Abstract
Osteopontin (OPN) is a protein, involved in various pathophysiological events. OPN has been studied as a secreted protein, but recent reports showed that OPN can be found in the cytoplasm and the nucleus. Therefore, some OPN molecules are not secreted and stay in cells. Such intracellular OPN (iOPN) has biological functions distinct from secreted OPN (sOPN). iOPN is involved in cytoskeletal rearrangement and in signal transduction pathways downstream of innate immune receptors, such as Toll-like receptors (TLRs), as an adaptor or scaffolding protein. Although sOPN and iOPN are generated from the same Opn mRNA species, biological outcomes mediated by two isoforms can be different. It would be necessary to delineate which isoform of OPN is responsible for pathophysiological events.
Keywords: osteopontin (OPN), intracellular OPN (iOPN), sOPN, innate immunity, EAE, MS, autoimmune diseases, T helper cell polarization, dendritic cells (DCs)
1. What is osteopontin (OPN)?
Osteopontin (OPN), also known as early T lymphocyte activation 1 (Eta-1), is a glycoprotein expressed by a variety of tissues and cell types. OPN is multifunctional and its functions are linked to various physiological and pathological events. OPN was first described by Hynes and colleagues [1] as a phosphoprotein secreted by transformed cells lines. Heinegard’s group [2] cloned the bone-specific sialoprotein, which plays a role as a matrix protein, from the rat osteosarcoma phage λgt11 library and termed the protein “osteopontin”. The mouse Opn gene was first cloned by Denhardt’s group as a tumor promoter (TPA) inducible gene [3]. Cantor and colleagues [4] independently identified OPN as Eta-1, which is abundantly expressed in activated T cells. This finding was the first report to directly connect OPN to immunity. OPN was identified and has been studied as an extracellular protein. Therefore, unless specifically noted, any previous studies were performed as OPN to be a secreted protein. We will discuss a novel intracellular isotype of OPN (iOPN) later in this review.
OPN can act as modulators of cell adhesion as well as autocrine and paracrine factors by interacting with cell surface receptors such as integrins. OPN contains an integrin-binding RGD motif that is recognized by integrins αvβ3, αvβ1, αvβ5, αvβ6, α5β1, and α8β1 [5–10]. In contrast, integrins α9β1 and α4β1 bind to the cryptic epitope (SLAYGLR motif in mice) of OPN that is exposed upon cleavage by thrombin [11–14]. OPN is also detected by CD44, specifically by isotypes containing v6-v7 regions, and by integrin αxβ2 (14–16). Function of OPN is regulated by post-translational modifications, such as phosphorylation, glycosylation, sulfation, and proteolytic cleavage [15–17]. Phosphorylation of OPN is required for integrin binding and generation of certain biological activities, such as induction and secretion of cytokines, macrophage migration and subsequent activation [18, 19]. Tumor-derived OPN is likely less phosphorylated than OPN derived from normal cells. Ras-transformed fibroblasts are known to produce OPN that is significantly under-phosphorylated [17, 20]. Proteolytic cleavage of OPN by thrombin or matrix metalloproteinase (MMP) changes the biological functions of OPN in diseases, such as rheumatoid arthritis (RA) and prostate cancer [14, 21].
Genetic polymorphisms in the human Opn locus have been characterized. Such polymorphisms in the human Opn locus contribute to host susceptibility to infections, autoimmune diseases, and cancer [22–29]. Furthermore, human Opn mRNA splicing variants, such as deletions of exon 4 (OPN-c) or exon 5 (OPN-b), are known to exist in human cells, and considered to be used for cancer biomarkers [30–32].
In summary, the Opn (Spp1) locus generates various forms of OPN protein by splicing, posttranslational modification, and cleavage by proteases; and different forms of OPN can have distinct biological functions.
2. Secreted OPN (sOPN) in immunity
sOPN is expressed in various tissues including bone, mammary gland, kidney, smooth muscle, brain, and immune organs. Various immune cells express sOPN; those cells include macrophages, dendritic cells (DCs), neutrophils, eosinophils, NK cells, NKT cells, and T and B lymphocytes. sOPN expressed by immune cells promotes cell proliferation, adhesion, migration, activation, anti-apoptosis, angiogenesis, and cytokine expression as well as cytoskeletal-related functions including cell motility, cell fusion, and survival [33, 34]. NF-κB is known to mediate at least some of these outcomes upon cellular detection of sOPN by integrin αvβ3 [35, 36].
sOPN induces Th1 responses by upregulation of IL-12 and downregulation of IL-10 through αvβ3 integrin and CD44, respectively, expressed on the macrophage cell surface [18]. In turn, in vitro Th1 cytokine milieu induces Opn mRNA expression by CD4+ T cells and increases sOPN protein levels in culture supernatants of CD4+ T cells activated by CD3/28 antibodies [37]. A major Th1 cytokine that is responsible for the induction of Opn expression is IL-12 (Shinohara et al. unpublished data). On the other hand, at least in mice, IFNγ does not appear to induce Opn expression; rather, high concentrations of IFNγ suppress Opn expression [37, 38]. It is notable that T-bet, the key transcription factor in the induction of IFNγ, positively regulates Opn expression [37]. Therefore, Opn upregulation may be an early event in Th1 polarization. As Th1 responses progress and IFNγ levels in the milieu increase, expression of Opn may be downregulated. Such feedback regulation of Opn appears to exist during the course of Th1 polarization progression.
Later studies also suggested that sOPN induces expression of IL-17, in addition to IFNγ; i.e. Th1 and Th17 responses are induced by sOPN [39]. Since iOPN expressed in antigen-presenting cells, not in T cells, also enhances Th17 responses [40], sOPN appears to get iOPN’s help from innate immune cells in inducing Th17 responses.
3. OPN and immune-related diseases
A number of studies have demonstrated that sOPN is critical for the development of several autoimmune diseases. Elevated plasma levels of sOPN was observed in many chronic inflammatory diseases, including rheumatoid arthritis (RA) [41], Crohn’s disease [42], fulminant hepatitis [43], allergic cutaneous disease [44], and multiple sclerosis (MS)[45, 46].
sOPN induces progression of RA by recruiting inflammatory cells to arthritic joints. sOPN is indeed required for progression of arthritis induced by injection with a mixture of type II collagen mAb and LPS [47]. Uede and colleagues [14] further demonstrated that thrombin-cleaved form of sOPN, which bind to integrins α9β1 and α4β1, is critically involved in the pathogenesis of RA induced by type II collagen mAb and LPS. OPN antibody that recognized the OPN cryptic epitope inhibited the proliferation of synovium, bone erosion, and inflammatory cell infiltration in arthritic joints [14]. In addition, it is notable that the same OPN antibody ameliorated concanavalin A (ConA)-induced hepatitis by suppressing NKT activity and the following neutrophil infiltration and activation in livers [12].
OPN plays an important role in progression of MS and its mouse model, experimental autoimmune encephalomyelitis (EAE), by inducing Th1 and Th17 cell polarization [37, 39, 40, 48–51]. Steinman and colleagues [45] found that Opn is one of the most highly expressed genes in MS brain lesions and identified accumulation of OPN protein in perivascular inflammatory lesions in the CNS from MS patients. An report from Cantor and colleagues also showed similar results: OPN deficient mice displayed milder PLP172–183 peptide-induced EAE progression with no spontaneous relapse as well as a reduction of Th1-associated cytokine expression in vivo and ex vivo [48]. OPN appears to induce EAE progression by at least a couple of mechanisms. sOPN inhibits apoptosis of T cells and allows accumulation of autoreactive T cells to induce relapse and progression of EAE [50]. sOPN also induces Th17 responses through β3 integrin [39]. iOPN expressed in DCs and microglia also plays a role in induction of Th17 responses during EAE progression [40], as we discuss in later chapters of this review.
Weiss and colleagues [44] found that expression of OPN is upregulated in the skin and draining lymph nodes (DLNs) during the sensitization phase of allergic cutaneous contact hypersensitivity. sOPN plays a role in inducing chemotaxis of Langerhans cells/DCs to DLNs by interacting with CD44 and integrin. sOPN induces IL-12 by DCs, and promotes Th1 responses during skin inflammation [52].
OPN induces both Th1 and Th17 responses, which is profitable for hosts infected by viruses, bacteria, and fungi. However, Th1 and Th17 responses induce inflammation and are detrimental in autoimmune diseases. In addition to Th1 and Th17-associated diseases as mentioned above, OPN has also been implicated in Th2-associated disease such as airway hyper-responsiveness (AHR)[53]. In ovalbumin (OVA)-induced allergic airway inflammation, sOPN exerts opposite effects during primary sensitization and secondary challenge through differential inhibition of distinct DC subset migration to the site of the antigen response [53]. During the primary antigen sensitization, sOPN induced AHR by inhibiting the migration of plasmacytoid dendritic cells (pDCs), which prevent asthmatic reactions [53]. During the secondary antigenic challenge, however, sOPN ameliorated AHR by decreasing established Th2 responses, which was achieved by inhibiting migration of proinflammatory conventional DCs (cDCs) into DLNs [53]. Such sOPN-dependent suppression of DC migration may be mediated by, at least in part, downregulation of CCR7 in the DCs [54, 55], which enhances migration of mature DCs into regional LNs. Another study demonstrated that sOPN plays a regulatory role in systemic allergen sensitization by inducing IL-12 expression by DCs [56]. In contrast to OPN’s function in acute phase of AHR [53, 56], OPN appears to play a role as an inducer of AHR and lung remodeling in chronic phase by multiple allergen challenges [57].
As we have just discussed, it is becoming clear that the behavior of OPN can be quite different depending on pathological conditions. Although OPN is mainly considered an inflammatory molecule, Denhardt’s group and Kuo’s group have already made it clear that OPN can be anti-inflammatory by suppressing nitric oxide (NO) production [58–60]. In addition to NO production, OPN may exert other mechanisms to induce anti-inflammation. Some OPN studies show results that are opposite from what people generally expect, based on the notion that OPN is an inflammatory molecule. Such studies probably include important clues to explain the mechanism by which outcomes of OPN-mediated physiological responses are not always the same.
4. Discovery of intracellular OPN (iOPN)
Two lines of Opn knockout (KO) mice are currently available; one from a group led by Denhardt [61] and another by Hogan [62]. It goes without saying that those mice significantly contributed to the progression of OPN research. The Opn KO mice also revealed to us a new phenomenon. For example, it occasionally happened, although not always, that a treatment of the KO mice/cells with rOPN did not “correct” KO phenotypes and OPN depletion by antibodies in WT mice/cell culture also did not reproduce KO phenotypes [49, 63–67]. These studies were well performed with reliable reagents; thus, the involvement of extracelluar OPN alone may not explain the unexpected results. (We also need to take it into consideration that different protein expression systems generate rOPN of different posttranslational modifications, though.) This prompted us to look for intracellular molecule [49]. Here, a possible explanation of the unexpected results after treatments with rOPN and OPN antibody was the presence of intracellular OPN (iOPN), which cannot be replaced by rOPN or neutralized by OPN antibody because treatments with such reagents work extracellularly.
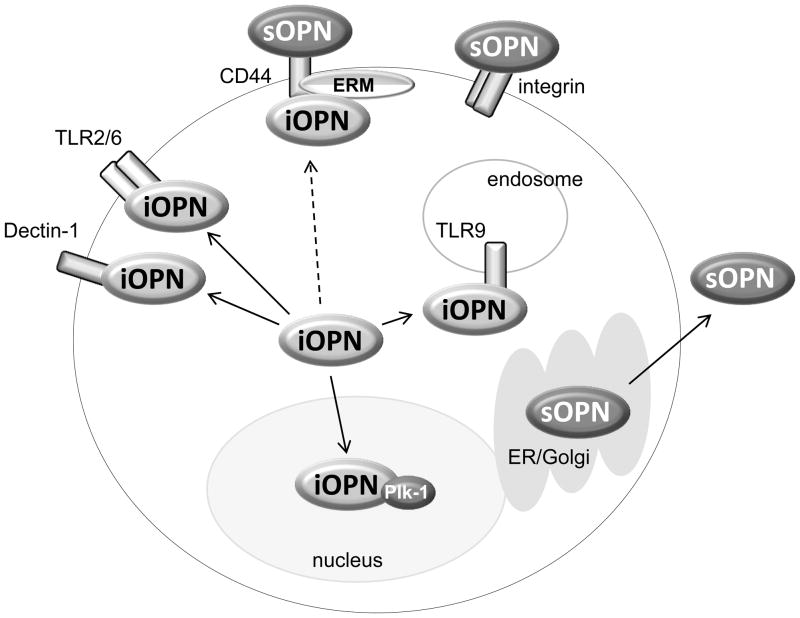
iOPN was initially found in rat calvarial cells and reported by Sodek’s group [68]. The study performed single cell analysis using confocal microscopy. They found two patterns of OPN staining in a cell; a perinuclear distribution that appeared to be in Golgi, and a perimembrane distribution that was reminiscent of focal adhesion staining [68]. The latter OPN that localized outside of the secretory vesicles was iOPN. The same group later demonstrated that iOPN at perimembrane regions co-localized with the CD44-ERM (ezrin-radixin-moesin) complex that played a role in cell motility [69](Fig. 1); a process likely mediated by ERM, which binds actin. In addition to the CD44-ERM complex, iOPN was found to colocalize with actin in macrophages and osteoclasts [67, 70]. Although there is currently no direct evidence of iOPN-mediated regulations in actin cytoskeletal rearrangement, our data also supported the possible involvement of iOPN. We demonstrated that iOPN expression in Opn−/− pDCs restored podosome formation [71], suggesting that iOPN induces cell reshaping and motility.
Fig. 1. Interaction of OPN and other molecules.
In unstimulated cells, iOPN appears to be distributed in cytoplasm of macrophages and dendritic cells. Once cells are stimulated with pattern-recognition receptor ligands, iOPN is recruited to the receptor that detects a ligand. Zymosan and fungal stimuli recruit iOPN to molecular complexes downstream of TLR2 (fungal-recognizing TLR2 makes heterodimer with TLR6) and dectin-1 (Inoue et al. Submitted). CpG DNA stimulation make iOPN to translocate to TLR9 (Shinohara et al., 2006). iOPN is also known to co-localize with CD44 and ezrin/radixin/moesin (ERM) protein ezrin (Zohar et al. 2000), although it is not known whether the colocalization is activation-specific (broken arrow). iOPN translocates into nuclei particularly during the G2/M period of mitosis, and interacts with Plk-1 (Junaid et al. 2007). sOPN is secreted through ER and Golgi. Receptors of sOPN are CD44 and various integrins.
iOPN was found at perimembrane regions of cells, but iOPN can also be distributed in cytoplasm [49, 71, 72]. Surprisingly, iOPN was detected in the nucleus [72](Shinohara et al., unpublished data). Although OPN does not have obvious nuclear localization signals, the last 100 amino acids at the C-terminal of OPN peptide appear to be responsible for OPN nuclear localization [72]. Nuclear iOPN is suggested to play a role in mitosis by physical association with polo-like kinase-1 (Plk-1)[72](Fig. 1), which triggers G2/M transition during mitosis.
iOPN was identified in the cytoplasm of pyramidal neurons in patients with advanced stages of Alzheimer’s disease (AD)[73]. The authors suggested that iOPN may play a role in cell cycle progression, neuronal remyelination, and/or the formation of protein aggregates in AD. iOPN is also found in cytoplasm of neuronal cells in both cortices and hippocampi during ischemia (Baliga et al., Submitted). Interestingly, only the right cortex exhibited elevated level of iOPN (Baliga et al., Submitted). It is of interest how iOPN is selectively expressed in the right cortex, instead of the left cortex.
Phagocytes, such as DCs and macrophages, produce high levels of Opn mRNA but do not secrete OPN as much as activated T cells do [37]. Phagocytes, therefore, tend to store OPN protein in cells (Shinohara et al. unpublished data). Although no study has documented the exact expression ratios of iOPN (in cytoplasm) vs. sOPN (in ER/Golgi and extracellular) in phagocytes and lymphocytes, it appears that phagocytes, or innate immune cells of myeloid origin, produce higher levels of iOPN than lymphocytes do. We compared levels of Opn mRNA (encoding both iOPN and sOPN) and secreted OPN in cell culture supernatants between activated T cells and LPS-stimulated macrophages [37]. Activated T cells could produce more than 50-fold of sOPN, detected in cell culture supernatants, per arbitrary unit of Opn mRNA than macrophages, regardless of the stimulation status of macrophages [37]. The data suggested that iOPN and sOPN participate mainly in innate and adaptive immunities, respectively [74]. Another difference between phagocytes and T cells is that unstimulated T cells produce little Opn mRNA, with the exception of CD4+ T memory cells from aged mice [75], whereas unstimulated macrophages and DCs constitutively express Opn mRNA and both iOPN and sOPN [37, 76].
5. How is OPN retained within cells?
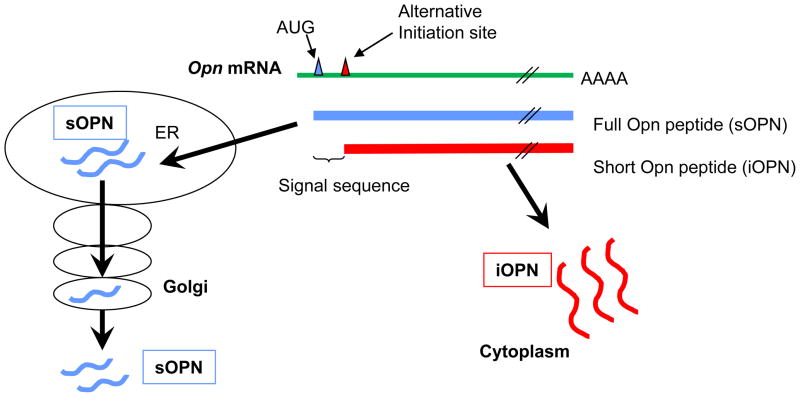
It has been a conundrum how OPN, which was considered to be a secreted protein, is localized in the cytoplasm. One explanation was generation of an isotype of OPN, which lacks the signal sequence but retains most of the OPN coding region [71](Fig. 2). Data suggested that such OPN cannot be formed by alternative transcription and/or alternative splicing. However, alternative translation initiation can produce an OPN protein without a signal sequence but with a majority of the coding region [71]. An Opn mutant construct lacking the first 39-nt of OPN peptide-encoding sequence produced a protein [71]. The 39-nt sequence encoded the N-terminal 13-aa of the OPN signal sequence consisting of 16-aa. Removing such a major part of signal sequence made the mutant OPN protein target to the cytoplasm, but not to Golgi [71]. Therefore, alternative translation initiation that produces an OPN peptide without the complete signal sequence is one explanation for the etiology of iOPN generation. Yet, there are still unanswered questions.
Figure 2. Alternative translation that generates iOPN.
Opn mRNA has the canonical AUG translation initiation site (blue triangle) and an alternative translation initiation site (red triangle). When translation starts from the canonical site, the peptide product (blue lines, sOPN) includes a signal sequence and is targeted to secretory vesicles. On the other hand, when translation starts from the alternative translation initiation site, the peptide product (red lines, iOPN) lacks a signal sequence and stays in the cell. Mouse OPN protein has 294-aa and the first 16-aa is a signal sequence. (Summarized from Shinohara et al. 2008, PNAS)
The first question is which amino acid initiates alternative translation to generate iOPN. Deletion of 48-nt completely abrogated the iOPN production [71], narrowing down to the 40~48-nt region as a sequence including either the alternative translation site or a sequence essential for alternative translation initiation. There is no AUG codon in the 40~48 nt sequence. Judging from immunoblotting data, the second methionine-encoding AUG does not appear to be the alternative translation site, because the second AUG is located too far downstream to produce the size of iOPN generated from the 39-nt deletion mutant Opn gene. Hence, the alternative translation start site must be non-AUG. Non-AUG alternative translation initiation codons were reported in genes encoding Fgf2 [77, 78], Fgf3 (Int-2)[79], Hck [80], and VEGF [81].
The second question is why the iOPN band generated from the 39-nt truncated Opn construct migrates almost at the same size as sOPN on SDS-PAGE gels (~5 kDa apart). iOPN is not supposed to have glycosylation, (because iOPN does not enter the Golgi) and is considered to migrate faster than sOPN.
The third unanswered question is how human cells produce iOPN. Based on histology data from human tissues, it appears that cytoplasmic OPN can be produced in human cells [45]. Indeed, Junaid et al. clearly detected intracellular OPN in human cells by confocal microscopy [72]. Thus, human cells appear to generate iOPN. Mouse OPN peptide has GIASS/LPVK (“/” denotes the cleave site by signal peptidase), whereas human OPN peptide has GITCA/IPVK, in the position 12–20 aa. Based on SDS-PAGE gel migration of iOPN generated from the 39-nt truncated Opn construct [71], the alternative translation initiation site of mouse iOPN is expected to be proximal to the signal sequence cleavage site. It is unknown where on an SDS-PAGE human iOPN appears. However, at least, three aa and one aa upstream and downstream (underlined) of the cleavage site, respectively, are not conserved between mice and humans. One possibility is that humans and mice do not share the same amino acid for translation initiation. Another possibility is that valine at the position 19-aa (Val19), or the codon position at 55~57-nt, could be an alternative translation site common to human and mouse OPN, and the 40~48-nt region is just essential for the translation initiation but not serving as the translation initiation site. GUG codon, encoding valine, is known to be an evolutionary conserved non-AUG alternative translation initiation site [82]. Although we have mutated GUG encoding Val19 to GUC and still observed the OPN doublet [71], the results are yet to be conclusive to determine whether the Val19 serves as the alternative translation initiation site. This is because iOPN (in the cytoplasm and in the nucleus) was not separated from sOPN (in secretory vesicles) before performing Western analysis. Here, separating total cell lysates on SDS-PAGE gels does not appear to discriminate iOPN from sOPN, because iOPN seems to migrate in a similar manner to sOPN does [71]. Therefore, separation of the cytoplasmic/nuclear fraction of the cells is necessary to identify iOPN by Western (Fig. 2).
We have discussed iOPN generation by alternative translation initiation, but the possibility of OPN retrotranslocation from the ER into the cytoplasm and/or incomplete targeting to secretory vesicles have not been ruled out yet.
6. Biological mechanisms that involve iOPN
Compared to sOPN, which has been extensively studied, the biological roles of iOPN have started to become clear only in the last several years. In addition to cell motility, cytoskeletal rearrangement, and mitosis [67–70, 72, 83, 84], iOPN plays a role as an adaptor molecule in signaling pathways downstream of innate immune receptors.
iOPN enhances IFNα expression through IRF7 activation upon TLR9 stimulation in pDCs [49]. TLR9 ligation promotes association of iOPN and MyD88, which can be co-immunoprecipitated [49](Fig. 1). The TLR9 pathway diverges into two different pathways, which activate IRF7 and NFκB, resulting in production of IFNα and pro-inflammatory cytokines, respectively. iOPN appears to be selectively involved in the IFNα production, because iOPN is essential to activate IRF7, a transcription factor inducing IFNα production. In contrast, the absence of iOPN does not alter NFκB activation by which proinflammatory cytokines are expressed [49]. Physiological outcomes of the iOPN-mediated IFNα production by TLR9-stimulated pDCs include enhancement of antigen cross-presentation, anti-viral activity against herpes simplex virus (HSV), and protection from lethal effects by B16N10 melanoma cells [49].
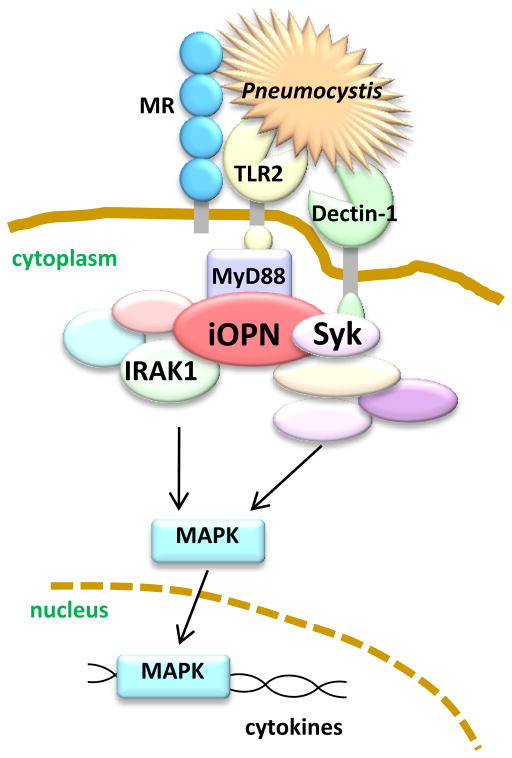
We have recently found that iOPN is also involved in TLR2 and dectin-1 pathways (Inoue et al., Submitted) in anti-fungal responses. We hypothesize that iOPN exerts dual functions upon detection of yeast (specifically the fungus, Pneumocystis murina). One function is to “anchor” multiple pattern-recognition receptors (PRRs) to form a receptor cluster (Fig. 3), while another is to induce anti-fungal responses as an adaptor molecule. We found that Opn−/− cells failed to form the PRR clusters, consisting of TLR2, dectin-1, and the mannose receptor (Fig. 3)(Inoue et al., Submitted). These three PRRs are known to be critical for Pneumocystis detection. In addition, iOPN associates with signaling molecules downstream of the TLR2 pathway (IRAK1) and a dectin-1 pathway (Syk)(Fig. 3); and, iOPN enhances MAPK activation (Inoue et al., Submitted).
Figure 3. Schematic model of iOPN clustering fungal PRRs and transducing signal to MAPK activation as an adaptor molecule.
Pneumocystis is simultaneously detected by dectin-1, MR, and TLR2. iOPN is essential for colocalization of dectin-1, TLR2, and MR. (It is likely that TLR2 is dimerized with TLR6, but we did not include TLR6 here for simplification.) In this figure, three PRRs co-localize and detect Pneumocystis for efficient detection and synergistic signal transduction. iOPN associates with IRAK1 (and MyD88 (Shinohara et al. 2000. Nat Immunol)) and Syk upon ligation of TLR2 and dectin-1, respectively. Simultaneous stimulation of TLR2 and dectin-1 requires the presence of iOPN for ERK MAPK activation to produce cytokines. (Inoue et al. Submitted)
It appears that iOPN is recruited to the TLR/MyD88 complex of certain TLRs upon ligation with a relevant ligand; for example, CpG-DNA for TLR9, and Pam2CSK4 for TLR2/6 (heterodimer of TLR2 and TLR6)[49](Inoue et al. submitted). Because the majority of TLR signaling pathways include MyD88, it is likely that iOPN is involved in additional TLR signaling pathways as well.
iOPN expressed by cDCs enhances IL-17-producing T helper (Th17) cell responses by suppressing the expression of IL-27, an IL-17 inhibitor produced by cDCs [40]. Since microglia in the central nervous system also showed iOPN-mediated IL-27 suppression, iOPN in cDCs and microglia is considered to induce Th17 responses upon autoantigen presentation during the progression of EAE [40]. This mechanism, at least in part, explains why IFNβ treatment controls progression of MS. This is because type-1 interferons, such as IFNβ, particularly suppress iOPN expression in cDCs [40, 85]. It must be noted that sOPN also plays a role in progression of EAE by enhancing survival of pathogenic T cells and induces Th17 responses [39, 50].
7. Future perspectives for iOPN in immunity
OPN has been studied as an extracellular molecule, but now iOPN, an intracellular isotype of OPN, is known to have various biological functions independent from those of sOPN. Since iOPN and sOPN are generated from the same Opn mRNA species, selective silencing of either iOPN or sOPN alone is not possible. Given this technical limitation, iOPN- or sOPN-specific knock-in mice will be useful to better understand distinct roles of iOPN and sOPN in vivo. Such knock-in mice will enable the evaluation of whether or not iOPN and sOPN could cause different biological outcomes, and will delineate some unexplained results. For example, OPN behaves as an anti-inflammatory molecule in an acute colitis model [86] and also as an anti-tumorigenic molecule [66]. That is -- why does OPN behaves in a manner that is opposite from expectations that are based upon earlier studies? It is possible that iOPN, which was not characterized before, may play a role in these findings. If sOPN exacerbates certain diseases and iOPN ameliorates the diseases, selective depletion of sOPN with an OPN neutralization antibody or OPN aptamer [87] would be a superior approach than silencing Opn mRNA, which will deplete both sOPN and iOPN. Thus, sOPN and iOPN may need to be independently targeted in therapies.
Based on recent studies, iOPN, like sOPN, can associate with various different proteins. In addition, iOPN is recruited to signaling complexes only when the signaling is triggered [49](Inoue et al. Submitted). Here, questions remain how iOPN is recruited and what biochemical activities (if any) it performs. A review article by Fedarko’s group [88] wrote that three-dimensional structure of the SIBLING protein family, including OPN, is “extended and flexible in solution, a property shared by a number of proteins that have multiple binding partners and that are involved in bridging macromolecular components.” It was 1986 when Heinegard and colleagues [2] coined the name “osteopontin,” because OPN was “a product of cells in the osteoid matrix and that it can form a bridge (Latin, pons) between cells and the mineral in the matrix.” One of the major biological roles of iOPN may literally be to bridge between proteins, as an adaptor or scaffold molecule in innate immune cells. Therefore, the name, osteopontin, indeed makes sense for iOPN.
Acknowledgments
We thank Dr. David Denhardt and Mr. Michael Brown for critical reading of our manuscript.
Citations
- 1.Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885–893. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- 2.Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig AM, Smith JH, Denhardt DT. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989;264:9682–9689. [PubMed] [Google Scholar]
- 4.Patarca R, Freeman GJ, Singh RP, Wei FY, Durfee T, Blattner F, et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989;170:145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saitoh Y, Kuratsu J, Takeshima H, Yamamoto S, Ushio Y. Expression of osteopontin in human glioma. Its correlation with the malignancy. Lab Invest. 1995;72:55–63. [PubMed] [Google Scholar]
- 6.Denhardt DT, Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl. 1998;30–31:92–102. [PubMed] [Google Scholar]
- 7.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liaw L, Almeida M, Hart CE, Schwartz SM, Giachelli CM. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994;74:214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- 9.Barry ST, Ludbrook SB, Murrison E, Horgan CM. Analysis of the alpha4beta1 integrin-osteopontin interaction. Exp Cell Res. 2000;258:342–351. doi: 10.1006/excr.2000.4941. [DOI] [PubMed] [Google Scholar]
- 10.Barry ST, Ludbrook SB, Murrison E, Horgan CM. A regulated interaction between alpha5beta1 integrin and osteopontin. Biochem Biophys Res Commun. 2000;267:764–769. doi: 10.1006/bbrc.1999.2032. [DOI] [PubMed] [Google Scholar]
- 11.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, et al. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 12.Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D, et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21:539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Sato I, Yamamoto N, Yamazaki H, Hashimoto S, Hino M, Sakai F, et al. Prevention of the cryptic epitope SLAYGLR within osteopontin does not influence susceptibility to Candida albicans infection. Antimicrob Agents Chemother. 2005;49:3053–3055. doi: 10.1128/AAC.49.7.3053-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003;112:181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 16.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 17.Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sorensen ES. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem. 2007;282:19463–19472. doi: 10.1074/jbc.M703055200. [DOI] [PubMed] [Google Scholar]
- 18.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 19.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72:752–761. [PubMed] [Google Scholar]
- 20.Kazanecki CC, Kowalski AJ, Ding T, Rittling SR, Denhardt DT. Characterization of anti-osteopontin monoclonal antibodies: Binding sensitivity to post-translational modifications. J Cell Biochem. 2007;102:925–935. doi: 10.1002/jcb.21487. [DOI] [PubMed] [Google Scholar]
- 21.Castellano G, Malaponte G, Mazzarino MC, Figini M, Marchese F, Gangemi P, et al. Activation of the osteopontin/matrix metalloproteinase-9 pathway correlates with prostate cancer progression. Clin Cancer Res. 2008;14:7470–7480. doi: 10.1158/1078-0432.CCR-08-0870. [DOI] [PubMed] [Google Scholar]
- 22.Chiocchetti A, Indelicato M, Bensi T, Mesturini R, Giordano M, Sametti S, et al. High levels of osteopontin associated with polymorphisms in its gene are a risk factor for development of autoimmunity/lymphoproliferation. Blood. 2004;103:1376–1382. doi: 10.1182/blood-2003-05-1748. [DOI] [PubMed] [Google Scholar]
- 23.Mochida S, Hashimoto M, Matsui A, Naito M, Inao M, Nagoshi S, et al. Genetic polymorphims in promoter region of osteopontin gene may be a marker reflecting hepatitis activity in chronic hepatitis C patients. Biochem Biophys Res Commun. 2004;313:1079–1085. doi: 10.1016/j.bbrc.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 24.D’Alfonso S, Barizzone N, Giordano M, Chiocchetti A, Magnani C, Castelli L, et al. Two single-nucleotide polymorphisms in the 5′ and 3′ ends of the osteopontin gene contribute to susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2005;52:539–547. doi: 10.1002/art.20808. [DOI] [PubMed] [Google Scholar]
- 25.Chiocchetti A, Comi C, Indelicato M, Castelli L, Mesturini R, Bensi T, et al. Osteopontin gene haplotypes correlate with multiple sclerosis development and progression. J Neuroimmunol. 2005;163:172–178. doi: 10.1016/j.jneuroim.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Marciano R, D’Annunzio G, Minuto N, Pasquali L, Santamaria A, Di Duca M, et al. Association of alleles at polymorphic sites in the Osteopontin encoding gene in young type 1 diabetic patients. Clin Immunol. 2009;131:84–91. doi: 10.1016/j.clim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 27.de las Fuentes L, Gu CC, Mathews SJ, Reagan JL, Ruthmann NP, Waggoner AD, et al. Osteopontin promoter polymorphism is associated with increased carotid intima-media thickness. J Am Soc Echocardiogr. 2008;21:954–960. doi: 10.1016/j.echo.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao B, Yasui T, Itoh Y, Li Z, Okada A, Tozawa K, et al. Association of osteopontin gene haplotypes with nephrolithiasis. Kidney Int. 2007;72:592–598. doi: 10.1038/sj.ki.5002345. [DOI] [PubMed] [Google Scholar]
- 29.Schultz J, Lorenz P, Ibrahim SM, Kundt G, Gross G, Kunz M. The functional -443T/C osteopontin promoter polymorphism influences osteopontin gene expression in melanoma cells via binding of c-Myb transcription factor. Mol Carcinog. 2009;48:14–23. doi: 10.1002/mc.20452. [DOI] [PubMed] [Google Scholar]
- 30.Mirza M, Shaughnessy E, Hurley JK, Vanpatten KA, Pestano GA, He B, et al. Osteopontin-c is a selective marker of breast cancer. Int J Cancer. 2008;122:889–897. doi: 10.1002/ijc.23204. [DOI] [PubMed] [Google Scholar]
- 31.He B, Mirza M, Weber GF. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene. 2006;25:2192–2202. doi: 10.1038/sj.onc.1209248. [DOI] [PubMed] [Google Scholar]
- 32.Young MF, Kerr JM, Termine JD, Wewer UM, Wang MG, McBride OW, et al. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN) Genomics. 1990;7:491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]
- 33.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Buback F, Renkl AC, Schulz G, Weiss JM. Osteopontin and the skin: multiple emerging roles in cutaneous biology and pathology. Exp Dermatol. 2009;18:750–759. doi: 10.1111/j.1600-0625.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- 35.Philip S, Kundu GC. Osteopontin induces nuclear factor kappa B-mediated promatrix metalloproteinase-2 activation through I kappa B alpha/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J Biol Chem. 2003;278:14487–14497. doi: 10.1074/jbc.M207309200. [DOI] [PubMed] [Google Scholar]
- 36.Das R, Philip S, Mahabeleshwar GH, Bulbule A, Kundu GC. Osteopontin: it’s role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression. IUBMB Life. 2005;57:441–447. doi: 10.1080/15216540500159424. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci U S A. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 2008;181:7480–7488. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohshima S, Yamaguchi N, Nishioka K, Mima T, Ishii T, Umeshita-Sasai M, et al. Enhanced local production of osteopontin in rheumatoid joints. J Rheumatol. 2002;29:2061–2067. [PubMed] [Google Scholar]
- 42.Sato T, Nakai T, Tamura N, Okamoto S, Matsuoka K, Sakuraba A, et al. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut. 2005;54:1254–1262. doi: 10.1136/gut.2004.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, et al. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension. 2004;43:1195–1201. doi: 10.1161/01.HYP.0000128621.68160.dd. [DOI] [PubMed] [Google Scholar]
- 44.Weiss JM, Renkl AC, Maier CS, Kimmig M, Liaw L, Ahrens T, et al. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J Exp Med. 2001;194:1219–1229. doi: 10.1084/jem.194.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 46.Vogt MH, Lopatinskaya L, Smits M, Polman CH, Nagelkerken L. Elevated osteopontin levels in active relapsing-remitting multiple sclerosis. Ann Neurol. 2003;53:819–822. doi: 10.1002/ana.10606. [DOI] [PubMed] [Google Scholar]
- 47.Yumoto K, Ishijima M, Rittling SR, Tsuji K, Tsuchiya Y, Kon S, et al. Osteopontin deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Proc Natl Acad Sci U S A. 2002;99:4556–4561. doi: 10.1073/pnas.052523599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 51.Chen TH, Chang CF, Yu SC, Wang JC, Chen CH, Chan P, et al. Dipyridamole inhibits cobalt chloride-induced osteopontin expression in NRK52E cells. Eur J Pharmacol. 2009;613:10–18. doi: 10.1016/j.ejphar.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 52.Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, et al. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106:946–955. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 53.Xanthou G, Alissafi T, Semitekolou M, Simoes DC, Economidou E, Gaga M, et al. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13:570–578. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shinohara ML, Cantor H. The bridge between dendritic cells and asthma. Nat Med. 2007;13:536–538. doi: 10.1038/nm0507-536. [DOI] [PubMed] [Google Scholar]
- 55.Begum MD, Umemura M, Kon S, Yahagi A, Hamada S, Oshiro K, et al. Suppression of the bacterial antigen-specific T cell response and the dendritic cell migration to the lymph nodes by osteopontin. Microbiol Immunol. 2007;51:135–147. doi: 10.1111/j.1348-0421.2007.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 56.Kurokawa M, Konno S, Takahashi A, Plunkett B, Rittling SR, Matsui Y, et al. Regulatory role of DC-derived osteopontin in systemic allergen sensitization. Eur J Immunol. 2009;39:3323–3330. doi: 10.1002/eji.200838970. [DOI] [PubMed] [Google Scholar]
- 57.Simoes DC, Xanthou G, Petrochilou K, Panoutsakopoulou V, Roussos C, Gratziou C. Osteopontin deficiency protects against airway remodeling and hyperresponsiveness in chronic asthma. Am J Respir Crit Care Med. 2009;179:894–902. doi: 10.1164/rccm.200807-1081OC. [DOI] [PubMed] [Google Scholar]
- 58.Wai PY, Guo L, Gao C, Mi Z, Guo H, Kuo PC. Osteopontin inhibits macrophage nitric oxide synthesis to enhance tumor proliferation. Surgery. 2006;140:132–140. doi: 10.1016/j.surg.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Guo H, Wai PY, Mi Z, Gao C, Zhang J, Kuo PC. Osteopontin mediates Stat1 degradation to inhibit iNOS transcription in a cecal ligation and puncture model of sepsis. Surgery. 2008;144:182–188. doi: 10.1016/j.surg.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang SM, Lopez CA, Heck DE, Gardner CR, Laskin DL, Laskin JD, et al. Osteopontin inhibits induction of nitric oxide synthase gene expression by inflammatory mediators in mouse kidney epithelial cells. J Biol Chem. 1994;269:711–715. [PubMed] [Google Scholar]
- 61.Rittling SR, Matsumoto HN, McKee MD, Nanci A, An XR, Novick KE, et al. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 1998;13:1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- 62.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katagiri YU, Murakami M, Mori K, Iizuka J, Hara T, Tanaka K, et al. Non-RGD domains of osteopontin promote cell adhesion without involving alpha v integrins. J Cell Biochem. 1996;62:123–131. doi: 10.1002/(sici)1097-4644(199607)62:1<123::aid-jcb13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 64.Sato I, Yamamoto N, Rittling SR, Denhardt DT, Hino M, Morimoro J, et al. Osteopontin is dispensable for protection against high load systemic fungal infection. Int Immunopharmacol. 2008;8:1441–1448. doi: 10.1016/j.intimp.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Iwata D, Kitamura M, Kitaichi N, Saito Y, Kon S, Namba K, et al. Prevention of experimental autoimmune uveoretinitis by blockade of osteopontin with small interfering RNA. Exp Eye Res. 90:41–48. doi: 10.1016/j.exer.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Bourassa B, Monaghan S, Rittling SR. Impaired anti-tumor cytotoxicity of macrophages from osteopontin-deficient mice. Cell Immunol. 2004;227:1–11. doi: 10.1016/j.cellimm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, et al. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–167. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 68.Zohar R, Lee W, Arora P, Cheifetz S, McCulloch C, Sodek J. Single cell analysis of intracellular osteopontin in osteogenic cultures of fetal rat calvarial cells. J Cell Physiol. 1997;170:88–100. doi: 10.1002/(SICI)1097-4652(199701)170:1<88::AID-JCP10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 69.Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, et al. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki K, Takeyama S, Sakai Y, Yamada S, Shinoda H. Current topics in pharmacological research on bone metabolism: inhibitory effects of bisphosphonates on the differentiation and activity of osteoclasts. J Pharmacol Sci. 2006;100:189–194. doi: 10.1254/jphs.fmj05004x2. [DOI] [PubMed] [Google Scholar]
- 71.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci U S A. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Junaid A, Moon MC, Harding GE, Zahradka P. Osteopontin localizes to the nucleus of 293 cells and associates with polo-like kinase-1. Am J Physiol Cell Physiol. 2007;292:C919–926. doi: 10.1152/ajpcell.00477.2006. [DOI] [PubMed] [Google Scholar]
- 73.Wung JK, Perry G, Kowalski A, Harris PL, Bishop GM, Trivedi MA, et al. Increased expression of the remodeling- and tumorigenic-associated factor osteopontin in pyramidal neurons of the Alzheimer’s disease brain. Curr Alzheimer Res. 2007;4:67–72. doi: 10.2174/156720507779939869. [DOI] [PubMed] [Google Scholar]
- 74.Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol. 2009;9:137–141. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimatani K, Nakashima Y, Hattori M, Hamazaki Y, Minato N. PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc Natl Acad Sci U S A. 2009;106:15807–15812. doi: 10.1073/pnas.0908805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nystrom T, Duner P, Hultgardh-Nilsson A. A constitutive endogenous osteopontin production is important for macrophage function and differentiation. Exp Cell Res. 2007;313:1149–1160. doi: 10.1016/j.yexcr.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 77.Bugler B, Amalric F, Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991;11:573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnaud E, Touriol C, Boutonnet C, Gensac MC, Vagner S, Prats H, et al. A new 34-kilodalton isoform of human fibroblast growth factor 2 is cap dependently synthesized by using a non-AUG start codon and behaves as a survival factor. Mol Cell Biol. 1999;19:505–514. doi: 10.1128/mcb.19.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acland P, Dixon M, Peters G, Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990;343:662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- 80.Lock P, Ralph S, Stanley E, Boulet I, Ramsay R, Dunn AR. Two isoforms of murine hck, generated by utilization of alternative translational initiation codons, exhibit different patterns of subcellular localization. Mol Cell Biol. 1991;11:4363–4370. doi: 10.1128/mcb.11.9.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huez I, Bornes S, Bresson D, Creancier L, Prats H. New vascular endothelial growth factor isoform generated by internal ribosome entry site-driven CUG translation initiation. Mol Endocrinol. 2001;15:2197–2210. doi: 10.1210/mend.15.12.0738. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi K, Maruyama M, Tokuzawa Y, Murakami M, Oda Y, Yoshikane N, et al. Evolutionarily conserved non-AUG translation initiation in NAT1/p97/DAP5 (EIF4G2) Genomics. 2005;85:360–371. doi: 10.1016/j.ygeno.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki K, Zhu B, Rittling SR, Denhardt DT, Goldberg HA, McCulloch CA, et al. Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts. J Bone Miner Res. 2002;17:1486–1497. doi: 10.1359/jbmr.2002.17.8.1486. [DOI] [PubMed] [Google Scholar]
- 84.Zohar R, Cheifetz S, McCulloch CA, Sodek J. Analysis of intracellular osteopontin as a marker of osteoblastic cell differentiation and mesenchymal cell migration. Eur J Oral Sci. 1998;106 (Suppl 1):401–407. doi: 10.1111/j.1600-0722.1998.tb02206.x. [DOI] [PubMed] [Google Scholar]
- 85.Chen M, Chen G, Nie H, Zhang X, Niu X, Zang YC, et al. Regulatory effects of IFN-beta on production of osteopontin and IL-17 by CD4+ T Cells in MS. Eur J Immunol. 2009;39:2525–2536. doi: 10.1002/eji.200838879. [DOI] [PubMed] [Google Scholar]
- 86.Heilmann K, Hoffmann U, Witte E, Loddenkemper C, Sina C, Schreiber S, et al. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mi Z, Guo H, Russell MB, Liu Y, Sullenger BA, Kuo PC. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol Ther. 2009;17:153–161. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]