Abstract
Hemophilia A is a common X chromosome-linked genetic bleeding disorder caused by abnormalities in the coagulation factor VIII gene (F8). Hemophilia A patients suffer from a bleeding diathesis, such as life-threatening bleeding in the brain and harmful bleeding in joints and muscles. Because it could potentially be cured by gene therapy, subhuman animal models have been sought. Current mouse hemophilia A models generated by gene targeting of the F8 have difficulties to extrapolate human disease due to differences in the coagulation and immune systems between mice and humans. Here, we generated a porcine model of hemophilia A by nuclear transfer cloning from F8-targeted fibroblasts. The hemophilia A pigs showed a severe bleeding tendency upon birth, similar to human severe hemophiliacs, but in contrast to hemophilia A mice which rarely bleed under standard breed conditions. Infusion of human factor VIII was effective in stopping bleeding and reducing the bleeding frequency of a hemophilia A piglet but was blocked by the inhibitor against human factor VIII. These data suggest that the hemophilia A pig is a severe hemophilia A animal model for studying not only hemophilia A gene therapy but also the next generation recombinant coagulation factors, such as recombinant factor VIII variants with a slower clearance rate.
Introduction
Hemophilia A is an inherited X-linked bleeding disorder caused by abnormalities in the coagulation factor VIII (FVIII) gene (F8). The genetic abnormalities result in FVIII deficiency, which in turn creates a bleeding diathesis, such as life-threatening bleeding in the brain and harmful bleeding in joints and muscles. The morbidity of hemophilia A is one in 5,000 male live births [1]. The current standard therapy for hemophilia A is intravenous injection of recombinant FVIII or monoclonal antibody-purified FVIII from plasma. Prophylactic administration of FVIII is effective in preventing harmful bleeding; however, hemophilia A patients are still not free from the risks of life-threatening intracranial and other harmful bleeding [1] [2]. In addition, severe hemophilia A patients develop antibody against FVIII (inhibitor) upon infusion of FVIII frequently [1].
Gene therapy, that enables sustained elevation of coagulation factor levels, will provide the next-generation therapy for hemophilia [1], [3], [4]. In fact, gene and cell therapy for hemophilia clinical trials were conducted. Compared with clinical trials of the gene therapy for hemophilia B [5], [6], gene and cell therapies for hemophilia A have had limited successes [7], [8]. Upcoming therapeutic alternatives for hemophilia A are FVIII variants with a slower clearance rate. Therapeutic factors, such as recombinant activated factor VII and plasma-derived activated prothrombin complex, are used for the treatment of hemophilia A patients with inhibitors, and the second generation therapeutic factors for hemophilia A patients with inhibitors are also currently under development. For studying next-generation therapeutics, good animal models are required. Hemophilia A mice generated by targeted ablation of mouse F8 [9] have been the mainstay for assessment of hemophilia A gene therapy and evaluation of FVIII variants. However, there are significant species differences between mice and humans. For example, the half-life of human FVIII in the mouse circulation is very short, making it difficult to analyze the efficacy of human FVIII-expressing vectors for gene therapy or novel FVIII variants. As alternatives, there are natural hemophilia A dogs and hemophilia A sheep. Hemophilia A dogs have been used for gene therapy studies [10], [11], [12]. Hemophilia A sheep would be an alternative model [13]. There may be interspecies differences, such as body size, physiology, disease progression and chromosome structure homology, between these animal models and humans.
Pigs are excellent biomedical models of human diseases [14], [15]. The porcine blood coagulation system is very similar to that in humans, because of the high homology between the coagulation factor amino acid sequences [16], [17], [18]. In addition, porcine FVIII has been used to treat hemophilia A patients with FVIII inhibitors [19], [20], [21]. Therefore, the hemophilia A pig could be a good animal model to study the next-generation therapeutics for hemophilia A. Moreover, a miniature pig strain exists, and thus, cloned pigs could be downsized to an adequate size, approximately 20–30 kg in weight. For these reasons, we decided to generate hemophilia A pigs by cloning technology.
Results
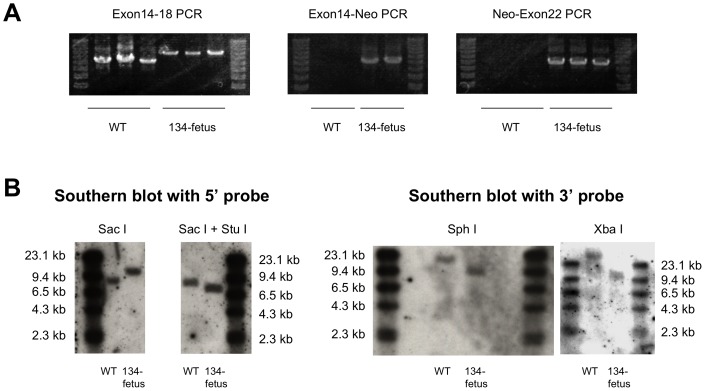
Firstly, we constructed a F8 targeting vector (Figure 1A ) and targeted F8 in male porcine embryonic fibroblasts (PEF) with the F8-targeting vector as shown in Figure 1. The DNA fragment amplified from the non-transfected PEF DNA migrated at 6.5 kb on agarose gel electrophoresis, whereas two DNA fragments, migrating at 6.5 kb and 8.3 kb, were amplified from PEF colony 134. The 8.3 kb DNA was not amplified from genomic DNA of PEF colonies 135–137. The 8.3 kb fragment PCR-amplified from PEF colony 134 was cleaved into a 2.4-kb fragment and a 5.9-kb fragment by Stu I, whereas the PCR-amplified 6.5-kb DNA fragment was not susceptible to Stu I digestion. This supports that the PCR-amplified 8.3-kb fragment is derived from the F8-targeted genome because a Stu I recognition sequence present in the neo-resistant gene but not in the PCR amplified DNA fragment from the wild-type F8. The expected DNA fragments were amplified by PCR with Neo primers from genomic DNA from PEF colony 134, but not from wild-type genomic DNA (WT). PCR analysis of genomic DNA with three primer sets revealed a recombination event in F8 of a colony, 134 (PEF-134). PEF-134 nuclei were then injected into enucleated oocytes. After an electrical pulse, the oocytes were transplanted into the oviduct of a female pig [22], [23]. Transplantation of nucleus-transferred oocytes to the oviducts of female pigs was repeated four times. Three months later, a fetus was obtained by induced abortion. Dermal fibroblasts from this PEF-134-derived fetus (134-fetus) were isolated and cultured, and genomic DNA was isolated for analysis by PCR and by Southern blotting (Figure 2). The PCR amplified wild-type (WT) F8 exon 14–18 fragment migrated at 6.5 kb, whereas the 8.3-kb targeted DNA fragment was amplified solely from 134-fetus fibroblast DNA. PCR-amplified DNA fragments using an F8 exonic primer and a Neo primer were obtained only from 134-fetus DNA. The PCRs demonstrated insertion of the neomycin-resistance gene in F8 (Figure 2A ). Southern blotting showed that the 5′ probe hybridized with the 8.1 kb DNA fragment of Sac I-digested wild-type DNA while the 5′ probe hybridized with 9.9 kb DNA fragment of Sac I-digested 134-fetus DNA. Southern blotting with the 3′ probe confirmed recombination in the F8 gene in the 134-fetus genome because a Sph I recognition sequence and a Xba I recognition sequence located in the 3′ end of the Neo resistant gene of the targeted allele (Figure 2B ). Therefore, five transfers of fetal fibroblast nuclei to oocytes followed by oocyte transplantation were performed. Four females became pregnant and each produced a full-term delivery.
Figure 1. F8 targeting of porcine fetal fibroblasts (PEF).
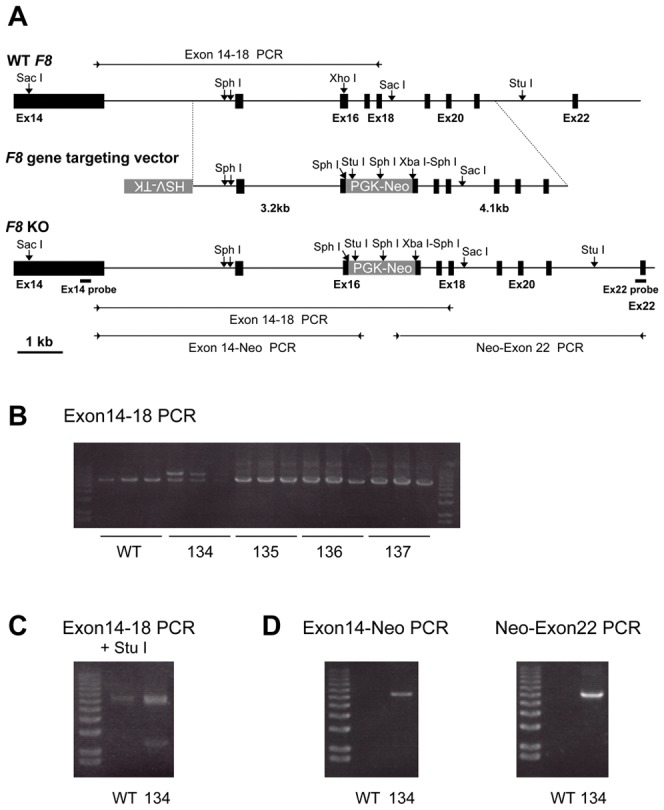
(A) Schematic diagram of part of porcine F8, the positions of the restriction endonuclease sites, the F8 targeting vector structure, and the targeted F8 (F8 KO) allele are shown. The neomycin-resistance gene (PGK-neo) was inserted in the exon 16 DNA fragment with deletion of a part of exon 16 and was flanked by two F8 DNA fragments (5′ arm: 3.2 kb; 3′ arm: 4.1 kb) in F8 targeting vector. The positions of PCR primers (arrowheads), expected amplified DNA fragments (bars), and restriction endonuclease sites used for the Southern blot analysis are indicated in the schema for F8 KO. (B) F8 exon 14–18 PCR on genomic DNA from non-transfected PEF (WT), PEF colony 134 (134), and three other PEF colonies (135–137) was shown. (C) The F8 exon 14–18 PCR products were treated with Stu I and analyzed by agarose gel electrophoresis. (D) PCR analyses with two sets of primer pairs for exon 14 and the neomycin resistance gene and for the neomycin resistance gene and exon 22 were shown.
Figure 2. F8 targeting and genetic analysis of the colony 134-derived fetus.
PCR analysis of genomic DNA of 134-fetus was shown. (A) Two or three independent PCR reactions were carried out for detection of recombination in F8 of 134-fetus. (B) Southern blotting with a 5′ exon 14 probe (on Sac I− or Sac I + Stu I-digested DNA) and with a 3′ exon 22 probe (on Sph I− or Xba I-digested DNA) showed correct targeting of the F8 in 134-fetus.
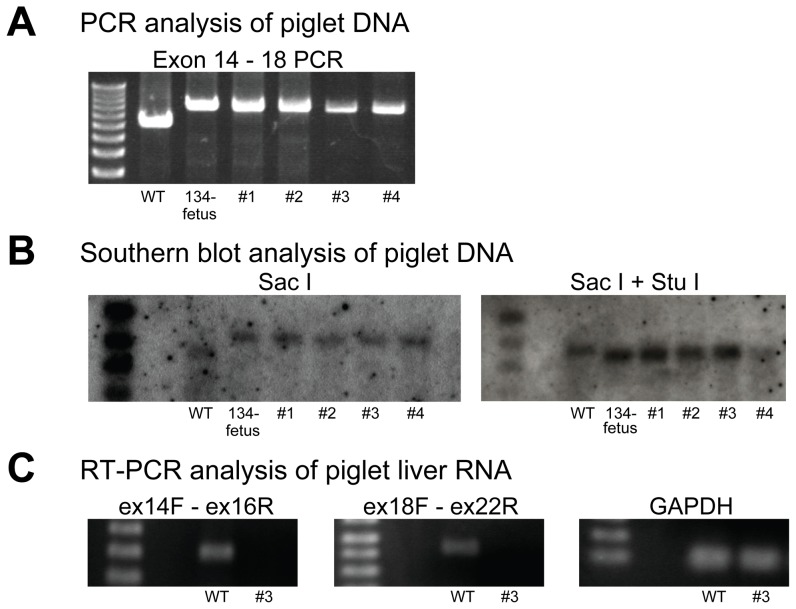
Four live offspring were obtained and PCR analysis and Southern blotting were carried out. As shown in Figure 3A , the 8.3 kb DNA fragments were PCR amplified from piglets DNA as same as that of 134-fetus (Figure 2). Similarly, Southern blotting of Sac I-treated and Sac I and Stu I-treated DNA of the piglets with the 5′ probe confirmed the recombination of F8 of piglets and showed that each piglet had a single copy of the targeted F8 (Figure 3, A & B ). RT-PCR analysis revealed that FVIII mRNA was not detected in the liver of piglet #3 (Figure 3C ). Analysis of the blood of piglets #3 and #4 confirmed that the FVIII level was severely decreased to less than 1%, using an activated partial thromboplastin time (APTT)-based coagulation assay for human FVIII (Table 1). Other coagulation factors were moderately decreased (Table 1). The levels of albumin and cholinesterase of these piglet blood were also measured as the references to study whether the decreased level of coagulation factors II, V, VII, IX, and X were specific or not. The albumin levels of piglet #3 and #4 were decreased significantly compared with the wild type piglets. However, the cholinesterase activities of piglets #3 and #4 were not decreased. The data suggested that synthesis of some proteins in the liver of the cloned piglets was altered. The precise mechanism of the moderately decreased levels of coagulation factors II, V, VII, IX, and X, and albumin was not elucidated in this study.
Figure 3. Analysis of the F8 in cloned piglets.
(A) PCR analysis of genomic DNA of piglet DNA was shown. Genomic DNA of wild-type, 134-fetus, piglet #1, piglet #2, piglet #3, and piglet #4 was subjected to PCR analysis with primers Exon 14 sF and Exon 18 sR as in Figure 1. The 8.3 kb exon 14–18 band was amplified from the 134-fetus DNA and the cloned piglet DNA. (B) Southern blotting with a 5′ exon 14 probe (on Sac I− or Sac I + Stu I-digested DNA) showed the same mobility shifts of the bands as those in Figure 2B and confirmed the insertion of the Neo resistant gene in F8 of the cloned piglets. (C) RT-PCR analysis of piglet liver RNA was shown. Two independent PCRs (exons 14–16 and exons 18–22) revealed the absence of FVIII mRNA from the liver of cloned piglet #3. Control GAPDH mRNA was detected in the liver RNA of piglet #3 as in the wild type (WT).
Table 1. Coagulation factor activity of piglets #3 and #4.
| Coagulation factor | Wild type (n = 4) | Piglet #3 | Piglet #4 |
| Fibrinogen (µmol/L) | 2.67±1.39 | 1.56 | ND |
| Factor II (%) | 75.7±3.9 | 53 | 47 |
| Factor V (%) | >200 | 118 | 168 |
| Factor VII (%) | 68.5±3.4 | 19 | 19 |
| Factor VIII (%) | >200 | 1> | 1> |
| Factor IX (%) | >200 | 96 | 69 |
| Factor X (%) | 134±7.0 | 72 | 64 |
The coagulation factor levels of piglet #3 and #4 are shown with the control coagulation factor levels of wild-type piglets. Each coagulation factor activity was calculated from the standard curve generated with normal human plasma and expressed as the percentage of the respective coagulation factor activity in normal human plasma.
ND: not determined.
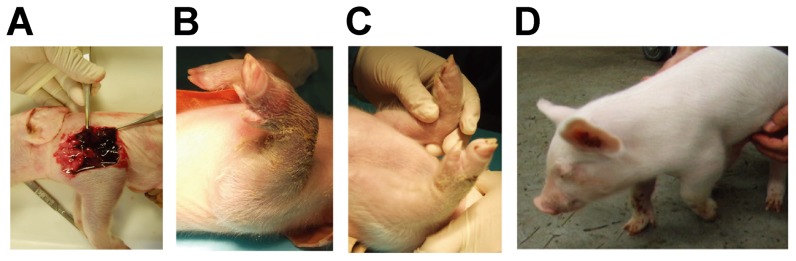
Two of the piglets (#1 and #2) found dead the next day (day 2) after delivery. The cause of death of these two piglets was not certain. Early deaths of cloned piglets after birth are not uncommon as described [24], [25]. Accidental bleeding might affect the condition of piglet #1 since large hematomas were observed in piglet #1 (Figure 4). Massive traumatic intramuscular bleeding was thought to affect the death of piglet #3 on day 3 because the general condition of piglet #3 became severe immediately after the bleeding took place and piglet #3 died. Piglet #4 was born with bleeding in the left forelimb, thus, human FVIII concentrate (150 U/kg) was injected intravenously on day 2 after delivery, which cured the bleeding in the limb (Figure 4). However, because this piglet still showed a bleeding in the limbs and the tongue, which was cured with human FVIII infusion, it was given a prophylactic infusion of human FVIII (150 U/kg) twice a week, which was effective in reducing the bleeding frequency. The human FVIII activity at 12.1% (average of two points; day 10 and day 23 after birth) was detected in the piglet #4 plasma obtained two days after the injection. However, spontaneous bleeding still occurred in piglet #4, in particular repeated bleeding in the left forelimb, causing limping (Figure 4 and video S1). Piglet #4 died due to gastric bleeding from a gastric ulcer on day 38 after birth. Inhibitor (856 BU/mL) against human FVIII was detected in the plasma obtained on the day when piglet #4 died. The development of inhibitor might explain why human FVIII injected two days before was not effective to reduce bleeding from the gastric ulcer.
Figure 4. The bleeding phenotype of cloned F8 KO piglets.
(A) A part of macroscopic picture of cloned piglet #1, which died by day 2 after birth is shown. Ecchymosis was seen in the cheek, the forelimb, and the hind limb (not shown). Pathological examination revealed hematomas in these areas of piglet #1. (B) Forelimb of cloned piglet #4 on day 1 after delivery was shown. Ecchymosis had been seen in the left forelimb of cloned piglet #4 since delivery. (C) On day 5 after administration of human FVIII (150 U/kg), the bleeding in the left forelimb was not observed. Macroscopic picture of cloned piglet #4 on day 28 after birth showed that the left forelimb was swollen because of the repeated bleeding (D), causing the piglet to limp (also see video 1).
Discussion
Advances in cloning technology have allowed us to generate genetically modified animals [22], [26], [27]. Among these, a few gene-targeted pigs have been reported, such as cystic fibrosis pigs [28] and heterozygous fumarylacetoacetate hydrolase deficient pigs [29]as a disease model, and α1, 3-galactosyltransferase gene-knockout (KO) pigs [30] for organ transplantation [30], [31]. Considering the limitations in studying human disease in murine models, gene-targeted pigs are thought to be preferred for studying human diseases and for translational research. We explore the possibility of F8 KO pigs (hemophilia A pigs) for studying the next generation therapy for hemophilia A in the current study. The genotype of cloned pigs showed the proper recombination in the F8 of the pigs and the blood coagulation factor levels of cloned pigs confirmed severe FVIII deficiency. The precise mechanism of moderately decreased other coagulation factor levels in piglets #3 and #4 was not elucidated yet, these changes may not be specific to the coagulation factors since the level of albumin was decreased but the cholinesterase level was not decreased (both albumin and cholinesterase are synthesized in the liver). One possible mechanism of the changes could be the epigenetic effect genome DNA methylation and histone acetylation, which alter gene expression in cloned pigs [24], [32], [33], [34]. Hemophilia A pigs generated by the nuclear transfer technology did show a severe bleeding phenotype that is in contrast to F8 KO mice that rarely exhibit spontaneous bleeding into the muscles and joints under standard breed conditions [9]. Therefore, hemophilia A pig can be used to evaluate an efficacy of novel therapy such as gene therapy for hemophilia A in a standard breed condition. Moreover, prophylactic infusion of human FVIII was effective in reducing bleeding in F8 KO piglet #4 thought its therapeutic effect was not perfect. This suggests that the F8 KO pig is a subhuman animal model of severe hemophilia A for the study of upcoming therapeutic factors, such novel FVIII variants. Piglet #4 died because of bleeding from a gastric ulcer. Since inhibitor against human FVIII was detected in the plasma sample obtained on the day when piglet #4 died, the therapeutic effect of human FVIII no longer existed at the time, resulting in severe bleeding from the gastric ulcer. It is possible that F8 KO pigs might develop antibodies against porcine FVIII as against human FVIII. The possibility of the use of F8 KO pigs as a model for studying immune tolerance induction therapy for FVIII inhibitor remains to be studied.
Methods
Construction of the F8 targeting vector
Porcine genomic DNA was isolated from porcine embryonic fibroblasts (LW; Landrace – Large White, ED65). The F8 targeting vector was constructed by inserting two genomic DNA fragments into the plasmid vector pHSV-TK/PGK-Neo. The F8 targeting vector was designed by referring to the F8 exon 16 gene-targeting vector used to generate hemophilia A mice [9]. F8 DNA fragments from exons 14–22 were isolated by PCR using primers (Table S1) based on the Sus scrofa coagulation factor VIII mRNA sequence (accession number: NM_214167) and sequenced. The two homologous arms of the gene-targeting vector were generated by reference to this sequence. The 5′ DNA fragment spanning intron 15 to exon 21 of F8 was PCR-amplified, digested with Xho I to generate an 11-nucleotide deletion of exon 16, and inserted into pHSV-TK/PGK-Neo. The 3′ DNA fragment was PCR-amplified from exon 16 to intron 21, and cloned into pHSV-TK/PGK-Neo containing the 5′ F8 DNA fragment. The herpes simplex virus thymidine kinase gene was located in the opposite orientation on the 5′ end of the 5′ arm. The targeting vector was linearized with Not I before transfection.
Isolation of porcine embryonic fibroblasts and isolation of F8-targeted cells
Porcine embryonic fibroblasts (PEF) were isolated from a male fetus of the LW strain as described [22]. PEFs (1×107 cells) were transfected with the F8 targeting vector by electroporation (Gene Pulser II; Bio-Rad, Hercules, CA) at 278 V and 950 µF. After transfection, cells were cultured in Dulbecco's modified Eagle's medium with low-glucose (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum. After 48 h incubation, cells were selected with 800 µg/ml G418 (Nacalai Tesque, Inc., Kyoto, Japan) and 2 µM gancyclovir (Tanabe-Mitsubishi Pharma, Tokyo, Japan). On the eighth day following selection, G418-resistant colonies had grown. Cells from these colonies were grown in 24-well plates (Corning) in medium containing 4 ng/ml bFGF, and expanded for genomic DNA extraction and storage. DNA isolated from three wells of each colony was analyzed by three independent PCR reactions for recombination in the porcine F8 (Table S1).
Southern blotting
Southern blotting for the F8 recombination was performed by the standard procedure. Digoxigenin (DIG)-labeled 5′ and 3′ probes were generated by PCR (497 bp from exon 14 and 469 bp from exon 22, respectively) (Table S1). Signals were visualized using a DIG detection module (anti-DIG-alkaline phosphatase and a CSPD) (Roche Diagnostics GmbH., Mannheim, Germany).
RT-PCR of porcine FVIII mRNA
Total RNA was isolated from piglet liver using an RNeasy Mini kit (Invitrogen), converted to cDNA and PCR amplified using the SuperScript One-Step RT-PCR System (Invitrogen) with primer pairs specific for FVIII mRNA (Table S1) [35], [36].
Nuclear transfer and transplantation of manipulated embryos to recipients
Production of clone piglets by nuclear transfer was performed as described previously [22], [23]. In brief, metaphase II oocytes were enucleated by gentle aspiration of the first polar body and adjacent cytoplasm using a beveled pipette (25 to 30 µm) in PZM3 medium containing 5.0 µg/ml cytochalasin B. Enucleated oocytes were washed in PZM3 medium lacking cytochalasin B and nuclei of the F8-targeted cells introduced by direct intracytoplasmic injection using a piezo-actuated micromanipulator (Prime Tech., Tsuchiura, Japan). Oocytes were then stimulated with a direct current pulse of 1.5 kV/cm for 100 µS using a somatic hybridizer (SSH-10, Shimadzu, Kyoto, Japan) and transferred to PZM3 supplemented with cytochalasin B to prevent extrusion of a pseudo-second polar body. The nuclear transferred oocytes were then cultured in PZM3 medium in an atmosphere of 5% CO2, 5% O2 and 90% air at 38.5°C for 2 days until reaching the two-to-eight-cell stage. Cleaved embryos were transferred to the oviducts (200 embryos per recipient) of an anesthetized pseudopregnant surrogate mother (matured LWD; a Landrace×Large White×Duroc triple cross). Following embryo transfer, mother pigs were observed daily to confirm pregnancy by checking estrus. Farrowing was synchronized by injection of the prostaglandin F2α analog, (1)-cloprostenol (Planate, Osaka, Japan) on day 113–116 of gestation.
Coagulation factor activity measurement
Activities of porcine coagulation factors were measured at a clinical laboratory (SRL, Tokyo, Japan) by the standard clotting time method with respective coagulation factor-deficient human plasma. Normal human plasma was used as the standard for each test. The coagulation factor activity in piglet plasma was expressed as the percentage of the coagulation factor activity in normal pooled plasma, except for fibrinogen. The fibrinogen concentration was determined by the thrombin time method. von Willebrand factor levels in pig plasma were measured with an enzyme immunoassay with latex particle conjugated antibody (performed at SRL, Tokyo, Japan) since the von Willebrand factor activity (Ristocetin cofactor activity) in pig plasma was unable to be measured with human platelets. The von Willebrand factor antigen levels in pig plasma were expressed as percentages of the normal human plasma. An inhibitor assay for human FVIII was performed as described [36].
Blood chemistry analysis
The levels of albumin and choline esterase of piglet blood were measured at the Nagahama Life-science Laboratory of Oriental East Co. Ltd (Hagahama, Shiga-ken, Japan). Choline esterase activities of blood samples were measured with p-hydroxy benzoyl choline iodide as the substrate [37].
Animal experiments
All the animal experiments and surgical procedures were carried out in accordance with guidelines approved by the Institutional Animal Care and Concern Committees of Jichi Medical University and the National Institute of Agrobiological Sciences. Protocols for the use of animals in this study were approved by the review boards of Animal Care Committees of Jichi Medical University and the National Institute of Agrobiological Sciences. Wild type pigs used in this study were bred under a standard condition according to the institutional guideline of Animal Care Committee of National Institute of Agrobiological Sciences. After delivery, cloned F8KO pigs were separated from mother pigs and each cloned F8KO pig was bred by artificial suckling in a cage with protection of soft buffers to avoid traumas. All the experimental procedures including injection of FVIII were carried out under inhalation anesthesia with isoflurane and monitoring of body temperature. The endpoint of this study was to generate F8KO pigs and analyze the genotype and the phenotype of the F8KO pig precisely to investigate whether the F8KO pig can be a subhuman model of severe hemophilia A.
Supporting Information
Sequences of primers used in this study.
(DOC)
Piglet #4 (day 28 after birth) to limp in the left forelimb.
(MOV)
Funding Statement
This study was supported by Grants-in-Aid for Scientific Research (20591155, 21591249 and 21790920) and the Support Program for Strategic Research Infrastructure from the Japanese Ministry of Education, Culture, Sports, Science and Technology; and Health, Labour and Science Research Grants for Research on HIV/AIDS and Research on Intractable Diseases from the Japanese Ministry of Health, Labour and Welfare. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mannucci PM, Tuddenham EG (2001) The hemophilias–from royal genes to gene therapy. N Engl J Med 344: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 2. Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, et al. (2007) Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 357: 535–544. [DOI] [PubMed] [Google Scholar]
- 3. Hasbrouck NC, High KA (2008) AAV-mediated gene transfer for the treatment of hemophilia B: problems and prospects. Gene Ther 15: 870–875. [DOI] [PubMed] [Google Scholar]
- 4. VandenDriessche T, Collen D, Chuah MK (2003) Gene therapy for the hemophilias. J Thromb Haemost 1: 1550–1558. [DOI] [PubMed] [Google Scholar]
- 5. Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, et al. (2006) Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 12: 342–347. [DOI] [PubMed] [Google Scholar]
- 6. Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, et al. (2011) Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365: 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roth DA, Tawa NE Jr, O'Brien JM, Treco DA, Selden RF (2001) Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med 344: 1735–1742. [DOI] [PubMed] [Google Scholar]
- 8. Powell JS, Ragni MV, White GC 2nd, Lusher JM, Hillman-Wiseman C, et al. (2003) Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood 102: 2038–2045. [DOI] [PubMed] [Google Scholar]
- 9. Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, et al. (1995) Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet 10: 119–121. [DOI] [PubMed] [Google Scholar]
- 10. Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL, et al. (2004) Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood 103: 804–810. [DOI] [PubMed] [Google Scholar]
- 11. Finn JD, Ozelo MC, Sabatino DE, Franck HW, Merricks EP, et al. (2010) Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood 116: 5842–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabatino DE, Freguia CF, Toso R, Santos A, Merricks EP, et al. (2009) Recombinant canine B-domain-deleted FVIII exhibits high specific activity and is safe in the canine hemophilia A model. Blood 114: 4562–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Porada CD, Sanada C, Long CR, Wood JA, Desai J, et al. (2010) Clinical and molecular characterization of a re-established line of sheep exhibiting hemophilia A. J Thromb Haemost 8: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lunney JK (2007) Advances in swine biomedical model genomics. Int J Biol Sci 3: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bendixen E, Danielsen M, Larsen K, Bendixen C (2010) Advances in porcine genomics and proteomics–a toolbox for developing the pig as a model organism for molecular biomedical research. Brief Funct Genomics 9: 208–219. [DOI] [PubMed] [Google Scholar]
- 16. Massicotte P, Mitchell L, Andrew M (1986) A comparative study of coagulation systems in newborn animals. Pediatr Res 20: 961–965. [DOI] [PubMed] [Google Scholar]
- 17. Reverdiau-Moalic P, Watier H, Vallee I, Lebranchu Y, Bardos P, et al. (1996) Comparative study of porcine and human blood coagulation systems: possible relevance in xenotransplantation. Transplant Proc 28: 643–644. [PubMed] [Google Scholar]
- 18. Chen Y, Qiao J, Tan W, Lu Y, Qin S, et al. (2009) Characterization of porcine factor VII, X and comparison with human factor VII, X. Blood Cells Mol Dis 43: 111–118. [DOI] [PubMed] [Google Scholar]
- 19. Morrison AE, Ludlam CA (1991) The use of porcine factor VIII in the treatment of patients with acquired hemophilia: the United Kingdom experience. Am J Med 91: 23S–26S. [DOI] [PubMed] [Google Scholar]
- 20. Toschi V (2010) OBI-1, porcine recombinant Factor VIII for the potential treatment of patients with congenital hemophilia A and alloantibodies against human Factor VIII. Curr Opin Mol Ther 12: 617–625. [PubMed] [Google Scholar]
- 21. Barrow RT, Lollar P (2006) Neutralization of antifactor VIII inhibitors by recombinant porcine factor VIII. J Thromb Haemost 4: 2223–2229. [DOI] [PubMed] [Google Scholar]
- 22. Onishi A, Iwamoto M, Akita T, Mikawa S, Takeda K, et al. (2000) Pig cloning by microinjection of fetal fibroblast nuclei. Science 289: 1188–1190. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki S, Iwamoto M, Saito Y, Fuchimoto D, Sembon S, et al. (2012) Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell 10: 753–758. [DOI] [PubMed] [Google Scholar]
- 24. Cho SK, Kim JH, Park JY, Choi YJ, Bang JI, et al. (2007) Serial cloning of pigs by somatic cell nuclear transfer: restoration of phenotypic normality during serial cloning. Dev Dyn 236: 3369–3382. [DOI] [PubMed] [Google Scholar]
- 25. Umeyama K, Watanabe M, Saito H, Kurome M, Tohi S, et al. (2009) Dominant-negative mutant hepatocyte nuclear factor 1alpha induces diabetes in transgenic-cloned pigs. Transgenic Res 18: 697–706. [DOI] [PubMed] [Google Scholar]
- 26. Chesne P, Adenot PG, Viglietta C, Baratte M, Boulanger L, et al. (2002) Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat Biotechnol 20: 366–369. [DOI] [PubMed] [Google Scholar]
- 27. Shin T, Kraemer D, Pryor J, Liu L, Rugila J, et al. (2002) A cat cloned by nuclear transplantation. Nature 415: 859. [DOI] [PubMed] [Google Scholar]
- 28. Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, et al. (2008) Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321: 1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hickey RD, Lillegard JB, Fisher JE, McKenzie TJ, Hofherr SE, et al. (2011) Efficient production of Fah-null heterozygote pigs by chimeric adeno-associated virus-mediated gene knockout and somatic cell nuclear transfer. Hepatology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, et al. (2002) Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295: 1089–1092. [DOI] [PubMed] [Google Scholar]
- 31. Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, et al. (2005) Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 11: 32–34. [DOI] [PubMed] [Google Scholar]
- 32. Tian XC, Park J, Bruno R, French R, Jiang L, et al. (2009) Altered gene expression in cloned piglets. Reprod Fertil Dev 21: 60–66. [DOI] [PubMed] [Google Scholar]
- 33. Shen CJ, Cheng WT, Wu SC, Chen HL, Tsai TC, et al. (2012) Differential differences in methylation status of putative imprinted genes among cloned swine genomes. PLoS One 7: e32812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim YJ, Ahn KS, Kim M, Shim H (2011) Comparison of potency between histone deacetylase inhibitors trichostatin A and valproic acid on enhancing in vitro development of porcine somatic cell nuclear transfer embryos. In Vitro Cell Dev Biol Anim 47: 283–289. [DOI] [PubMed] [Google Scholar]
- 35. Ishiwata A, Mimuro J, Kashiwakura Y, Niimura M, Takano K, et al. (2006) Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene. Thromb Res 118: 627–635. [DOI] [PubMed] [Google Scholar]
- 36. Ishiwata A, Mimuro J, Mizukami H, Kashiwakura Y, Takano K, et al. (2009) Liver-restricted expression of the canine factor VIII gene facilitates prevention of inhibitor formation in factor VIII-deficient mice. J Gene Med 11: 1020–1029. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka H, Igarashi T, Lefor AT, Kobayashi E (2009) The effects of fasting and general anesthesia on serum chemistries in KCG miniature pigs. J Am Assoc Lab Anim Sci 48: 33–38. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences of primers used in this study.
(DOC)
Piglet #4 (day 28 after birth) to limp in the left forelimb.
(MOV)