Abstract
The NCI-60 panel of 60 human cancer cell-lines of nine different tissues of origin has been extensively characterized in biological, molecular and pharmacological studies. Analyses of data from such studies have provided valuable information for understanding cellular processes and developing strategies for the diagnosis and treatment of cancer. Here, Affymetrix® GeneChip™ miRNA version 1 oligonucleotide microarrays were used to quantify 847 microRNAs to generate an expression dataset of 495 (58.4%) microRNAs that were identified as expressed in at least one cell-line of the NCI-60 panel. Accuracy of the microRNA measurements was partly confirmed by reverse transcription and polymerase chain reaction assays. Similar to that seen among the four existing NCI-60 microRNA datasets, the concordance of the new expression dataset with the other four was modest, with mean Pearson correlation coefficients of 0.37–0.54. In spite of this, comparable results with different datasets were noted in clustering of the cell-lines by their microRNA expression, differential expression of microRNAs by the lines’ tissue of origin, and correlation of specific microRNAs with the doubling-time of cells or their radiation sensitivity. Mutation status of the cell-lines for the TP53, PTEN and BRAF but not CDKN2A or KRAS cancer-related genes was found to be associated with changes in expression of specific microRNAs. The microRNA dataset generated here should be valuable to those working in the field of microRNAs as well as in integromic studies of the NCI-60 panel.
Introduction
Systematic studies of sets of cell-lines have provided insights on biological processes and their mechanisms, and have proved useful for devising diagnostic and therapeutic approaches. For instance, phosphatase and tensin homologue (PTEN)-dependent and -independent tumor suppressor mechanisms have been identified using a panel of melanoma cell-lines [1], and genetic markers of sensitivity to the anti-cancer drug, trastuzumab (Herceptin™) have been delineated using a panel of breast cancer cell-lines [2]. The NCI-60 panel of 60 human tumor cell-lines of diverse histologies and nine different tissues of origin was developed by the National Cancer Institute (NCI) of USA in the late 1980s for the purpose of screening anti-cancer drugs [3]. Over the last two decades, the NCI-60 cells have been used to test more than 150,000 chemical compounds and natural product extracts for drug discovery [4]. Numerous studies have profiled the cells for phenotypic features, such as doubling time, drug efflux, and radiation sensitivity, and for characterization of their genomes and levels of RNA, proteins and metabolites [5], [6], [7]. Analyses of data integrated from multiple studies have also yielded significant insights of biological and clinical importance [8].
MicroRNAs, short non-coding RNAs, play significant roles in cellular processes by targeting protein-encoding mRNA transcripts to inhibit translation or cause their degradation. In humans, 2,042 species of mature microRNAs have been identified as per the latest version (19; August 2012) of the miRBase microRNA sequence repository [9]. Dysregulated expression of microRNAs occurs in many diseases, including cancer [10], and this observation has led to multiple studies demonstrating therapeutic and biomarker utility of these molecules [11], [12]. Evaluation of the microRNAs expressed in the NCI-60 panel of cell-lines has proved useful in identifying, among other things, biological roles of specific microRNAs [13], [14], the mechanistic bases and biomarker potential of microRNAs in drug sensitivity [15], [16], genetic signatures for diseases [17], [18], and the structural underpinnings of microRNA regulatory networks [19], [20].
The expression of microRNAs in the NCI-60 cells were first quantified in 2007 by Gaur, et al. [21] and Blower, et al. [22] in studies that respectively examined 241 and 321 species of the molecules. The current study was designed to expand the NCI-60 microRNA expression profiles to cover the many hundreds of microRNAs that have since been discovered. While this study has been in progress, two other groups have published data quantifying >700 microRNAs in the NCI-60 panel [23], [24]. Here, besides the generation and characterization of a new microRNA expression dataset, such multiple datasets are compared, and analyses that integrate information on cell proliferation, mRNA expression, oncogene mutations, or radiation sensitivity from studies of the cell-line panel are reported.
Materials and Methods
Isolation of RNA from Cells of the NCI-60 Panel
For each of the 60 cell-lines of the NCI-60 panel (table S1), one ml of frozen cells (1–2×106) in cell-line-specific culture medium containing 10% dimethylsulfoxide was obtained from the Developmental Therapeutics Program (DTP) of the Division of Cancer Treatment and Diagnosis of the NCI. Immediately prior to RNA isolation, cells were thawed in a water-bath at 37 °C for five minutes and collected by centrifugation at 200 g for five minutes at room temperature. Isolation of total RNA from cells using the miRVana™ kit (Ambion®, Austin, TX) was done in randomized batches of 12–24 cell-lines over four days within 10 days of obtaining the cells. RNA concentration was measured by absorbance spectrophotometry on a NanoDrop™ 2000 instrument (Thermo Scientific®, Waltham, MA).
Quantification of microRNAs by Reverse Transcription-PCR (RT-PCR)
Levels of mature microRNAs miR-16, −21, −30a-5p, −106a and −200b in 50 ng total RNA were measured using TaqMan™ microRNA reverse transcription kit and RT-PCR assays (Applied Biosystems®, Foster City, CA) as per protocols suggested by the manufacturer. Identification numbers of the microRNA assays were 391, 397, 417, 578, and 1800, respectively. RT and PCR reactions for all RNA samples were performed together for each microRNA assay. Cycle quantification (Cq) values were calculated as the average of Cq values identified by SDS software (version 2.3; Applied Biosystems®) for triplicate 40-cycle PCR reactions that were performed in a 7900HT thermocycler (Applied Biosystems®).
Microarray Hybridization
The GeneChip™ miRNA array (version 1; Affymetrix®, Santa Clara, CA) platform was used to quantify microRNAs in total RNA isolated from the NCI-60 cells. One µg RNA was polyadenylated and ligated with biotinylated 3DNA™ dendrimers using the FlashTag™ Biotin HSR RNA Labeling Kit (Genisphere®, Hatfield, PA). The labeled RNA, in a volume of 80 µl, was heated to 99°C for 5 minutes and then to 45°C for 5 minutes for loading on an array using material and methods provided with the Affymetrix® GeneChip™ Hybridization, Wash and Stain kit. RNA-array hybridization was performed with agitation at 60 rotations per minute for 16–18 hours at 48°C on an Affymetrix® 450 Fluidics Station, after which arrays were washed and a streptavidin-phycoerythrin conjugate was used for the detection of bound RNAs on the arrays with a GeneChip™ Systems 3000Dx2 (Affymetrix®) confocal laser-scanning microscope. Signal values were computed using the Affymetrix® GeneChip™ Command Console software. Data from all the arrays had low background noise, a similar distribution of signals for endogenous RNAs, and a similar and concentration-dependent signal distribution for specific RNAs that were spiked in the RNA samples before they were labeled. The raw signal data has been deposited with accession number E-MTAB-327 in the ArrayExpress database of the European Bioinformatics Institute [25].
The GeneChip™ miRNA version 1.0 array has 11 µm-sized 46,228 probe-spots for 7,815 DNA oligonucleotide probes including those against 922 non-microRNA human RNAs, 847 human microRNAs, and 5,856 microRNAs of 70 other species. Probes to detect microRNAs are spotted on the array in quadruplicate. The array also has 95 non-specific probes, each spotted up to 94 times, that are binned by GC content. Signals from these background probes are used to make ‘absent’ or ‘present’ detection calls. In total, 72 array hybridizations were performed in nine batches, each with eight arrays, over three days, with two, three and four batches used on the first, second and third day, respectively (table S1). Quadruplicate hybridizations were performed for four arbitrarily chosen cell-lines to assess technical replicability (table S1).
Microarray Data Processing
Raw data files from the 72 array hybridizations were processed together using Affymetrix® miRNA QC Tool software (version 1.0.33) with the following steps in order as suggested by Genisphere®: background detection, background correction with the robust multichip average (RMA) method [26], quantile normalization, and median polish summarization [27]. Quality control and inter-replicate correlation analyses were used to identify the best of the four replicates for four of the RNA preparations (four cell-lines) that were assayed in quadruplicate. Data from the remaining three replicates were excluded from the final expression dataset. At this stage, the expression data matrix had signal values for 7,635 human as well as non-human RNAs. Values for 324 non-microRNA RNAs and 2,282 microRNAs with ‘absent’ detection calls for all 60 cell-lines were then removed from the data matrix. Expression of RNAs with signal values <4x the average inter-quartile range of signal values for all RNAs was considered as absent, and RNAs whose expression was absent in all cell-lines were then excluded from further analyses.
Comparison with Existing NCI-60 MicroRNA Expression Datasets
Pre-processed microRNA expression datasets generated by Blower, et al. [22], Gaur, et al. [21], Liu, et al. [24], and Sokilde, et al. [23] were respectively obtained using NCI’s CellMiner™ web application [28], the DTP website, the authors, and Gene Expression Omnibus of the National Center for Biotechnology Information, USA, with accession number GSE26375. For the dataset of Gaur, et al., the row-names for microRNAs were standardized to the names used in version 15 of the miRBase database [9] as has been described previously [23]. Pearson correlation coefficients between untransformed microRNA measurement values in these datasets and in the one generated in this study were calculated using R.
Correlation Analyses of Levels of microRNAs and their Target mRNAs
Raw gene expression data acquired using Affymetrix® GeneChip™ HG-U133A DNA oligonucleotide microarrays for the expression of mRNAs in 57 of the 60 NCI-60 cell-lines of this study could be obtained using the CellMiner™ web application. A list of 10,392 conserved mRNAs (unique gene symbols) predicted by the TargetScan 5.1 algorithm [29] to be targets of 153 conserved microRNA families was obtained online at http://www.targetscan.org. The mRNA expression data was pre-processed using the RMA method with the Affy Bioconductor package [30] in R, to obtain normalized and log2-transformed microarray signal values. A filter was then applied to retain values for only those probes for which the sample range of signal values was ≥1. Of the 495 microRNAs considered expressed in this study, 192, belonging to a total of 121 conserved microRNA families, were identified as targeting 6,465 of the 10,392 mRNAs. Gene symbols of the 6,465 mRNAs were converted to 10,612 Affymetrix® probe identifiers using the biomaRt Bioconductor package [31] in R. A total of 117,004 Pearson correlation coefficients between expression levels of the 192 microRNAs and their target mRNAs as detected by the 10,612 probes were then computed in R. Two-tailed t tests with Benjamini-Hochberg correction for a false discovery rate (FDR) of <5% were used to assess significance of the correlations. Correlations were also computed for 10 random permutations of class labels (identifying the cell-lines) of the mRNA expression dataset.
Other
The limma (version 3.10.0) Bioconductor package [32] was used in R for analysis of differential expression using log2-transformed expression values. Statistical analyses and graphical plotting were done using Excel™ (version 12; Microsoft®, Redmond, WA), Prism™ (version 5.0c; GraphPad®, La Jolla, CA), and R (version 2.12.1). TM4 [33] MultiExperiment Viewer (version 4.6) was used for hierarchical clustering analysis with optimization for leaf-ordering. Unless stated otherwise, a P value below 0.05 in statistical tests was used to deem statistical significance. Details on correction for multiple testing are provided with the results of the analyses where such a correction was applied.
Results
MicroRNA Expression Profiling of NCI-60 Cells Using Affymetrix® Microarrays
GeneChip® miRNA 1.0 microarrays from Affymetrix® were used to examine the expression of 847 microRNAs in total RNA of 60 cell-lines of the NCI-60 cancer cell-line panel (table S1). This work was performed over three days in a total of nine batches of eight RNA samples each (table S1). RNA used for the expression profiling was obtained from frozen cells, similar to a previous study of microRNA expression in the NCI-60 panel [21]. The yield of RNA from 1–2×106 cells of the cell-lines ranged from 11 to 60 ug (mean = 37; standard deviation [SD] = 11).
Technical replicability of the expression profiling method was confirmed by examination of hybridization data for four RNA preparations, one each from the KM12, PC-3, RPMI-8226 and OVCAR-8 cell-lines that were assayed in quadruplicate. The mean (and SD) of the 75th percentiles of log2-transformed microarray signal values for the quadruplicate sets for the 1,779 probes (847 for microRNAs) annotated as human-specific were 3.58 (0.07), 3.61 (0.12), 3.62 (0.04) and 3.50 (0.10), respectively. For the 95th percentiles, the values were 9.60 (0.10), 9.43 (0.09), 9.66 (0.11) and 9.60 (0.21), respectively. Mean (and SD) of the 12 self-self Pearson correlation coefficients for the four cell-lines were 0.98 (<0.01), 0.97 (0.01), 0.98 (<0.01) and 0.98 (0.01), respectively. For each cell-line, the quadruplicate hybridizations were performed on different days except that for OVCAR-8, one pair of the quadruplicates was assayed in the same batch (table S1). The Pearson correlation coefficient was 0.980 for this pair of intra-batch replicates.
At least one of the 60 cell-lines expressed 523 (63.4%) of the 847 microRNAs detectable by the microarray platform as indicated by the significantly higher signal values from the microRNA-specific probes compared to non-specific mismatch probes of similar GC content. Although the inter-replicate correlations described above appear excellent, a closer examination revealed that correlations were not good at low microarray signal values. This is shown in figure S1 for four inter-replicate comparisons. Since this indicated high noise levels at low signal values, a signal value-based filtering was done to identify RNAs that could be considered as being more precisely quantified. Accordingly, the expression of RNAs with signal values <4x the mean (18.3) of the inter-quartile ranges of signal values for all hybridizations (range = 13.3–26.3; SD = 2.7) was considered as absent. Twenty-eight microRNAs with expression absent in all 60 cell-lines as per this criteria were excluded from further analyses. Thus, 495 (58.4%) of the 847 microRNAs detectable with the microarray platform were considered expressed and the data for them was used for subsequent analyses.
Correlation between Array- and RT-PCR-based microRNA Quantifications
To at least partially confirm the accuracy of the array-based microRNA expression measurements, RT-PCR was used to quantify levels of microRNAs miR-16, −21, −30a-5p, −106a, and −200b in 50 ng each of the RNA preparations for 12 cell-lines. The cell-lines were randomly selected and the microRNAs were arbitrarily picked from those for which at least half of the cell-lines had log2-transformed microarray signal value >5. As shown in figure 1, the two sets of measurements for all five microRNAs had significant correlations (P<0.01) in Pearson tests, with absolute correlation coefficient ≥0.70. The values of the slope of linear regression lines (least squares method) varied from −0.40 (miR-16) to −0.93 (miR-21) for four microRNAs, indicating better detectability of the microRNAs with the RT-PCR assay. For miR-200b, this is also notable with the five RNA samples with poor microarray signal values (21.2–21.7) but detectable RT-PCR Cq values in a good range (27.8–34.3). For miR-30a-5p, the slope of the regression line was −2.2, suggesting better detectability with the microarray platform.
Figure 1. Confirmation of microarray-based microRNA quantifications by reverse transcription-PCR assays.
Pearson correlation coefficients (r), P values, and best-fitting (least squares) lines are shown for RT-PCR Cq and log2-transformed microarray signal values for microRNAs miR-16, −21, −30a-5p, −106a, and −200b (n = 12).
Comparison with Existing NCI-60 microRNA Expression Datasets
Of the 495 microRNAs considered expressed in the set of 60 cell-lines in this study, 135 (27%), 144 (29%), 299 (60%) and 359 (73%) were also quantified in 59 of the 60 cell-lines in the studies of Blower, et al. [22], Gaur, et al. [21], Liu, et al. [24], and Sokilde, et al. [23], respectively. These four studies respectively used custom 40-mer DNA microarrays, TaqMan™ RT-PCR assays, Agilent® 60-mer DNA microarrays (version 2), and Exiqon® locked nucleic acid miRCURY™ microarrays (Dx, version 9.2) to assess the expression of 321, 241, 723 and 955 microRNAs, respectively. In Pearson correlation analyses of untransformed expression measurements of microRNAs common to this and the Blower, Gaur, Liu or Sokilde studies, the mean (and SD) of the Pearson coefficients were 0.44 (0.30), 0.47 (0.28), 0.54 (0.29) and 0.45 (0.34), respectively. The fractions of microRNAs with Pearson coefficient >0.75 were 0.18, 0.16, 0.29 and 0.24, respectively (figure 2). In all four comparisons, correlation coefficients were higher for microRNAs with high signal values than for those with low values (data not shown). Similar analyses were also performed to compare the datasets of Sokilde, et al. and Liu, et al. against others (figure S2). When correlating the Sokilde dataset with the Blower, Gaur and Liu datasets respectively, the mean (and SD) of the Pearson coefficients were 0.37 (0.32), 0.39 (0.33) and 0.44 (0.30), respectively. Values were 0.47 (0.31) and 0.42 (0.22) in comparisons of the Liu dataset with the Blower and Gaur datasets, respectively. Thus, judging by the correlations seen with existing datasets, the ‘correctness’ of the microRNA expression dataset generated in the current study is similar to the ones generated in previous studies. Because of variable sensitivity and specificity of the five quantification platforms of these studies for different microRNAs, a cross-platform comparison for cell-line correlations was not performed.
Figure 2. Correlations between different NCI-60 cell-line microRNA expression datasets.
Cumulative frequency distributions are shown for Pearson correlation coefficients (r) with a bin-size of 0.025 for microRNAs quantified in this study and in the studies of Blower, et al. [22], Gaur, et al. [21], Liu, et al. [24], and Sokilde, et al [23]. The distribution of the coefficients with the expression measurements of Liu, et al. resampled is also shown.
MicroRNA Expression and Tissues of Origin
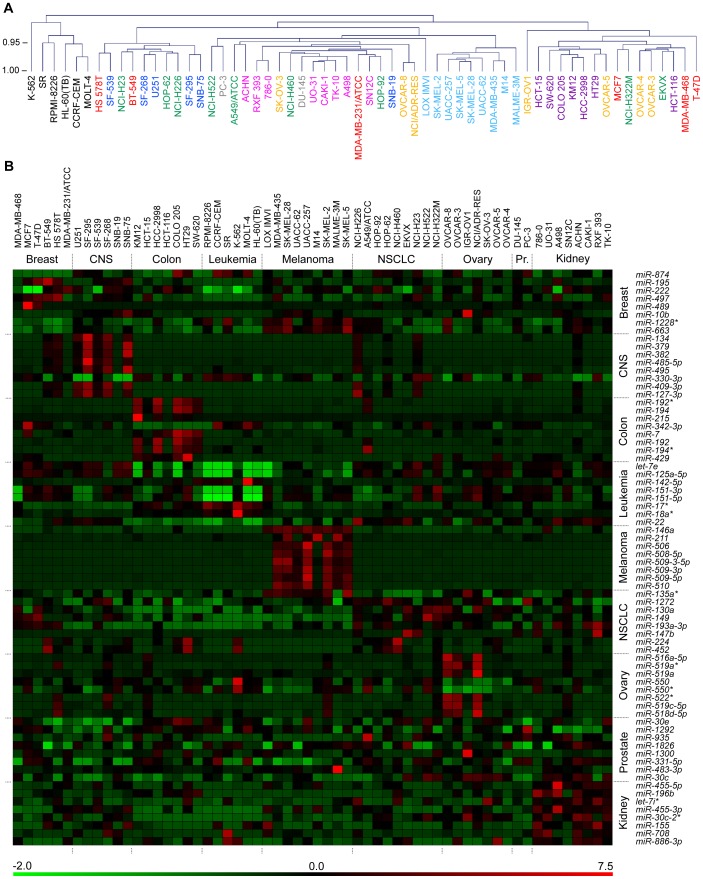
To assess if microRNA expression among the cell-lines was associated with the tissue of origin of the cell-lines, unsupervised hierarchical clustering based on average uncentered Pearson correlation was performed using log2-transformed microarray signal values for the 495 expressed microRNAs (figure 3A). The groups of leukemia and melanoma cell-lines completely segregated into their own independent clusters. All the seven but one (HCT-116) colon cancer cell-lines too segregated into one independent cluster. A similarly robust clustering of cell-lines for the remaining six tissues of origin was not seen. The OVCAR-8 ovarian cancer cell-line and the NCI/ADR-RES cell-line derived from it [34], as well as the M14 melanoma cell-line and the MDA-MB-435 cell-line derived from it [35], clustered as neighbors as expected. However, this was not seen for the third pair of homologous cell-lines in the NCI-60 panel, the U251 cell-line and its derivative, SNB-19 [36]. DNA fingerprinting suggests that, compared to the 94%–97% genetic similarity of the other two pairs, this pair of lines is only 81% similar.
Figure 3. MicroRNA expression in the NCI-60 cell-lines by tissue of origin.
A. Unsupervised clustering of 60 NCI-60 cell-lines by log2-transformed microarray signal values of the 495 expressed microRNAs is shown as a dendrogram. The different types of tissues of origin of the cell-lines are indicated by their color (Pr., prostate). The tree is drawn from uncentered Pearson correlations, with average linkages used for joining clusters. The scale on the left represents node-heights. B. Heat-map, with its pseudo-color scale underneath, of Z scaled microarray signal values of the 60 cell-lines for the sets of eight microRNAs each with lowest P values in tests of differential expression in cell-lines of a specific tissue of origin compared to all the other cell-lines. Both cell-lines and microRNAs are grouped by tissue of origin. Z scaling was done along rows (by microRNA).
To identify microRNAs differentially expressed in the cell-lines of a specific tissue-origin compared to all the other cell-lines, empirical Bayes moderated t-statistics and Benjamini-Hochberg correction for FDR <5% were used. The groups of cell-lines from CNS cancer (n = 6), colon cancer (n = 7), leukemia (n = 6), melanoma (n = 9), ovarian cancer (n = 7) and renal cancer (n = 8), respectively, showed differential expression of 83 (17%), 37 (7%), 137 (28%), 74 (15%), 14 (3%) and 3 (0.6%) of the 495 microRNAs present in the full dataset. No differentially expressed microRNAs could be identified in the groups of cell-lines from breast (n = 6), NSCLC (n = 9) or prostate (n = 2) cancer though the groups had 10 (2%), 24 (5%) and 4 (0.8%) microRNAs for which the P values were <0.05 without adjustment for false discovery. The heat-maps in figures 3B and S6 shows the expression of eight microRNAs for each of the nine groups of cell-lines with the lowest P values. Detailed statistics for the 72 total microRNAs are provided in table S2.
MicroRNA Expression and Radiation Sensitivity
Amundson, et al. have examined the gamma radiation sensitivity of 59 of the 60 cell-lines of this study to quantify seven different radiation survival parameters [37]. The parameters include SF2, SF5 and SF8, the fraction of cell population that survives after exposure to 2, 5 and 8 Gy of radiation, respectively. To check if the NCI-60 cell-lines can be assorted into two radiation-sensitive and -resistant groups of similar size, as has been done for drug sensitivity (e.g., [6], [38], [39]), a non-parametric method was used to estimate Gaussian kernel densities of the radiation response data after log2 transformation and z-score normalization. A bimodal distribution with similarly-sized sensitive and resistant groups was not observed for any of the seven radiation survival parameters (figure S3). The radiation parameters were therefore considered as continuous variables and Pearson correlation analyses of log2-transformed parameter values with log2-transformed microRNA expression values were performed. Though significant correlations with absolute Pearson coefficient >0.5 were not seen for four of the radiation sensitivity parameters, they were present for 30 (6%), 27 (5%) and 33 (7%) of the 495 expressed microRNAs of this study for SF2, SF5 and SF8, respectively (figure S4). However, the correlations for all three parameters were largely driven by the six highly radiation-sensitive leukemia cell-lines of the NCI-60 panel. When the leukemia lines were excluded from the correlation analyses, expression of none of the 495 microRNAs correlated with absolute Pearson coefficient >0.5 for any of the three radiation sensitivity parameters. Similar results were seen when the microRNA expression datasets of Liu, et al. and Sokilde, et al. were analyzed (figure S4). As listed in table S3, 10, four and 14 microRNAs in the datasets of the current study, Liu, et al., and Sokilde, et al., respectively, had absolute Pearson coefficient >0.4 for at least one of SF2, SF5 and SF8. MicroRNAs let-7i, miR-142–5p and miR-193b were common to the results with the three datasets.
Association of microRNAs with Oncogene Mutations
The mutation status of 24 oncogenes is known for 59 of the 60 cell-lines of this study [40]. For some of the genes – BRAF, CDKN2A (p16), KRAS, PTEN, and TP53– oncogenic mutations exist in >10 of the cell-lines, which allows for a comparison of microRNA expression levels between the groups of mutant and wild-type cell-lines. Using empirical Bayes moderated t-statistics and Benjamini-Hochberg correction for FDR <5%, microRNAs miR-29b and miR-769-3p were found to be the only two microRNAs that were differentially expressed between the 47 mutant PTEN and 12 wild-type PTEN lines (P<0.01 for both microRNAs). In a similar analysis, microRNAs miR-34a and miR-34a* (miR-34a-3p) were found to be the only two microRNAs that were differentially expressed between the 43 mutant TP53 and 16 wild-type TP53 lines (P<0.01 for both microRNAs). The differential expression of the four PTEN- or TP53-associated microRNAs is depicted in figure 4. For the BRAF gene, for which 11 cell-lines carry mutations, 40 microRNAs were differentially expressed, with miR-146a and miR-509-3p overexpressed and miR-149 and mR-192b underexpressed the most (table S5). This high number of differentially expressed microRNAs may be because eight of the 11 BRAF mutant cell-lines are melanoma cell-lines. No differentially expressed microRNAs were identified in such analyses for CDKN2A (33 mutant cell-lines) or KRAS (11 mutant cell-lines).
Figure 4. MicroRNAs and PTEN or TP53 mutations in NCI-60 cell-lines.
Dot plots with medians and inter-quartile ranges are shown for the expression of microRNAs miR-29b, −34a, −34a* and −769-3p in 59 NCI-60 cell-lines grouped by mutation status for the PTEN or TP53 genes.
Correlation between Levels of microRNAs and their Target mRNAs
Significant correlations between expression of microRNAs and mRNAs were detected for 3,483 (3%) of 117,004 possible pairs of microRNAs and their target mRNAs as predicted by the TargetScan algorithm. The 3,483 pairs consisted of 167 microRNAs and 1,232 target mRNAs with unique gene symbols. Among the 3,483 significant correlations, 1,997 (57%) were negative and 1,486 (43%) were positive (figure S5). In contrast, the mean and SD for the number of significant correlations seen with 10 random permutations of the mRNA expression dataset to switch cell-line identifiers were 21.3 and 6.6, respectively. With Benjamini-Hochberg correction for FDR below 5%, a mean value of 174 was expected. Furthermore, the values of 117,004 correlation coefficients obtained with the mRNA expression dataset were significantly different in Wilcoxon rank sum tests (P<2×10−16) from those obtained with any of the 10 permutated datasets.
Discussion
The expression of 847 microRNAs in the NCI-60 panel of human tumor cell-lines was examined in this study using Affymetrix® GeneChip™ miRNA 1.0 DNA oligonucleotide microarrays to generate a dataset of 495 microRNAs identified as expressed in at least one of the 60 lines of the panel. Thus, the study expands the microRNA coverage of three of the four existing NCI-60 expression datasets. These sets were independently generated using different high throughput platforms – custom 40-mer DNA microarray, TaqMan™ RT-PCR, Agilent® 60-mer DNA microarray, or Exiqon® locked nucleic acid miRCURY™ microarray – to assess levels of 321, 241, 723 or 955 microRNAs, respectively [21], [22], [23], [24]. As depicted in figures 2 and S2, the correlation of microRNA expression measurements among the five datasets is modest, with mean Pearson coefficients varying from 0.37 to 0.54. These values, likely reflecting a known discordance among different microRNA quantification platforms [41], [42], highlight the usefulness of the dataset produced in this study in ascertaining results obtained using another dataset. Vast amount of information for a multitude of features exist and continue to be generated (e.g., [43], [44]) for the NCI-60 lines, and, as cited in the introduction section, integrated analysis of microRNA expression with other features of the cells has the potential to yield significant insights to generate and test hypotheses.
Unsupervised hierarchical clustering of the cell-lines by the microRNA expression patterns quantified in this study shows segregation by tissue of origin, and thus the relative homogeneity, of the lines derived from leukemia, melanoma or colon tumors (figure 3A). Similar clustering results are seen with the other NCI-60 microRNA datasets, even with that of Gaur, et al. which has expression measures for only 241 microRNAs [21], [22], [23], [24]. It is noteworthy in this regard that an assay of only 64 microRNAs has been found to quite accurately identify the tissue of origin of 42 different types of human tumors [45]. Analysis of the current study’s dataset for tissue-specific microRNA expression (figure 3B and table S2) shows that the highest degree of tissue-specific differences are seen for the leukemia, melanoma and colon cancer cell-lines, which interestingly cluster well by tissue of origin (figure 3A). Comparable tissue-specific microRNA expression patterns are identified using the five different NCI-60 microRNA expression datasets in spite of the modest inter-dataset correlations mentioned above. For instance, higher expression of microRNAs miR-192 and miR-194 in colon cancer cell-lines, and of miR-708 and miR-886-3p in renal cancer cell-lines (table S2) was also observed by Sokilde, et al [23]. Similarly, higher expression of miR-382 in the CNS cell-lines can be observed in the datasets of this (table S2) as well as the Blower and Gaur studies [21], [22]. The association of microRNA expression profiles with tissues of origin of the NCI-60 cell-lines, however, has been noted to be somewhat weaker than the association of mRNA profiles [22], [24]. With human tumor tissues, both mRNA and microRNA expression profiles appear to be similarly informative of tissue of origin [46], [47].
To compare the similarity of results obtained using different datasets, we also examined the association with population doubling-times of the NCI-60 cell-lines with their microRNA expression as measured by us or by Liu, et al [24]. Of the 28 microRNAs of our dataset that were identified as associated with doubling-time and were also quantified in the Liu study, 21 (75%) continued to be associated, none with a reversal of expression trend, when measurements were taken from the Liu dataset (table S4). Such similarity between the multiple NCI-60 microRNA datasets was also observed in correlation analysis of microRNA levels and radiation sensitivity of the cell-lines, in which microRNAs let-7i, miR-142-5p and miR-193b were identified as associated with at least one of the SF2, SF5 and SF8 radiation sensitivity parameters in datasets of this (table S3) as well as the Liu and Sokilde studies [23], [24]. Expression of let-7i and miR-193b positively correlated with SF2, SF5 and SF8, indicating radiation resistant cell-lines had higher levels of the two microRNAs. Expression of these two microRNAs has been shown to be induced by ionizing radiation [48], [49], [50]. Though microRNA miR-142-5p levels from all three datasets were negatively correlated with radiation sensitivity, expression of the microRNA has been observed to increase in response to ionizing radiation [50], [51], [52]. All three microRNAs, let-7i, miR-142-5p and miR-193b, are known to have growth-suppressive effects [53], [54], [55]. Unlike for expression of mRNAs [37], [56], microRNA expression patterns strongly associated with radiation sensitivity of the NCI-60 panel could not be discerned in the current study (figure S4).
As might be expected, mutations in the NCI-60 cells in three of the five oncogenes that were examined were found to be associated with microRNA expression changes (figure 4 and table S5). For example, TP53 mutant cell-lines have higher levels of miR-34a and miR-34a* than the wild-type lines. The TP53 protein activates transcription of the MIR34A gene that encodes for the two mature microRNAs [57], [58]. For the BRAF gene, presence of an oncogenic mutation was found to be associated with 40 microRNAs (table S5). In the NCI-60 panel, there are 11 BRAF mutant cell-lines, including all eight melanoma lines. Indeed, except for miR-29b-1* (miR-29b-1–5p) and miR-768-3p, all microRNAs differentially expressed between the mutant and wild-type lines were also differentially expressed between the eight melanoma and 52 non-melanoma cell-lines. Nevertheless, the observed associations may indicate a universal role of the BRAF pathway in regulation of certain microRNAs. This is supported by the observation that miR-146a, whose levels are higher in BRAF mutant cell-lines (table S5), is upregulated upon activation of the BRAF-encoded B-raf kinase protein in non-melanoma cells [59]. Though miR-29b has been shown to target PTEN transcripts [60], a basis for the differential expression of the two microRNAs, miR-29b and miR-769-3p, between the PTEN mutant and wild-type lines (figure 4) is not evident in published literature. This exemplifies the value of NCI-60 integromics to generate testable hypotheses. Another potential use of the NCI-60 microRNA expression datasets such as the one generated here is for the identification of microRNA targets and molecular pathways through integrated analysis of gene expression data. One such analysis, depicted in figure S5, shows the presence of many significantly correlated pairs of microRNAs and target mRNAs predicted by nucleotide sequence comparison. As has been observed by others [61], a large fraction of such pairs had positive correlation values, which is paradoxical considering the prevalent notion of microRNA-mediated gene regulation, that microRNAs either abate target mRNA levels or not but they do not increase them. Such contradiction, which has also been observed with the NCI-60 datasets with other analytical approaches for microRNA-mRNA interaction [62], could reflect co-expression of microRNA and mRNA genes or indirect gene regulation, imperfectness of the TargetScan microRNA target prediction algorithm [63], or the fact that an mRNA molecule can be targeted by multiple species of microRNAs.
Supporting Information
Technical replication of microarray hybridizations. Scatter-plots for four pairs of replicate hybridizations for RNA from four cell-lines are shown with ordered × values of log2-transformed microarray signal values (dots) and inter-replicate Pearson correlation coefficients, r (lines) for 1779 human RNA-specific probes. A rolling window of width 99 along the × axis was used to calculate r at the mid-window abscissa. Horizontal gray lines represent Y = 0.5 along the right Y axis. Names of the cell-lines and the batches of microarray hybridizations are noted.
(TIF)
Correlations between different NCI-60 cell-line microRNA expression datasets. Cumulative frequency distributions are shown for Pearson correlation coefficients (r) with a bin-size of 0.025 for microRNAs quantified in the study of Sokilde, et al. [23] (left) or that of Liu, et al. [24] (right) and in other similar studies including this one (Patnaik) and those of Blower, et al. [22] and Gaur, et al [21]. The distributions of the coefficients with the expression measurements of Liu, et al. resampled are also shown. Numbers within parentheses in the legends indicate sample sizes, i.e., the number of microRNAs quantified in both of the compared datasets.
(TIF)
Gaussian kernel density estimates of seven radiation survival parameter values of 59 NCI-60 cell-lines. Data on the parameters SF2, SF5, SF8, D0, n, Casp8 and Casp16 were obtained from the study of Amundson, et al. [37]. Parameter values were log2-transformed and then z-score-normalized. The ks package (version 1.8.3) for R was used for density estimation with the package’s default settings. The short gray ticks along the × axis indicate parameter values of the 59 cell-lines.
(TIF)
Distribution of Pearson coefficients in correlation analyses of microRNA expression and radiation sensitivity of 59 NCI-60 cell-lines. Data on the radiation sensitivity parameters SF2, SF5, SF8, D0, n, Casp8 and Casp16 were obtained from the study of Amundson, et al. [37]. Expression measurements of 365, 495 and 896 microRNAs were respectively from the study of Liu, et al. [24], current study (Patnaik), and the study of Sokilde, et al. [23] as indicated in the figure. Leukemia cell-lines (n = 6) were excluded from the analyses depicted in the bottom half of the figure. All values were log2-transformed before correlation analyses. Pearson coefficients (r) are binned with a width of 0.02. Curve smoothing was done using four neighboring values and a zero-order polynomial.
(TIF)
Distribution of Pearson coefficients for significant correlations of expression levels of microRNAs and their target mRNAs in 57 NCI-60 cell-lines. Pearson coefficients (r) are binned with a width of 0.02. Curve smoothing was done using four neighboring values and a zero-order polynomial.
(TIF)
Heat-map, with its pseudo-color scale underneath, of log2-transformed microarray signal values of the 60 cell-lines for the sets of eight microRNAs each with lowest P values in tests of differential expression in cell-lines of a specific tissue of origin compared to all the other cell-lines. Both cell-lines and microRNAs are grouped by tissue of origin.
(TIF)
The 60 NCI-60 cell-lines examined in this work listed along with the day and batch of microarray hybridizations.
(PDF)
Differential expression of microRNAs in NCI-60 cell-lines by tissue of origin.
(PDF)
MicroRNAs whose expression correlates with an absolute Pearson correlation coefficient >0.4 for at least one of the radiation sensitivity parameters SF2, SF5 or SF8 in 53 non-leukemia NCI-60 cell-lines.
(PDF)
MicroRNAs whose expression correlates with doubling-time of 59 NCI-60 cell-lines.
(PDF)
MicroRNAs differentially expressed between NCI-60 cell-lines with and without BRAF gene mutations.
(PDF)
Funding Statement
The work was funded by Roswell Park Cancer Institute, Buffalo, New York, United States of America, and Medical Prognosis Institute, Hørsholm, Denmark. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lin WM, Baker AC, Beroukhim R, Winckler W, Feng W, et al. (2008) Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer research 68: 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, et al. (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer cell 10: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shoemaker RH, Monks A, Alley MC, Scudiero DA, Fine DL, et al. (1988) Development of human tumor cell line panels for use in disease-oriented drug screening. Progress in clinical and biological research 276: 265–286. [PubMed] [Google Scholar]
- 4. Shoemaker RH (2006) The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6: 813–823. [DOI] [PubMed] [Google Scholar]
- 5. Lee JS, Paull K, Alvarez M, Hose C, Monks A, et al. (1994) Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol Pharmacol 46: 627–638. [PubMed] [Google Scholar]
- 6. Savas S, Briollais L, Ibrahim-zada I, Jarjanazi H, Choi YH, et al. (2010) A whole-genome SNP association study of NCI60 cell line panel indicates a role of Ca2+ signaling in selenium resistance. PLoS One 5: e12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishizuka S, Charboneau L, Young L, Major S, Reinhold WC, et al. (2003) Proteomic profiling of the NCI-60 cancer cell lines using new high-density reverse-phase lysate microarrays. Proceedings of the National Academy of Sciences of the United States of America 100: 14229–14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinstein JN (2006) Spotlight on molecular profiling: “Integromic” analysis of the NCI-60 cancer cell lines. Molecular cancer therapeutics 5: 2601–2605. [DOI] [PubMed] [Google Scholar]
- 9. Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ (2006) miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34: D140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iorio MV, Croce CM (2012) microRNA involvement in human cancer. Carcinogenesis 33: 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandoval J, Esteller M (2012) Cancer epigenomics: beyond genomics. Current opinion in genetics & development 22: 50–55. [DOI] [PubMed] [Google Scholar]
- 12. Murray MY, Rushworth SA, MacEwan DJ (2012) Micro RNAs as a new therapeutic target towards leukaemia signalling. Cellular signalling 24: 363–368. [DOI] [PubMed] [Google Scholar]
- 13. Park SM, Gaur AB, Lengyel E, Peter ME (2008) The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes & development 22: 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moes M, Le Bechec A, Crespo I, Laurini C, Halavatyi A, et al. (2012) A novel network integrating a miRNA-203/SNAI1 feedback loop which regulates epithelial to mesenchymal transition. PloS one 7: e35440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blower PE, Chung JH, Verducci JS, Lin S, Park JK, et al. (2008) MicroRNAs modulate the chemosensitivity of tumor cells. Molecular cancer therapeutics 7: 1–9. [DOI] [PubMed] [Google Scholar]
- 16. Gmeiner WH, Reinhold WC, Pommier Y (2010) Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP [10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Molecular cancer therapeutics 9: 3105–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang Y (2008) An expression meta-analysis of predicted microRNA targets identifies a diagnostic signature for lung cancer. BMC medical genomics 1: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, et al. (2009) MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer research 69: 7569–7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu J, Li CX, Li YS, Lv JY, Ma Y, et al. (2011) MiRNA-miRNA synergistic network: construction via co-regulating functional modules and disease miRNA topological features. Nucleic acids research 39: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bisognin A, Sales G, Coppe A, Bortoluzzi S, Romualdi C (2012) MAGIA2: from miRNA and genes expression data integrative analysis to microRNA-transcription factor mixed regulatory circuits (2012 update). Nucleic acids research 40: W13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, et al. (2007) Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 67: 2456–2468. [DOI] [PubMed] [Google Scholar]
- 22. Blower PE, Verducci JS, Lin S, Zhou J, Chung JH, et al. (2007) MicroRNA expression profiles for the NCI-60 cancer cell panel. Mol Cancer Ther 6: 1483–1491. [DOI] [PubMed] [Google Scholar]
- 23. Sokilde R, Kaczkowski B, Podolska A, Cirera S, Gorodkin J, et al. (2011) Global microRNA analysis of the NCI-60 cancer cell panel. Mol Cancer Ther 10: 375–384. [DOI] [PubMed] [Google Scholar]
- 24. Liu H, D’Andrade P, Fulmer-Smentek S, Lorenzi P, Kohn KW, et al. (2010) mRNA and microRNA expression profiles of the NCI-60 integrated with drug activities. Mol Cancer Ther 9: 1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brazma A, Parkinson H, Sarkans U, Shojatalab M, Vilo J, et al. (2003) ArrayExpress–a public repository for microarray gene expression data at the EBI. Nucleic Acids Research 31: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193. [DOI] [PubMed] [Google Scholar]
- 27. Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264. [DOI] [PubMed] [Google Scholar]
- 28. Shankavaram UT, Varma S, Kane D, Sunshine M, Chary KK, et al. (2009) CellMiner: a relational database and query tool for the NCI-60 cancer cell lines. BMC Genomics 10: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome research 19: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307–315. [DOI] [PubMed] [Google Scholar]
- 31. Durinck S, Moreau Y, Kasprzyk A, Davis S, De Moor B, et al. (2005) BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 21: 3439–3440. [DOI] [PubMed] [Google Scholar]
- 32.Smyth G (2005) Limma: linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Dudoit S, Irizarry RA, editors. Bioinformatics and Computational Biology Solutions using R and Bioconductor. New York: Springer. 397–420.
- 33. Saeed AI, Sharov V, White J, Li J, Liang W, et al. (2003) TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34: 374–378. [DOI] [PubMed] [Google Scholar]
- 34. Liscovitch M, Ravid D (2007) A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer letters 245: 350–352. [DOI] [PubMed] [Google Scholar]
- 35. Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD (2007) MDA-MB-435 cells are derived from M14 melanoma cells–a loss for breast cancer, but a boon for melanoma research. Breast cancer research and treatment 104: 13–19. [DOI] [PubMed] [Google Scholar]
- 36. Lorenzi PL, Reinhold WC, Varma S, Hutchinson AA, Pommier Y, et al. (2009) DNA fingerprinting of the NCI-60 cell line panel. Molecular cancer therapeutics 8: 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amundson SA, Do KT, Vinikoor LC, Lee RA, Koch-Paiz CA, et al. (2008) Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res 68: 415–424. [DOI] [PubMed] [Google Scholar]
- 38. Savas S, Azorsa DO, Jarjanazi H, Ibrahim-Zada I, Gonzales IM, et al. (2011) NCI60 cancer cell line panel data and RNAi analysis help identify EAF2 as a modulator of simvastatin and lovastatin response in HCT-116 cells. PLoS One 6: e18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jarjanazi H, Kiefer J, Savas S, Briollais L, Tuzmen S, et al. (2008) Discovery of genetic profiles impacting response to chemotherapy: application to gemcitabine. Hum Mutat 29: 461–467. [DOI] [PubMed] [Google Scholar]
- 40. Ikediobi ON, Davies H, Bignell G, Edkins S, Stevens C, et al. (2006) Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther 5: 2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Git A, Dvinge H, Salmon-Divon M, Osborne M, Kutter C, et al. (2010) Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA 16: 991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sato F, Tsuchiya S, Terasawa K, Tsujimoto G (2009) Intra-platform repeatability and inter-platform comparability of microRNA microarray technology. PloS one 4: e5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, et al. (2012) The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483: 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, et al. (2012) Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483: 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meiri E, Mueller WC, Rosenwald S, Zepeniuk M, Klinke E, et al. (2012) A Second-Generation MicroRNA-Based Assay for Diagnosing Tumor Tissue Origin. The oncologist 17: 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma XJ, Patel R, Wang X, Salunga R, Murage J, et al. (2006) Molecular classification of human cancers using a 92-gene real-time quantitative polymerase chain reaction assay. Arch Pathol Lab Med 130: 465–473. [DOI] [PubMed] [Google Scholar]
- 47. Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, et al. (2008) MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol 26: 462–469. [DOI] [PubMed] [Google Scholar]
- 48. Niemoeller OM, Niyazi M, Corradini S, Zehentmayr F, Li M, et al. (2011) MicroRNA expression profiles in human cancer cells after ionizing radiation. Radiation oncology 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simone NL, Soule BP, Ly D, Saleh AD, Savage JE, et al. (2009) Ionizing radiation-induced oxidative stress alters miRNA expression. PloS one 4: e6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chaudhry MA (2009) Real-time PCR analysis of micro-RNA expression in ionizing radiation-treated cells. Cancer biotherapy & radiopharmaceuticals 24: 49–56. [DOI] [PubMed] [Google Scholar]
- 51. Chaudhry MA, Sachdeva H, Omaruddin RA (2010) Radiation-induced micro-RNA modulation in glioblastoma cells differing in DNA-repair pathways. DNA and cell biology 29: 553–561. [DOI] [PubMed] [Google Scholar]
- 52. Cui W, Ma J, Wang Y, Biswal S (2011) Plasma miRNA as biomarkers for assessment of total-body radiation exposure dosimetry. PloS one 6: e22988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao Y, Deng C, Wang J, Xiao J, Gatalica Z, et al. (2011) Let-7 family miRNAs regulate estrogen receptor alpha signaling in estrogen receptor positive breast cancer. Breast cancer research and treatment 127: 69–80. [DOI] [PubMed] [Google Scholar]
- 54. Liu X, Sempere LF, Galimberti F, Freemantle SJ, Black C, et al. (2009) Uncovering growth-suppressive MicroRNAs in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 15: 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rauhala HE, Jalava SE, Isotalo J, Bracken H, Lehmusvaara S, et al. (2010) miR-193b is an epigenetically regulated putative tumor suppressor in prostate cancer. International journal of cancer Journal international du cancer 127: 1363–1372. [DOI] [PubMed] [Google Scholar]
- 56. Torres-Roca JF, Eschrich S, Zhao H, Bloom G, Sung J, et al. (2005) Prediction of radiation sensitivity using a gene expression classifier. Cancer research 65: 7169–7176. [DOI] [PubMed] [Google Scholar]
- 57. Fabbri M, Bottoni A, Shimizu M, Spizzo R, Nicoloso MS, et al. (2011) Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA : the journal of the American Medical Association 305: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bhatt K, Zhou L, Mi QS, Huang S, She JX, et al. (2010) MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Molecular medicine 16: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, et al. (2010) p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell death and differentiation 17: 236–245. [DOI] [PubMed] [Google Scholar]
- 60. Wang C, Bian Z, Wei D, Zhang JG (2011) miR-29b regulates migration of human breast cancer cells. Molecular and cellular biochemistry 352: 197–207. [DOI] [PubMed] [Google Scholar]
- 61. Wang YP, Li KB (2009) Correlation of expression profiles between microRNAs and mRNA targets using NCI-60 data. BMC Genomics 10: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Engelmann JC, Spang R (2012) A Least Angle Regression Model for the Prediction of Canonical and Non-Canonical miRNA-mRNA Interactions. PloS one 7: e40634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Witkos TM, Koscianska E, Krzyzosiak WJ (2011) Practical Aspects of microRNA Target Prediction. Current molecular medicine 11: 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical replication of microarray hybridizations. Scatter-plots for four pairs of replicate hybridizations for RNA from four cell-lines are shown with ordered × values of log2-transformed microarray signal values (dots) and inter-replicate Pearson correlation coefficients, r (lines) for 1779 human RNA-specific probes. A rolling window of width 99 along the × axis was used to calculate r at the mid-window abscissa. Horizontal gray lines represent Y = 0.5 along the right Y axis. Names of the cell-lines and the batches of microarray hybridizations are noted.
(TIF)
Correlations between different NCI-60 cell-line microRNA expression datasets. Cumulative frequency distributions are shown for Pearson correlation coefficients (r) with a bin-size of 0.025 for microRNAs quantified in the study of Sokilde, et al. [23] (left) or that of Liu, et al. [24] (right) and in other similar studies including this one (Patnaik) and those of Blower, et al. [22] and Gaur, et al [21]. The distributions of the coefficients with the expression measurements of Liu, et al. resampled are also shown. Numbers within parentheses in the legends indicate sample sizes, i.e., the number of microRNAs quantified in both of the compared datasets.
(TIF)
Gaussian kernel density estimates of seven radiation survival parameter values of 59 NCI-60 cell-lines. Data on the parameters SF2, SF5, SF8, D0, n, Casp8 and Casp16 were obtained from the study of Amundson, et al. [37]. Parameter values were log2-transformed and then z-score-normalized. The ks package (version 1.8.3) for R was used for density estimation with the package’s default settings. The short gray ticks along the × axis indicate parameter values of the 59 cell-lines.
(TIF)
Distribution of Pearson coefficients in correlation analyses of microRNA expression and radiation sensitivity of 59 NCI-60 cell-lines. Data on the radiation sensitivity parameters SF2, SF5, SF8, D0, n, Casp8 and Casp16 were obtained from the study of Amundson, et al. [37]. Expression measurements of 365, 495 and 896 microRNAs were respectively from the study of Liu, et al. [24], current study (Patnaik), and the study of Sokilde, et al. [23] as indicated in the figure. Leukemia cell-lines (n = 6) were excluded from the analyses depicted in the bottom half of the figure. All values were log2-transformed before correlation analyses. Pearson coefficients (r) are binned with a width of 0.02. Curve smoothing was done using four neighboring values and a zero-order polynomial.
(TIF)
Distribution of Pearson coefficients for significant correlations of expression levels of microRNAs and their target mRNAs in 57 NCI-60 cell-lines. Pearson coefficients (r) are binned with a width of 0.02. Curve smoothing was done using four neighboring values and a zero-order polynomial.
(TIF)
Heat-map, with its pseudo-color scale underneath, of log2-transformed microarray signal values of the 60 cell-lines for the sets of eight microRNAs each with lowest P values in tests of differential expression in cell-lines of a specific tissue of origin compared to all the other cell-lines. Both cell-lines and microRNAs are grouped by tissue of origin.
(TIF)
The 60 NCI-60 cell-lines examined in this work listed along with the day and batch of microarray hybridizations.
(PDF)
Differential expression of microRNAs in NCI-60 cell-lines by tissue of origin.
(PDF)
MicroRNAs whose expression correlates with an absolute Pearson correlation coefficient >0.4 for at least one of the radiation sensitivity parameters SF2, SF5 or SF8 in 53 non-leukemia NCI-60 cell-lines.
(PDF)
MicroRNAs whose expression correlates with doubling-time of 59 NCI-60 cell-lines.
(PDF)
MicroRNAs differentially expressed between NCI-60 cell-lines with and without BRAF gene mutations.
(PDF)