rickettsia rickettsii. Infect Immun. 1977 Feb;15(2):668–669. doi: 10.1128/iai.15.2.668-669.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe M. E., Burton P. R., Shankel D. M. Isolation and characterization of two cell types of Coxiella burneti phase I. J Bacteriol. 1972 Apr;110(1):368–377. doi: 10.1128/jb.110.1.368-377.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Ormsbee R. A., Tallent G., Peacock M. G. Effects of various suspending media on plaque formation by rickettsiae in tissue culture. Infect Immun. 1972 Oct;6(4):550–556. doi: 10.1128/iai.6.4.550-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial cell water and membrane permeability determined by a micro space technique. Appl Environ Microbiol. 1976 Jan;31(1):146–149. doi: 10.1128/aem.31.1.146-149.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Silverman D. J. In vitro studies on Rickettsia-host cell interactions: lag phase in intracellular growth cycle as a function of stage of growth of infecting Rickettsia prowazeki, with preliminary observations on inhibition of rickettsial uptake by host cell fragments. Infect Immun. 1976 Jun;13(6):1749–1760. doi: 10.1128/iai.13.6.1749-1760.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]