Abstract
Pulmonary hypertension is a common complication of sickle cell disease (SCD) and a risk factor for early death. Hemolysis may participate in its pathogenesis by limiting nitric oxide (NO) bioavailability and producing vasculopathy. We hypothesized that hemoglobin mutations that diminish hemolysis in SCD would influence pulmonary hypertension susceptibility. Surprisingly, coincident α-thalassemia (OR = 0.95, 95% CI = 0.46 – 1.94, P = NS) was not associated with pulmonary hypertension susceptibility in homozygous SCD. However, pulmonary hypertension cases were less likely to have hemoglobin SC (Odds Ratio [OR] = 0.18, 95% confidence interval [CI] = 0.06 to 0.51, P = 0.0005) or Sβ+ thalassemia (OR = 0.25, 95% CI = 0.06 to 1.16, P = 0.10). These compound heterozygotes may be protected from pulmonary hypertension because of reduced levels of intravascular hemolysis, but develop this complication at a lower rate possibly due to the presence of non-hemolytic risk factors such as renal dysfunction, iron overload and advancing age. Despite this protective association, patients with SC who did develop pulmonary hypertension remained at significant risk for death during 49 months of follow-up (Hazard Ratio=8.20, P=0.0057).
INTRODUCTION
Sickle cell disease (SCD) is a common Mendelian disease caused by mutations in the β-globin subunit of hemoglobin. Homozygosity for sickle hemoglobin results in a series of cellular alterations of red cell morphology and function that shorten red cell lifespan and lead to vascular occlusion by rigid or sickled erythrocytes with consequent tissue damage in target organs.1 Chronic hemolytic anemia is also a feature of SCD that may be associated with the development of a progressive vasculopathy, pulmonary hypertension, cutaneous leg ulceration, priapism and possibly stroke.2-5 Ultimately, target organ damage results in organ dysfunction and premature death, typically in the fifth decade of life.6
Among the manifestations of target organ damage, pulmonary hypertension has been identified as a frequent complication. Recent screening studies using Doppler echocardiography and N-terminal pro-BNP (NTproBNP) suggest that pulmonary hypertension occurs in approximately one third of adults with SCD. Furthermore, elevated pulmonary pressures are associated with an approximate ten-fold increased risk of early death, making this complication a leading predictor of mortality.5,7-10 Intravascular hemolysis, which inhibits nitric oxide (NO) bioavailability via the release of red cell hemoglobin and arginase into plasma, has been proposed as one of several etiologic mechanisms.5,11,12 Other independent risk factors associated with pulmonary hypertension include anemia, hepatic congestion, iron overload, renal insufficiency, and possibly hyposplenism.5
Genetic determinants, in addition to environmental exposures, are hypothesized to influence the risk of developing this and other associated manifestations of SCD.13 To date, genetic factors have not been shown to contribute to the development of SCD pulmonary hypertension, although some have speculated that mutations known to affect the rate of SCD related hemolysis like α-thalassemia might influence susceptibility to this complication.14,15 In anticipation of an emerging era of low cost whole genome re-sequencing and personalized medicine16,17, we have performed targeted sequencing and genotyping of globin genes containing hemoglobin mutations on 2 chromosomes to determine their role in susceptibility to pulmonary hypertension in a well-characterized adult SCD cohort.
RESULTS
Cohort and Effect of Age on Pulmonary Hypertension
Of 282 individuals screened, 260 unrelated subjects had both a DNA sample and echocardiogram for analysis (Table I). Overall, pulmonary hypertension defined solely by TRV was identified in 36.5% of subjects, and pulmonary hypertension cases were significantly older than the SCD control group (P<0.0001, Table I). Typing at 15 unlinked STR loci and the Amelogenin (AMEL) locus confirmed DNA from 260 unique individuals used in the case control study. In addition, 5 pairs of first degree relatives were identified by family history and by calculating a cumulative sibship index (CSI) using the 15 STR markers, where a CSI > 3.0 suggests a sibling (mean CSI for relatives = 1,235,693, range 15,225-3,468,704). Thus, of 22 (7.8%) cases excluded from further genetic analyses, 11 (3.9%) did not submit a DNA sample, 4 (1.4%) did not have an echocardiogram, and 7 (2.5%) were censored from analysis (2 for known HIV infection [an additional risk factor for pulmonary hypertension], and 5 were first degree relatives).
Table I.
NIH-Howard University SCD Population Demographics.
| Characteristic | PH Screening Population |
|---|---|
| Total subjects screened (n, %) | 282 (100.0%) |
| Total subjects, genetic analysis (n, %) | 260 (92.2%) |
| Subjects censored from analysis (n, %) | 22 (7.8%)a |
| First degree relatives (n, %) | 10 (3.5%)b |
| Age, overall (yrs., mean and SD) | 36.1 (11.5) |
| Age, PH cases (yrs., mean and SD) | 41.0 (12.4)c |
| Age, controls (yrs., mean and SD) | 33.8 (10.3) |
| Female (n, %) | 163 (58.0%) |
| African American/African (n, %) | 266 (94.7%) |
| Hydroxyurea treatment (n, %) | 89 (35.9%)d |
| Presence of Hb A (>5% Hb A) | 98 (38.1%)d |
| PH cases by TRV (n, %) | 95 (36.5%)e |
| PH cases by NTproBNP (n, %) | 56 (24.4%)f |
| Concordant PH phenotypes (n, %) | 184 (80.3%)g |
PH=pulmonary hypertension.
Subjects censored from analysis included 4 (1.4%) with no echocardiogram, 11 (3.9%) with no available DNA, 5 (1.8%) first degree relatives of another study subject, and 2 (0.7%) with known HIV infection.
PH cases defined by TRV are significantly older than SCD controls, Alternate Welch's t-test P<0.0001.
248 subjects had available data regarding hydroxyurea use and 257 subjects had quantitative HPLC.
Percentage of 260 subjects available for genetic analysis with PH defined by a TRV greater than or equal to 2.5 m/s.
Percentage of 229 subjects available for genetic analysis with PH retrospectively defined as an NTproBNP ≥ 160 pg/mL.
Percentage of 229 subjects with concordant phenotypic assignments as a PH case or a control without PH based upon both echocardiogram TRV and NTproBNP.
Allelic Spectrum and Prevalence of HBB Mutations in SCD
From a total of 271 samples (inclusive of the subjects censored from analysis above), 13 mutations underlying the diagnosis of SCD (Table SI) and an additional 16 single nucleotide variants were identified by sequencing the entire HBB gene (Figure 1). Homozygous SCD (SS) was most frequent (203 cases, 74.9%), in addition to 68 compound heterozygotes (25.1%; Table SI). The presence of coincident α-thalassemia (α3.7 deletion) was also determined in 260 of all SCD patients (96%, n=271): 7 ( 2.7%) had the -α/-α genotype, 73 (28.1%) with -α/αα, 179 (68.8%) with αα/αα, and 1 individual or 0.4% with ααα/αα. No α4.2 deletions were detected (93%, n=252 out of total=271).
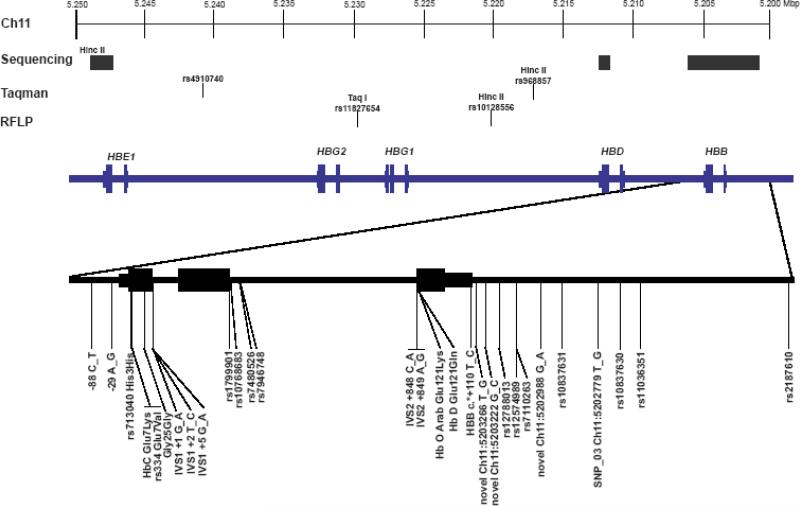
Figure 1. Overview of Chromosome 11 Markers.
The region of Chromosome 11 investigated in this study is schematically represented. At the top of the figure, chromosomal coordinates in the human genome are presented (March 2006 build, hg18). Below these coordinates, the methods utilized to type specific markers are presented (either sequencing, Taqman, or RFLP). Finally, the locations of the individual globin genes are illustrated on the blue line with exons presented as larger blocks on the line with the gene name presented above the exon blocks. The sequenced region of the HBB gene (with the 3 exons represented as blocks on the line) is expanded below with the locations of 29 individual single nucleotide variants (including 4 new variants that are not presently included in the dbSNP database).
Comparison of Hemoglobin Phenotypes by HPLC to Sequence Verified HBB Genotypes
A high prevalence of detectable HbA (38.1%) by high performance liquid chromatography (HPLC) due to either recent transfusion therapy or Sβ+ thalassemia was noted when reviewing the demographics of the study cohort (Table I). Ten (5.3%) out of 190 hemoglobin phenotypes assigned by HPLC with hemoglobin electrophoresis previously published for the initial cohort subjects were incorrect when compared to the sequence verified genotypes (P=0.0017, Table SII).5 The high prevalence of detectable HbA resulted in a positive predictive value of only 65% (P<0.0001) for the diagnosis of Sβ thalassemia in this original cohort.
HBB Mutations in SCD Pulmonary Hypertension
The effect of the β globin locus on pulmonary hypertension was first examined using 17 markers present across 46,465 base pairs on chromosome 11 (Figure 2A). Four haplotype blocks are defined by 14 informative markers (Figure 2A and 2B).18,19 Nearly every subject carries the Hb C mutation on a single haplotype that spans all 4 blocks (Block 1 CGTAT, Block 2 AA, Block 3 GG, and Block 4 CGTTA; Figure 2B). Significant associations with pulmonary hypertension defined by TRV are observed for Markers 6 (rs334 or Hb S, P=0.0439, age adjusted P=0.0032), 7 (HbC, P=0.0163, age adjusted P=0.0050), 15 (SNP_02, P=0.0369, age adjusted P=0.0150), and 17 (SNP_01, P=0.1770, age adjusted P=0.0425) (Figure 2C). The age adjusted analysis is presented due to the significant effect of this variable upon the prevalence of pulmonary hypertension (Table I). The occurrence of the hemoglobin C mutation on a single haplotype defined by markers 7 (HbC), 15, and 17 with strong linkage disequilibrium (LD) between the latter two markers and the hemoglobin C mutation (LOD=91 or r2=0.78 and LOD=93 or r2=0.10, respectively) explains the pattern of individual associations attributable to the effect of a single haplotype. Consistent with this explanation, association analysis by TRV was more sensitive when analyzed by haplotype blocks comprising the hemoglobin C haplotype (Table SIII). Strong associations for Markers 6 (P=0.0014, age adjusted P=0.0059) and 7 (P=0.0633, age adjusted P=0.0010) are again observed when 56 pulmonary hypertension cases defined only by NTproBNP levels were compared to 178 SCD controls (Figure 2D).
Figure 2. Haplotypes of the β Globin Locus on Chromosome 11 in Adults with Sickle Cell Disease.
(A) Pattern of pairwise linkage disequilibrium (LD) for 17 SNP markers in 260 unrelated patients with SCD. Red squares indicate strong LD and values within each square are LD measurements by LOD score. The outlined blocks represent haplotype blocks. The sickle and hemoglobin C mutations are markers 6 and 7, respectively. (B) The haplotypes of the β globin locus observed in 260 adults with SCD as the blocks defined in panel A. Lines connecting each haplotype block are in bold when observed with a frequency of greater than 5% and the fraction adjacent to each block is the observed haplotype frequency. Note that the hemoglobin C mutation is carried on a nearly unique haplotype across the 4 blocks which is distinguished from all other haplotypes by markers 7 (HbC) and 15 (SNP_02). (C) Age corrected P values for each single marker association comparing PH cases defined by echocardiography to controls among 260 patients with SCD. P values are presented as the |Log10 P value| where P=0.05 is 1.3 (indicated by red dashed line). (D) Age corrected P values for each single marker association comparing PH cases defined by NTproBNP to controls among 177 patients with SCD.
The prevalence of hemoglobin phenotypes (SS, SC, and Sβ+ thalassemia) assigned by interpretation of specific HBB mutations was significantly different between cases and controls at the locus (Table II, 3 × 2 table, X2 = 7.445 with 2 degrees of freedom and no age adjustment, P = 0.0242). In Table II, age adjusted odds ratios for individual hemoglobin phenotypes suggest a trend where those with hemoglobin SC are less susceptible to pulmonary hypertension relative to other SCD patients (Odds Ratio 0.19, 95% confidence interval 0.07 to 0.51, P=0.0004), those with Sβ+ thalassemia are at intermediate risk (Odds Ratio 0.28, 95% confidence interval 0.06 to 1.11, P=0.08), and those with homozygous SCD are at the greatest risk as the reference group (Odds ratio=1.00). Furthermore, the effects for hemoglobin SC (P=0.0005) and sickle β+ thalassemia (P=0.0784) are essentially unchanged when the effect of co-incident α-thalassemia is also incorporated into this analysis (Table II).
Table II.
Hemoglobin Phenotype is Associated with Pulmonary Hypertension Susceptibility.
| Hb Phenotype | PH Cases Number (%) | Controls Number (%) | OR (95% CI)a | P value | OR (95% CI)b | P value |
|---|---|---|---|---|---|---|
| SC | 8 (8.4) | 35 (21.2) | 0.19 (0.07-0.51) | 0.0004 | 0.18 (0.06-0.51) | 0.0005 |
| Sβ+ thalassemia | 4 (4.2) | 12 (7.3) | 0.28 (0.06-1.11) | 0.0819 | 0.26 (0.06-1.09) | 0.0784 |
| SS | 79 (83.2) | 114 (69.7) | 1.00 (reference) | - | - | - |
| SS α- thalassemia | 23 (24.2) | 35 (21.2) | - | - | 0.94 (0.46-1.92) | 0.9941 |
| SS only | 55 (58.5) | 74 (44.8) | - | - | 1.00 (reference) | - |
| Sβ0 thalassemia | 2 (2.1) | 1 (0.6) | 3.27 (0.14-187.04) | 0.7688 | 3.09 (0.14-176.97) | 0.7905 |
| Totalc | 95 (100.0) | 165 (100.0) |
PH = pulmonary hypertension prospectively defined as a Doppler tricuspid regurgitant jet velocity of greater than or equa to 2.5 meters/second. OR=odds ratio. 95% CI=95% confidence interval. P values were determined by Mantel Haenzel summary chi-squares or Fisher exact tests where appropriate.
Age adjusted Odds Ratio set in reference to all HbSS subjects (inclusive of those with HbSS and coincident α-thalassemia).
Age adjusted OR set in reference to those with HbSS without coincident α- thalassemia (SS only).
Includes the uncharacterized Sβ thalassemia, Hb SD and SO Arab subjects.
X2 = 7.445 (3 × 2 table where all thalassemia heterzygotes were combined into 1 category, 2 df), P = 0.0242 for comparison of genotype distributions between pulmonary hypertension cases and controls at the locus.
Haplotype Blocks of the β-Globin Locus and Pulmonary Hypertension in Sickle Cell Anemia
Three common βS haplotypes predominate in this population in agreement with other studies20-22, and these β globin locus haplotypes do not appear to have a strong effect on the prevalence of pulmonary hypertension (Table III, Figure S1).
Table III.
Relationship Between Multimarker βS Globin Haplotypes Defined by this Study, Classical βS Haplotypes, and Pulmonary Hypertension.
| Haplotypea | Classical βS Haplotype | Overall Cohort (n=384)b | PH Cases (n=156)d | Controls (n=228) |
|---|---|---|---|---|
| GTTTCAGT0GCG | Benin | 211 (54.9%) | 92 (59.0%) | 119 (52.2%) |
| AGGCCGAC0CTA | CAR/Bantu | 67 (17.5%) | 28 (17.9%) | 39 (17.1%) |
| AGGTTGGC0CTA | Senegalc | 36 (9.4%) | 14 (9.0%) | 22 (9.6%) |
| GTTTCGGC0GTA | Cameroon | 16 (4.2%) | 4 (2.6%) | 12 (5.3%) |
| Recombinants | Atypicalc | 54 (14.0%) | 18 (11.5%) | 36 (15.8%) |
Haplotypes were determined by consistent runs of PHASE 2.1 using all 12 markers displayed (rsl 106351; SNP_03; rs7480526; rs968857; rs10128556; rs11827654; rs4910740;rs3759071; rs3834466; rs3759070; rs3759069; SNP_01).51,52 Overall, 30 different haplotypes were observed in the overall cohort with 86% representing the 4 classical βS haplotypes (defined by markers rs968857 [Hinc II], rs10128556 [Hinc II], rs11827654 [Taq 1], and rs3834466 [HBE Hinc II] as underlined above).46
n refers to the number of chromosomes.
The Senegal and Arabian/Indian βS haplotypes are classically reported to carry an XmnI RFLP site 5′ to the HBG2 gene (rs7482144) that was not typed in this study.46 rs3834466 (HBE Hinc II) is one marker that classically defines the Arabian/Indian βS haplotype. However, rs3834466 is carried on four different 12 marker haplotypes (or on non-Arabian/Indian atypical βS haplotypes) in this population, suggesting that only carriers of the classical Senegal haplotype would be expected to have the rs7482144 allele corresponding to the XmnI cut site.
PH=pulmonary hypertension as defined by TRV. The overall comparison of classical βS haplotypes between cases and controls was not significant (5 × 2 table, P=0.4528), and this was also observed for comparison of the 12 marker haplotypes between the cases and controls using the –c option in PHASE (1000 permutations, P=0.34).
Absence of a Strong Association of Coincident α-Thalassemia and Pulmonary Hypertension
For the initial analysis of the α-globin locus, Human Haplotype Map data (www.hapmap.org) was used to identify 4 haplotype tagged SNPs (rs8051004, rs3785309, rs45110012, and rs2858016) across 36,655 base pairs in the Yorbuan population using pairwise tagging in Haploview. Neither the 3 successfully genotyped htSNPs nor the α-thalassemia α3.7 deletion are significantly associated with pulmonary hypertension in homozygous SCD (Figure S2) or SCD overall (adjusted P=0.7078 for α3.7, data not shown). In homozygous SCD, 23 with pulmonary hypertension have α-thalassemia (29.5% combining -/- and -/α genotypes, n=78 with PH) versus 35 SS α-thalassemia controls (31.8%, n=110 controls) (P=0.9941, Table II).
Likelihood of False Positive Associations
Validity of the genetic associations in Figures 2, S1, and S2 was further assessed using the Bayesian FPRP method at 6 loci where significance was below our uncorrected threshold of α ≤ 0.05 (Table SIV).23 Associations for SNP_01, SNP_02, Hb C, and rs334 from Figure 2C yielded FPRP values below our criterion of 0.2, indicating that these are likely true positives. Protective associations for markers HbC and rs334 and the high NTproBNP phenotype (Figure 2D) are also unlikely false positive results.
Hemolysis and Other Clinical Risk Factors for Pulmonary Hypertension in Hemoglobin SS and SC
Previous studies have demonstrated that those with a high TRV are distinguished from other SCD patients by increasing age, systolic hypertension, low pulse oximetry measurements, a history of renal problems, iron overload, elevated alkaline phosphatase, decreased plasma transferrin levels, and markers of red cell hemolysis.4,5 Table III again illustrates this pattern of univariate associations with pulmonary hypertension in Hb SS, including a lower arginine:ornithine ratio (P=0.0219). Neither markers of inflammation (e.g. WBC) or nor the rate of vaso-occlusive pain events are different between the two groups as seen in prior studies (Table IV).
Table IV.
Clinical and Laboratory Characteristics of Hb SS Patients with and without Pulmonary Hypertension.
| Characteristic | Hb SS TRV greater than 2.5 m/s | Hb SS TRV less than 2.5 m/s | P valuea | ||
|---|---|---|---|---|---|
| n | Mean (SD) or n (%) | n | Mean (SD) or n (%) | ||
| Age (yrs.) | 79 | 38.9 (12.1) | 115 | 32.3 (9.5) | 0.0001b |
| Sex (female) | 79 | 45 (57.0%) | 115 | 66 (57.3%) | 1.0000d |
| Hemoglobin (gm/dL) | 74 | 8.4 (1.8) | 74 | 9.3 (1.4) | 0.0007 |
| Hematocrit (%) | 74 | 24.4 (5.6) | 112 | 27.1 (4.3) | 0.0009 |
| Hb F (gm/dL) | 71 | 0.61 (0.59) | 109 | 0.91 (0.73) | 0.0036 |
| Hb F (%) | 71 | 7.0 (5.8) | 109 | 9.6 (6.8) | 0.0102 |
| Reticulocytes, absolute (K/μL) | 71 | 270.7 (131.2) | 107 | 279.1 (127.8) | 0.7664b |
| WBC (K/μL) | 70 | 10.7 (3.4) | 111 | 10.7 (3.4) | 0.9499 |
| LDH (U/L) | 68 | 472.8 (224.7) | 100 | 352.8 (125.1) | <0.0001c |
| AST (U/L) | 69 | 51.1 (22.7) | 109 | 42.4 (21.9) | 0.0033 |
| ALT (U/L) | 70 | 28.7 (14.6) | 110 | 28.1 (19.1) | 0.5825 |
| Total bilirubin (mg/dL) | 73 | 3.2(1.7) | 110 | 2.9 (1.8) | 0.2034 |
| Direct bilirubin (mg/dL) | 73 | 0.7 (0.5) | 111 | 0.5 (0.2) | 0.0007b |
| Alkaline phosphatase (U/L) | 73 | 149.3 (14.6) | 111 | 99.9 (5.6) | 0.0013c |
| Haptoglobin (mg/dL) | 54 | 3.1 (3.2) | 87 | 6.0 (20.2) | 0.3650b |
| Transferrin (mg/dL) | 70 | 187.2 (46.9) | 110 | 205.1 (52.3) | 0.0029 |
| Ferritin (μg/L) | 74 | 1263.3 (1569.4) | 106 | 815.9 (1247.9) | 0.0056 |
| NTproBNP (pg/mL) | 61 | 2129.3 (6856.9) | 101 | 84.9 (144.6) | <0.0001c |
| Arginine:Ornithine ratio | 71 | 0.64 (0.33) | 100 | 0.78 (0.41) | 0.0219 |
| Systolic BP (mmHg) | 62 | 127.2 (19.9) | 97 | 114.4 (12.8) | <0.0001c |
| Diastolic BP (mmHg) | 62 | 66.9 (12.3) | 97 | 63.7 (9.1) | 0.0815c |
| SpO2 (%) | 44 | 94.4 (4.2) | 68 | 96.8 (3.0) | 0.0022b |
| MDRD GFR (mL/min./1.73m2) | 79 | 139.8 (80.1) | 113 | 171.3 (55.4) | 0.0022b |
| Pain (ER visits per year) | 58 | 4.9 (6.8) | 96 | 5.5 (11.0) | 0.7060b |
Students t-test unless otherwise indicated.
Mann-Whitney test.
Alternate Welch's t-test.
Fisher exact test.
To further examine why pulmonary hypertension develops in hemoglobin SC where chronic intravascular hemolysis is not a prominent feature of the disease, these same clinical parameters were examined among the 43 SC patients, where the strongest protective effect for susceptibility to pulmonary hypertension was observed. In this comparison, only seven of the associations overlapped with those described above, including increasing age, anemia, elevated alkaline phosphastase and NTproBNP levels, low transferrin levels, iron overload (as reflected by serum ferritin), and a lower calculated GFR (MDRD glomerular filtration rate) (Table V). None of the characteristics typical of chronic hemolysis, including elevations in direct bilirubin, AST, the ratio of plasma arginine to ornithine, or undetectable haptoglobin are significantly different between Hemoglobin SC cases and controls (Table V), consistent with the lower hemolytic rate of SC disease. In addition, hemoglobin SC patients with pulmonary hypertension may have more frequent vaso-occlusive pain events as quantified by average emergency department visits per year (10.0 visits per year in cases versus 4.0 for controls, P=0.0944), in contrast to the absence of this trend in Hb SS (Table IV, P=0.7060).
Table V.
Characteristics of Hemoglobin SC Patients with and without Pulmonary Hypertension.
| Characteristic | Hb SC TRV greater than 2.5 m/s | Hb SC TRV less than 2.5 m/s | P valuea | ||
|---|---|---|---|---|---|
| n | Mean (SD) or n (%) | n | Mean (SD) or n (%) | ||
| Age (yrs.) | 8 | 51.8 (15.1) | 35 | 37.7 (11.5) | 0.0053 |
| Sex (female) | 8 | 3 (37.5%) | 35 | 21 (60.0%) | 0.4319b |
| Hemoglobin (gm/dL) | 8 | 10.4 (1.3) | 35 | 11.9 (1.2) | 0.0026 |
| Hematocrit (%) | 8 | 30.2 (4.6) | 35 | 34.5 (3.4) | 0.0045 |
| Hb F (gm/dL) | 8 | 0.30 (0.38) | 35 | 0.20 (0.26) | 0.3387 |
| Hb F (%) | 8 | 3.0 (4.0) | 35 | 1.6 (2.0) | 0.1411 |
| Reticulocytes, absolute (K/μL) | 8 | 112.6 (35.2) | 31 | 140.6 (49.6) | 0.0705c,d |
| WBC (K/μL) | 8 | 8.8 (3.3) | 35 | 8.5 (3.6) | 0.8578 |
| LDH (U/L) | 6 | 235.7 (67.0) | 34 | 224.4 (71.8) | 0.7218d |
| AST (U/L) | 8 | 32.6 (21.9) | 35 | 29.4 (20.7) | 0.7262d |
| ALT (U/L) | 8 | 21.9 (12.8) | 35 | 24.8 (15.1) | 0.7196c |
| Total bilirubin (mg/dL) | 8 | 1.7 (1.4) | 35 | 1.6 (1.2) | 0.3331c |
| Direct bilirubin (mg/dL) | 8 | 0.6 (1.0) | 35 | 0.3 (0.2) | 0.7261c,d |
| Alkaline phosphatase (U/L) | 8 | 120.3 (60.4) | 35 | 82.3 (39.8) | 0.0421 |
| Haptoglobin (mg/dL) | 6 | 27.0 (21.7) | 26 | 14.4 (20.5) | 0.1077 |
| Transferrin (mg/dL) | 8 | 206.4 (47.9) | 34 | 244.7 (43.3) | 0.0330 |
| Ferritin (μg/L) | 8 | 573.1 (728.6) | 34 | 269.1 (553.7) | 0.0207 |
| proBNP (pg/mL) | 8 | 403.4 (689.7) | 35 | 48.4 (44.1) | 0.0394e |
| Arginine:Ornithine ratio | 8 | 0.72 (0.49) | 31 | 0.84 (0.46) | 0.4621c,d |
| Systolic BP (mmHg) | 6 | 133.5 (25.4) | 35 | 125.4 (17.1) | 0.3809d |
| Diastolic BP (mmHg) | 6 | 75.2 (11.4) | 35 | 73.2 (10.8) | 0.6834 |
| SpO2 (%) | 6 | 97.3 (1.6) | 25 | 98.4 (1.1) | 0.1078c,d |
| MDRD GFR (mL/min./1.73m2) | 8 | 95.8 (32.1) | 35 | 131.4 (31.3) | 0.0060 |
| Pain (ER visits per year) | 8 | 10.0 (13.0) | 31 | 4.0 (5.7) | 0.0944c |
Students t-test unless otherwise indicated.
Fisher exact test.
Mann-Whitney test.
Association significant for Hb SS but not Hb SC.
Alternate Welch's t-test.
Survival Analysis for Hemoglobin SS and SC
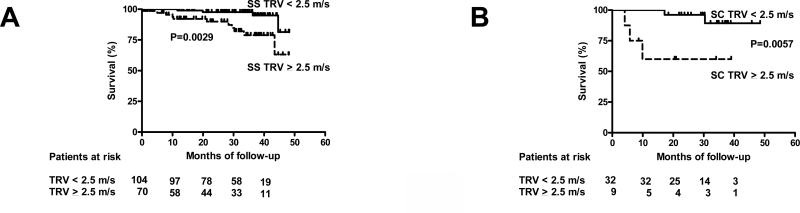
We next re-analyzed survival data for the two most frequent genotypes in this cohort, hemoglobin SS and hemoglobin SC in order to determine the effect of sequence verified hemoglobin phenotypes on survival for patients with pulmonary hypertension.4,5 Only patients followed during the first 49 months of study were included in this analysis (from a total of 252 consecutive patients; Hb SS n=174; Hb SC n=41). As previously observed for SCD overall, pulmonary hypertension (TRV ≥ 2.5 m/s) in Hb SS is a significant risk factor for mortality (Hazard Ratio = 4.81, Logrank test P=0.0029, Figure 3A). Pulmonary hypertension with hemoglobin SC is associated with at least as high a rate of mortality as observed in SS disease and in SCD overall (Hazard Ratio = 8.20, Logrank test P=0.0057, Figure 3B).
Figure 3. Kaplan-Meier Survival for Sickle Cell Anemia (HbSS) and Hemoglobin SC By Presence or Absence of Pulmonary Hypertension.
(A) Survival data published by Gladwin et al. and updated by Kato et al. was reanalyzed according the tricuspid jet velocity (TRV) for those with homozygous SCD (HbSS, n=176) from the first 252 consecutive patients screened with SCD during 49 months of follow-up.4,5 The presence of pulmonary hypertension (TRV ≥ 2.5 m/s) in HbSS is a significant risk factor for mortality (Hazard Ratio = 4.81, 95% CI 1.73-14.19, Logrank test P=0.0029). (B) Survival data for 41 patients with Hemoglobin SC with and without PH. PH is a significant risk factor for mortality in Hemoglobin SC (Hazard Ratio = 8.20, 95% Confidence Interval 2.71-352.10, Logrank test P=0.0057).
DISCUSSION
Sickle cell disease is a classical monogenic disorder characterized by chronic hemolytic anemia, the degree to which is known to be influenced by specific mutations in the genes for the subunits of hemoglobin A.15,24-26 In the present study, common polymorphisms and mutations within and flanking the HBB, HBA1, and HBA2 genes were analyzed for associations with pulmonary hypertension in SCD, where affected cases were identified by 2 validated non-invasive screening methods.5,7 In addition, the allelic spectrum of HBB mutations present within an unselected SCD cohort is documented as a necessary initial step for future studies to identify other genetic modifiers for pulmonary hypertension susceptibility.27 Overall, SCD compound heterozygosity is associated with a lower prevalence of pulmonary hypertension.
The chromosome 11 analysis suggests that non-sickle HBB mutations like Hb C that limit hemolytic rate in SCD are associated with a lower pulmonary hypertension risk, lending further support to the proposed hemolysis centered disease model of SCD pulmonary hypertension. These findings, together with prior studies of SC disease, indicate that the Hb C mutation is itself a significant genetic modifier of SCD. Specifically, Hb SC has a milder clinical course relative to homozygous SCD which is characterized by fewer vaso-occlusive events, a less intense hemolytic anemia, less frequent priapism, and a nearly normal average lifespan.2,6,28 However, some vaso-occlusive manifestations including retinopathy and osteonecrosis may be more frequent in SC disease.29,30 A similar protective association with pulmonary hypertension is also likely to be present in Sβ+ thalassemia. However, the statistical power for detecting this effect was below 80% and the high FPRP suggests a high likelihood of representing a false positive, which precludes a firm conclusion as to the validity of this suggested association.
Surprisingly, a higher hemolytic rate is not a distinguishing characteristic of pulmonary hypertension in Hb SC patients suggesting that this complication may be caused by etiologies unrelated to hemolysis. Perhaps intravascular hemolysis represents a lower fraction of total hemolysis in Hb SC than is the case in homozygous SCD. However, anemia, renal insufficiency, elevated alkaline phosphatase, and excess iron remain consistent distinguishing characteristics across all SCD pulmonary hypertension patients (Table V).5,10 Prior studies have documented phenotypic associations between pulmonary hypertension, hemolysis, and priapism in patients who may have less frequent vaso-occlusive manifestations.2,5,31 In fact, the low 8% prevalence of Hb SC among pulmonary hypertension cases at NIH nearly matches that reported for Hb SC (11%) observed among those with priapism in the Cooperative Study of Sickle Cell Disease.2 Taken together, SC pulmonary hypertension may be due to more prominent relative contributions by other mechanisms for remodeling the pulmonary vasculature including activation of the endothelin 1 and prostocyclin/thromboxane A2 pathways.32 In contrast, the contributions of these two pathways in the severe hemolytic anemias like homozygous SCD and β thalassemia major occur in addition to alterations in the NO signaling pathway.33-35 Moreover, these observations justify further study of SCD compound heterozygotes and their less intense hemolytic anemia as a more sensitive population for elucidating non-hemolytic mechanisms that may also contribute to the development of secondary pulmonary hypertension.
Despite speculation about differences in underlying mechanisms, pulmonary hypertension remains a strong predictor of death for all SCD patients. In particular both the absence of prominent laboratory features of hemolysis and the poor survival for patients with high TRV's should prompt clinicians to perform a comprehensive evaluation to rule out other etiologies of pulmonary hypertension in Hb SC (e.g. acute pulmonary embolism, chronic thromboembolic pulmonary hypertension or HIV infection).33 In addition, the significantly poor survival for Hb SC patients with pulmonary hypertension highlights the need for including this important subgroup of SCD patients in therapeutic clinical trials. Indeed, the efficacy of hydroxyurea in Hb SC still remains to be established today because these patients are typically excluded from multicenter placebo controlled trials for a variety of reasons.36-40
Another important distinguishing characteristic of SCD pulmonary hypertension observed in this and other studies is advancing age.5 Age has been reported to be a risk factor for increased pulmonary pressures in other diseases including primary pulmonary hypertension and systemic sclerosis.5 In the general population, the risk of developing primary pulmonary hypertension is an age dependent phenomenon, where the prevalence of the disease increases over 10% between the ages of 34 and 64 based upon estimates derived from the National Health and Examination Survey (NHANES).41 While the true prevalence of pulmonary hypertension in asymptomatic individuals may have been overestimated by NHANES, these findings highlight the importance of recognizing age as a significant variable confounding epidemiologic studies, just as we have observed with this genetic analysis of globin mutations. Thus with respect to our protective association in Hb SC, it remains unclear as to whether pulmonary hypertension in SC disease merely represents the background prevalence of pulmonary hypertension in otherwise healthy, aging African Americans or whether there is a true increase in risk secondary to their underlying hemoglobinopathy.
The final notable observations from this study are that neither α-thalassemia nor β-globin locus haplotypes have a strong association with pulmonary hypertension. The absence of a strong effect for α-thalassemia in SCD pulmonary hypertension was unexpected in contrast to the findings with Hb SC, despite the increased statistical power from the presence of the α3.7 allele at nearly three times the frequency of Hb C. Still, the magnitude of α3.7's effect may be sufficiently small to be below the threshold for detection for the statistical power in this study. Likewise, haplotypes of the β-globin locus have been used in the past to study both the variation in SCD severity and the evolutionary history of the βS mutation.42-46 In particular, some of the variability in Hb F expression is attributable to functional alleles in the fetal hemoglobin genes inherited on specific βS-globin haplotypes.43,47-49 Fetal hemoglobin expression may be relevant to pulmonary hypertension, as this and other studies have observed significantly lower levels of Hb F in SCD pulmonary hypertension cases (Table IV).9,10 However, the haplotype analysis would predict that the strongest loci underlying this effect would be present outside the β-globin locus. Together, the absence of associations with co-incident α-thalassemia or β-globin haplotypes might be attributed to an insufficient hematologic effect to reach a critical threshold that alters either the degree of hemolysis or the frequency of acute vaso-occlusive events. It is also possible that the natural history of the previously observed associations between these markers and SCD complications like priapism and leg ulcers have been altered by modern interventional therapies (e.g. ACE inhibitors, hydroxyurea, etc.) that have become commonplace since the completion of the CSSCD.2,50 Overall, further study in larger, independent populations is warranted.
In conclusion, mutations in the β-globin subunit of hemoglobin, but not a common α-thalassemia mutation, are associated with protection from SCD pulmonary hypertension. Despite this association, pulmonary hypertension remains a risk factor for death in carriers of modifying β-globin alleles. We speculate that the diminished relative contributions of hemolysis to the development of pulmonary hypertension in SCD compound heterozygotes is the likely mechanism underlying these protective associations. Future studies may be helpful to determine if other genetic factors influence risk for developing non-hemolytic forms of pulmonary hypertension in SCD.
METHODS
Patients
282 consecutive SCD patients at the National Institutes of Health (NIH) or Howard University were screened as previously described, including 195 patients4,5,12 and 250 patients7 whose clinical characteristics have previously been reported. All patients were evaluated with a history, physical examination, laboratory studies, and transthoracic Doppler echocardiography measurement of the triscuspid regurgitant jet velocity (TRV). Only patients providing a genomic DNA sample were evaluated in this study. Pulmonary hypertension cases were prospectively defined by a tricuspid regurgitant jet velocity (TRV) ≥ 2.5 m/s or retrospectively by an NTproBNP ≥ 160 pg/mL in a separate analysis. Informed consent was obtained from all subjects.
Genomic Alignments and Primer Design
Due to the high degree of sequence identity between globin genes on chromosome 11, individual genes of this region were aligned for gene specific primer design. 84,480 bp of sequence corresponding to the entire β-globin locus was downloaded from the UCSC genome browser (http://genome.ucsc.edu/; Human assembly, May 2004, hg17). 8000 base pair fragments corresponding to the HBE1, HBG1, HBG2, HBD, and HBB genes were aligned with ClustalW. Nine overlapping PCR primer pairs covering the promoter, exons, introns, and 3’ region of the HBB gene were designed using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Three additional primer pairs were designed for sequencing portions of the HBE1 and HBD genes.
Automated DNA Sequencing
Twelve PCR amplicons corresponding to the regions indicated above were sequenced (Big Dye chemistry, Applied Biosystems, Foster City, CA; ABI 3100 and 3730 platforms). Sequence data was analyzed using Sequencher 4.5 (Gene Codes Corporation, Michigan) or SeqScapev2.5 (Applied Biosystems, Foster City, CA).
Chromosome 11 and 16 Globin Locus Markers and STR Profiles
Genotypes were determined for the following 17 variant sites spanning the β globin locus (from 5’ to 3’) using either sequencing, Taqman (Pre-Designed/Validated assays, Applied Biosystems), or standard RFLPs42 (Figure 1): rs2187610, rs11036351, SNP_03 (Ch11 position 5202779; T to G SNP; identified by sequencing), rs10837631, rs7480526, rs334, HbC (Ch11 position 5204809), rs968857 (Hinc II site 3’ to ψβ), rs10128556 (Hinc II site 5’ to ψβ), rs11827654 (HBG1 TaqI site), rs4910740, rs3759071, rs3834466 (HBE gene Hinc II site), rs3759070, SNP_02 (Ch11 position 5248269; C to T SNP; identified by sequencing), rs3759069, and SNP_01 (Ch11 position 5248447; G to A SNP, identified by sequencing). Genotypes were also determined for the following markers on Chromosome 16: rs3785309, rs45110012, rs2858016, α-thalassemia (α3.7 and α4.2 deletions as previously described).51 Only common markers present with an allele frequency >5% were included in the linkage disequilibrium and association analysis (Figure 2). Fifteen simple tandem repeat markers (STRs) and 1 sex specific marker were typed using the AmpFlSTR Identifiler PCR amplification kit (Applied Biosystems, Foster City, CA, USA).
Statistical Analsysis
Statistical analysis was performed using Instat (Graph Pad Software, San Diego, CA), Prism (Graph Pad Software), PHASE version 2.152,53, DnaSP 3.5 (http://www.bio.ub.es/julio/DnaSP.html), Microsoft Excel (Microsoft Corporation, Redmond, WA) and Haploview 3.32 (wwwbroad.mit.edu/mpg/haploview).19 First degree relatives were identified by calculating cumulative sibship indices (CSIs) using a preliminary C++ analysis software (under development by Cobb and Taylor) and the STR allele data for the entire study population with frequencies provided with Identifiler.54,55 Only the first enrolled subject from a pair of first degree relatives was included in subsequent analyses. Comparison of categorical data was by Fisher exact test (2 × 2 table with 1 degree of freedom) including crude odds ratios (ORs) and 95% confidence intervals (CIs). Adjustment for age as a co-variable in the pulmonary hypertension case control analysis utilized age stratification with Mantel-Haenszel weighted Odds Ratios and Mantel-Haenszel summary Chi squares with corresponding adjusted P values. P values are presented without statistical correction using an α=0.05 as a significance threshold. Further scrutiny of associations below an α≤0.05 for the likelihood of representing false positive associations utilized a Bayesian method with a false positive report probability (FPRP) set at 0.2 and high π (prior probability of a true association, π=0.25-0.10,) based upon prior knowledge that the regions investigated contain mutations that are known to influence the rate of hemolysis in SCD.23 Continuous data were log transformed, if appropriate, and then analyzed by paired t test, Alternate Welch's t test, or Mann-Whitney test with P values.
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to the nursing staff of the OP7 and 5SES units of the NIH Clinical Center, Vincent Bekker MD/PhD, Wynona A. Coles, Mary K. Hall, James S. Nichols, Kristine Partovi, and the staff of the NCI Core Genotyping Facility for assisting this study.
This work was supported by the Intramural Research programs of the National Heart, Lung, and Blood Institute; the National Cancer Institute; and the Clinical Center of the National Institutes of Health.
REFERENCES
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–9. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106(9):3264–7. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lezcano NE, Odo N, Kutlar A, Brambilla D, Adams RJ. Regular transfusion lowers plasma free hemoglobin in children with sickle-cell disease at risk for stroke. Stroke. 2006;37(6):1424–6. doi: 10.1161/01.STR.0000221173.97108.01. [DOI] [PubMed] [Google Scholar]
- 4.Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM., Jr. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–85. doi: 10.1182/blood-2005-06-2373. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–95. doi: 10.1056/NEJMoa035477. others. [DOI] [PubMed] [Google Scholar]
- 6.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 7.Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, Taveira-DaSilva AM, Ballas SK, Blackwelder W, Xu X. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. Jama. 2006;296(3):310–8. doi: 10.1001/jama.296.3.310. others. [DOI] [PubMed] [Google Scholar]
- 8.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101(4):1257–61. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 9.Ataga KI, Sood N, De Gent G, Kelly E, Henderson AG, Jones S, Strayhorn D, Lail A, Lieff S, Orringer EP. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117(9):665–9. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134(1):109–15. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 11.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 12.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr., Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinberg MH. Predicting clinical severity in sickle cell anaemia. Br J Haematol. 2005;129(4):465–81. doi: 10.1111/j.1365-2141.2005.05411.x. [DOI] [PubMed] [Google Scholar]
- 14.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2006 doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgs DR, Aldridge BE, Lamb J, Clegg JB, Weatherall DJ, Hayes RJ, Grandison Y, Lowrie Y, Mason KP, Serjeant BE. The interaction of alpha-thalassemia and homozygous sickle-cell disease. N Engl J Med. 1982;306(24):1441–6. doi: 10.1056/NEJM198206173062402. others. [DOI] [PubMed] [Google Scholar]
- 16.Bentley DR. Whole-genome re-sequencing. Curr Opin Genet Dev. 2006;16(6):545–52. doi: 10.1016/j.gde.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Topol EJ, Frazer KA. The resequencing imperative. Nat Genet. 2007;39(4):439–40. doi: 10.1038/ng0407-439. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–9. doi: 10.1126/science.1069424. others. [DOI] [PubMed] [Google Scholar]
- 19.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder WA, Powars DR, Kay LM, Chan LS, Huynh V, Shelton JB, Shelton JR. Beta-cluster haplotypes, alpha-gene status, and hematological data from SS, SC, and S-beta-thalassemia patients in southern California. Hemoglobin. 1989;13(4):325–53. doi: 10.3109/03630268909003397. [DOI] [PubMed] [Google Scholar]
- 21.Oner C, Dimovski AJ, Olivieri NF, Schiliro G, Codrington JF, Fattoum S, Adekile AD, Oner R, Yuregir GT, Altay C. Beta S haplotypes in various world populations. Hum Genet. 1992;89(1):99–104. doi: 10.1007/BF00207052. others. [DOI] [PubMed] [Google Scholar]
- 22.Lemos Cardoso G, Farias Guerreiro J. African gene flow to north Brazil as revealed by HBB*S gene haplotype analysis. Am J Hum Biol. 2006;18(1):93–8. doi: 10.1002/ajhb.20467. [DOI] [PubMed] [Google Scholar]
- 23.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96(6):434–42. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mears JG, Lachman HM, Labie D, Nagel RL. Alpha-thalassemia is related to prolonged survival in sickle cell anemia. Blood. 1983;62(2):286–90. [PubMed] [Google Scholar]
- 25.Steinberg MH, Embury SH. Alpha-thalassemia in blacks: genetic and clinical aspects and interactions with the sickle hemoglobin gene. Blood. 1986;68(5):985–90. [PubMed] [Google Scholar]
- 26.Steinberg MH, Rosenstock W, Coleman MB, Adams JG, Platica O, Cedeno M, Rieder RF, Wilson JT, Milner P, West S. Effects of thalassemia and microcytosis on the hematologic and vasoocclusive severity of sickle cell anemia. Blood. 1984;63(6):1353–60. [PubMed] [Google Scholar]
- 27.Reich DE, Lander ES. On the allelic spectrum of human disease. Trends Genet. 2001;17(9):502–10. doi: 10.1016/s0168-9525(01)02410-6. [DOI] [PubMed] [Google Scholar]
- 28.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–6. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 29.Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev. 2003;17(3):167–78. doi: 10.1016/s0268-960x(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 30.Powars DR, Hiti A, Ramicone E, Johnson C, Chan L. Outcome in hemoglobin SC disease: a four-decade observational study of clinical, hematologic, and genetic factors. Am J Hematol. 2002;70(3):206–15. doi: 10.1002/ajh.10140. [DOI] [PubMed] [Google Scholar]
- 31.Nolan VG, Adewoye A, Baldwin C, Wang L, Ma Q, Wyszynski DF, Farrell JJ, Sebastiani P, Farrer LA, Steinberg MH. Sickle cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J Haematol. 2006;133(5):570–8. doi: 10.1111/j.1365-2141.2006.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655–65. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 33.Machado RF, Gladwin MT. Chronic sickle cell lung disease: new insights into the diagnosis, pathogenesis and treatment of pulmonary hypertension. Br J Haematol. 2005;129(4):449–64. doi: 10.1111/j.1365-2141.2005.05432.x. [DOI] [PubMed] [Google Scholar]
- 34.Morris CR, Kuypers FA, Kato GJ, Lavrisha L, Larkin S, Singer T, Vichinsky EP. Hemolysis-associated pulmonary hypertension in thalassemia. Ann N Y Acad Sci. 2005;1054:481–5. doi: 10.1196/annals.1345.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yung GL, Channick RN, Fedullo PF, Auger WR, Kerr KM, Jamieson SW, Kapelanski DP, Moser KM. Successful pulmonary thromboendarterectomy in two patients with sickle cell disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1690–3. doi: 10.1164/ajrccm.157.5.9710032. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. Jama. 2003;289(13):1645–51. doi: 10.1001/jama.289.13.1645. others. [DOI] [PubMed] [Google Scholar]
- 37.Charache S, Terrin ML, Moore RD, Dover GJ, McMahon RP, Barton FB, Waclawiw M, Eckert SV. Design of the multicenter study of hydroxyurea in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea. Control Clin Trials. 1995;16(6):432–46. doi: 10.1016/s0197-2456(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 38.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–22. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg MH, Nagel RL, Brugnara C. Cellular effects of hydroxyurea in Hb SC disease. Br J Haematol. 1997;98(4):838–44. doi: 10.1046/j.1365-2141.1997.3173132.x. [DOI] [PubMed] [Google Scholar]
- 40.Miller MK, Zimmerman SA, Schultz WH, Ware RE. Hydroxyurea therapy for pediatric patients with hemoglobin SC disease. J Pediatr Hematol Oncol. 2001;23(5):306–8. doi: 10.1097/00043426-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Rich S, Chomka E, Hasara L, Hart K, Drizd T, Joo E, Levy PS. The prevalence of pulmonary hypertension in the United States. Adult population estimates obtained from measurements of chest roentgenograms from the NHANES II Survey. Chest. 1989;96(2):236–41. doi: 10.1378/chest.96.2.236. [DOI] [PubMed] [Google Scholar]
- 42.Muralitharan S, Krishnamoorthy R, Nagel RL. Beta-globin-like gene cluster haplotypes in hemoglobinopathies. Methods Mol Med. 2003;82:195–211. doi: 10.1385/1-59259-373-9:195. [DOI] [PubMed] [Google Scholar]
- 43.Nagel RL, Fabry ME, Pagnier J, Zohoun I, Wajcman H, Baudin V, Labie D. Hematologically and genetically distinct forms of sickle cell anemia in Africa. The Senegal type and the Benin type. N Engl J Med. 1985;312(14):880–4. doi: 10.1056/NEJM198504043121403. [DOI] [PubMed] [Google Scholar]
- 44.Pagnier J, Mears JG, Dunda-Belkhodja O, Schaefer-Rego KE, Beldjord C, Nagel RL, Labie D. Evidence for the multicentric origin of the sickle cell hemoglobin gene in Africa. Proc Natl Acad Sci U S A. 1984;81(6):1771–3. doi: 10.1073/pnas.81.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orkin SH, Kazazian HH, Jr., Antonarakis SE, Goff SC, Boehm CD, Sexton JP, Waber PG, Giardina PJ. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982;296(5858):627–31. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- 46.Lapoumeroulie C, Dunda O, Ducrocq R, Trabuchet G, Mony-Lobe M, Bodo JM, Carnevale P, Labie D, Elion J, Krishnamoorthy R. A novel sickle cell mutation of yet another origin in Africa: the Cameroon type. Hum Genet. 1992;89(3):333–7. doi: 10.1007/BF00220553. [DOI] [PubMed] [Google Scholar]
- 47.Steinberg MH, Lu ZH, Nagel RL, Venkataramani S, Milner PF, Huey L, Safaya S, Rieder RF. Hematological effects of atypical and Cameroon beta-globin gene haplotypes in adult sickle cell anemia. Am J Hematol. 1998;59(2):121–6. doi: 10.1002/(sici)1096-8652(199810)59:2<121::aid-ajh4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Steinberg MH, Hsu H, Nagel RL, Milner PF, Adams JG, Benjamin L, Fryd S, Gillette P, Gilman J, Josifovska O. Gender and haplotype effects upon hematological manifestations of adult sickle cell anemia. Am J Hematol. 1995;48(3):175–81. doi: 10.1002/ajh.2830480307. others. [DOI] [PubMed] [Google Scholar]
- 49.Green NS, Fabry ME, Kaptue-Noche L, Nagel RL. Senegal haplotype is associated with higher HbF than Benin and Cameroon haplotypes in African children with sickle cell anemia. Am J Hematol. 1993;44(2):145–6. doi: 10.1002/ajh.2830440214. [DOI] [PubMed] [Google Scholar]
- 50.Koshy M, Entsuah R, Koranda A, Kraus AP, Johnson R, Bellvue R, Flournoy-Gill Z, Levy P. Leg ulcers in patients with sickle cell disease. Blood. 1989;74(4):1403–8. [PubMed] [Google Scholar]
- 51.Liu YT, Old JM, Miles K, Fisher CA, Weatherall DJ, Clegg JB. Rapid detection of alpha-thalassaemia deletions and alpha-globin gene triplication by multiplex polymerase chain reactions. Br J Haematol. 2000;108(2):295–9. doi: 10.1046/j.1365-2141.2000.01870.x. [DOI] [PubMed] [Google Scholar]
- 52.Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73(5):1162–9. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenk RE, Traver M, Chiafari FA. Determination of sibship in any two persons. Transfusion. 1996;36(3):259–62. doi: 10.1046/j.1537-2995.1996.36396182146.x. [DOI] [PubMed] [Google Scholar]
- 55.Tzeng CH, Lyou JY, Chen YR, Hu HY, Lin JS, Wang SY, Lee JC. Determination of sibship by PCR-amplified short tandem repeat analysis in Taiwan. Transfusion. 2000;40(7):840–5. doi: 10.1046/j.1537-2995.2000.40070840.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.