Abstract
22q11.2 Deletion Syndrome (22q11.2DS) is a common microdeletion syndrome with multisystem features. There is a strong association with psychiatric disorders. One in every four to five patients develop schizophrenia. Despite studies showing that early diagnosis and treatment are likely to lead to improved outcome, genetic counselors may be reluctant to discuss the risk of psychiatric illness. The aim of this research was to explore parental attitudes and genetic counselors’ perspectives and practice regarding disclosure of the clinical manifestations of 22q11.2DS, particularly the risk of psychiatric illness. We delivered a questionnaire to genetic counselors via established list-serves, 54 of which were completed. We also conducted interviews with four parents of adults with 22q11.2DS and schizophrenia. The majority of counselors and parents felt that the increased risk to develop a psychiatric illness is important to disclose. However, in the initial counseling session when the diagnosis was made in infancy genetic counselors were significantly less likely to discuss the risk of psychiatric disorders compared to other later onset features such as hypothyroidism (41 % vs. 83 %, p=0.001). When the diagnosis of 22q11.2DS was made in infancy, counselors’ responses in regard to timing of disclosure about psychiatric illnesses were fairly evenly divided between infancy, childhood and adolescence. In contrast, for other major features of 22q11.2DS, disclosure would predominantly be in infancy. The respondents reported that the discussion of psychiatric issues with parents was challenging due to the stigma associated with mental illness. Some also noted limited knowledge about psychiatric illness and treatment. These results suggest that genetic counselors could benefit from further education regarding psychiatric illness in 22q11.2DS and best strategies for discussing this important subject with parents and patients.
Keywords: 22q11.2 deletion syndrome, Velocardiofacial syndrome, Psychiatric illness, Schizophrenia, Genetic counseling
Introduction
22q11.2 Deletion Syndrome (22q11.2DS), also known as velo-cardio-facial syndrome (VCFS) or DiGeorge syndrome, is a common multi-system condition (Bassett et al. 2011; Botto et al. 2003). Cardiac defects, palatal anomalies (velopharyngeal incompetence and submucosal cleft palate), learning disabilities and a typical but subtle facial appearance characterize the common congenital malformations of this syndrome; however, the clinical presentation of 22q11.2DS is highly variable (Bassett et al. 2011). 22q11.2DS is associated with a 3.0 Mb hemizygous interstitial microdeletion of chromosome 22q11.2 in >85 % of individuals; others have smaller microdeletions at this locus (Emanuel 2008). The deletion usually occurs sporadically as a de novo mutation, though in 5–10 % of cases it is inherited from an affected parent (Bassett et al. 2008b; McDonald-McGinn et al. 2001). The estimated incidence of the 22q11.2 deletion is approximately 1/4000 births (reviewed in Kobrynski and Sullivan 2007). The incidence is thought to be an underestimate however, because mildly affected individuals may go undiagnosed (Kobrynski and Sullivan 2007) and inherited cases may be increasingly common (Costain et al. 2011b). Recognizing 22q11.2DS at any age has the potential to lead to significant changes in medical management, follow-up and genetic counseling that are helpful to the patient, family and clinicians (Bassett et al. 2011; Costain et al. 2011a).
Psychiatric disorders are one of the more prevalent clinical manifestations associated with 22q11.2DS (Bassett et al. 2005; Drew et al. 2011). These include generalized anxiety disorder, major depression, attention deficit disorder and obsessive-compulsive disorder (Antshel et al. 2010; Bassett et al. 2011; Fung et al. 2010). Most importantly, however, studies have consistently shown that 20–25 % of individuals with 22q11.2DS develop schizophrenia or related psychotic disorders such as schizoaffective disorder (Bassett et al. 2005; Fung et al. 2010; Murphy et al. 1999; Pulver et al. 1994), about 20 times the prevalence of schizophrenia in the general population. Schizophrenia is a serious psychiatric illness with onset most commonly in late teens to early twenties that is characterized by the hallucinations, delusions and disordered thinking encompassed by the term “psychosis” and by blunted affect, social withdrawal and other emotional and behavioral disturbances that significantly affect functioning.
Parents of children with 22q11.2DS have reported inadequate disclosure of the risk for mental illness by their health-care providers (Hercher and Bruenner 2008) and there is evidence of stigma associated with mental illness (Feret et al. 2011; Mann and Himelein 2004; Reavley and Jorm 2011). We hypothesized that genetic counselors may be reluctant to disclose the risk of mental illness associated with 22q11.2DS to parents. To our knowledge, this is the first study to assess genetic counselors’ attitudes and practice relating to disclosure of the risk of psychiatric illness in patients with 22q11.2DS.
Materials and Methods
Study Design
This was a mixed method study, with a qualitative component and a quantitative survey component. Both components of the study were approved by The Hospital for Sick Children and The Centre for Addiction and Mental Health Ethics Review Boards.
Participants and Procedures
Parents with an adult offspring with 22q11.2DS and a diagnosis of schizophrenia were recruited through the Clinical Genetics Research Program at the Centre for Addiction and Mental Health (CAMH). Four of five parents who were informed about the study and met the inclusion criteria participated in a one hour semi-structured interview conducted by the principal investigator who had no prior contact with the patients. The interview consisted of closed and open-ended questions developed by the research team that explored the participants’ views on the timing and method of information-giving regarding different manifestations of 22q11.2DS with a particular focus on schizophrenia.
The genetic counselor survey was distributed electronically through the Canadian Association of Genetic Counselors (CAGC) and the National Society of Genetic Counselors (NSGC) list-serves. The open-ended and closed-ended questions developed by the research team assessed genetic counselors’ experiences with 22q11.2DS, their current practices or what they would do regarding the disclosure process of 22q11.2DS manifestations, and personal and professional perspectives.
Analyses
The parental interviews and the open-ended questions on the survey distributed to the genetic counselors were analyzed using a variation of Diekelmann’s seven stages for analysis of qualitative text (Diekelmann 1992), which identified general themes, common patterns and shared aspects or impacts. Categorical data were analyzed using simple descriptive statistical analyses to generate frequencies and means. Contingency tables and chi-squared analyses or the Fisher’s exact probability test were used to analyze comparisons of frequencies of categorical variables using the SAS software package.
Results
Parental Attitudes—Semi-Structured Interviews—Qualitative Results
Four of the five parents (n=3 mothers; n=1 father) of adult offspring with classical features of 22q11.2DS who were invited, agreed to participate in the study. The average age of the parents was 55 years; they were of varying ethnic and educational backgrounds (high school to post graduate). Three of the four offspring with 22q11.2DS had experienced symptoms of psychiatric illness prior to the parents learning about the association of 22q11.2DS and the increased risk for psychiatric illness.
All parents identified behavioral and mental health issues as two of the main concerns they have regarding their offspring’s health and development in the past, present and future. All expressed they would have preferred to have been informed about the manifestations of 22q11.2DS including psychiatric illness prior to the onset of symptoms. Parents stressed awareness was important to limit the “surprise factor” and felt that information aids parents’ ability to cope. When asked about the best time to disclose the risk for psychiatric illness, generally parents said around school age is appropriate. However, 3 of the 4 parents reported they would have liked to have received the information at the time of initial diagnosis, even if there were other pertinent medical issues such as an upcoming heart surgery. Parents said information about the risk to develop a psychiatric illness prior to initial signs would allow parents to adjust emotionally. Furthermore, parents stated that learning about all the manifestations of 22q11.2DS enables parents to be proactive in the management of their child’s condition allowing them to address early signs and implement early intervention strategies if necessary.
All parents expressed higher anxiety levels over learning about psychiatric issues compared to learning about learning disabilities or thyroid problems. Parents were aware that thyroid problems are usually treated successfully with medication and learning disabilities was an area that parents felt that they could play an active role in advocating for early intervention strategies for their child. However, many parents did not have a good understanding of psychiatric illnesses. They indicated that their knowledge was based on the negative portrayal of psychiatric illness that is reported in the media.
The parents reported that learning about the early symptoms, early intervention strategies, treatment and information about resources for support were important factors in reducing anxiety about psychiatric illness. Another factor noted was the mode of delivery of information. It was important that the health professional delivering the information be equipped with the appropriate tools necessary to relay the essential information at an understandable level, and in a clear, straightforward and sensitive manner. Parents felt that using various strategies such as a narrative approach, allowing parents to ask questions and using supportive aids such as reading materials and videos would all be beneficial in aiding the delivery of the complex concepts that surround a diagnosis of a multisystem condition like 22q11.2DS. Finally, the parents felt that a multi-disciplinary approach was beneficial as was follow-up to review information and provide an opportunity to ask questions about newly arising issues.
Survey—Genetic Counselors’ Attitudes and Experiences—Quantitative Results
Fifty-four genetic counselors [n=52 (96 %) female; n=2 (4 %) male] with an average of 7.2 years of professional experience completed the survey. Most of the genetic counselors (n=42, 77 %) had seen patient populations that included infants, children and/or adolescents with 22q11.2DS; they counseled on average approximately 3 patients/families with 22q11.2DS per year and 89 % (n= 48) reported that their knowledge of 22q11.2DS was either adequate or substantial. Thirty-three (68 %) genetic counselors reported that their institution scheduled follow-up appointments for patients.
Patterns of Disclosure with Respect to Age at Initial Diagnosis of 22q11.2DS
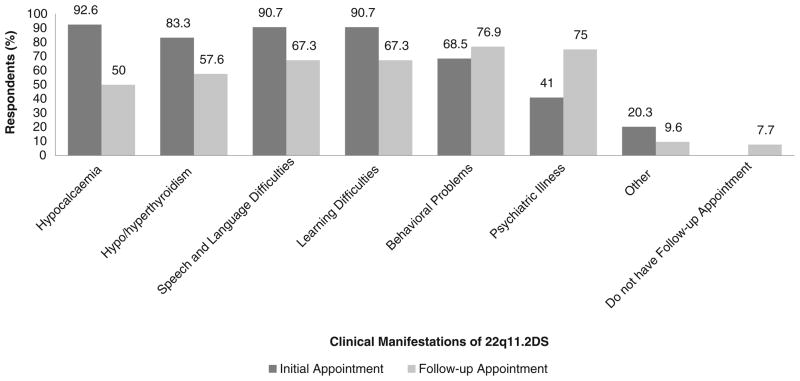
When patients were diagnosed as infants, genetic counselors reported that information about the risks to develop hypocalcemia, hypo/hyperthyroidism, speech and language problems, learning difficulties and behavioral problems was more likely to be discussed with parents in the initial information-giving session (92.6 %, 83.3 %, 90.7 %, 90.7 %, and 76.9 %, respectively) and then reviewed in a follow-up session (Fig. 1). In contrast, only 41 % of counselors reported they would disclose the risk to develop a psychiatric illness in the initial session, 44 % preferring to raise this issue for the first time in a follow-up appointment. Genetic counselors were significantly less likely to discuss psychiatric disorders at an initial session than another later onset manifestation of 22q11.2DS, such as thyroid disorders (83 % versus 41 %, p =0.001). Approximately 15 % of counselors would not discuss psychiatric risk, whereas non-disclosure of other manifestations ranged from 10 % for hyper/hypothyroidism to 2 % for speech and language problems.
Fig. 1.
Responses of 54 genetic counselors to survey questions regarding disclosure of the clinical manifestations of 22q11.2DS to parents with an offspring diagnosed in infancy
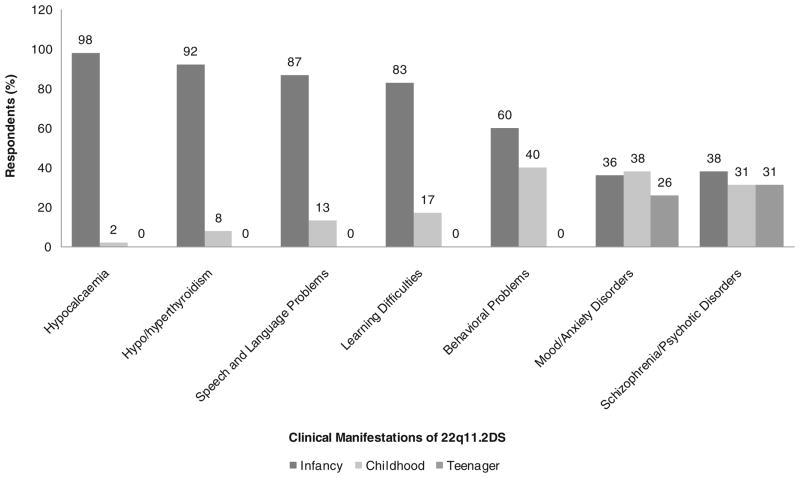
The disclosure results for diagnosis in infancy were supported by the counselors’ opinions on the timing of providing information regarding the various manifestations of 22q11.2DS. The vast majority of counselors felt that information regarding the risks to develop hypocalcemia, hypo/hyperthyroidism, speech and language problems, learning difficulties and behavioral problems should be disclosed to parents when the child with 22q11.2DS is an infant (Fig. 2). In contrast, the timing of disclosure about risk for mood and anxiety disorders and for schizophrenia and other psychiatric disorders, respectively, was fairly evenly divided between infancy (36 % and 38 %), childhood (38 % and 31 %) and adolescence (26 % and 31 %) (Fig. 2).
Fig. 2.
Responses of 54 genetic counselors to survey questions regarding timing (infancy, childhood, adolescence) preferences of disclosure for specific manifestations of 22q11.2DS to parents
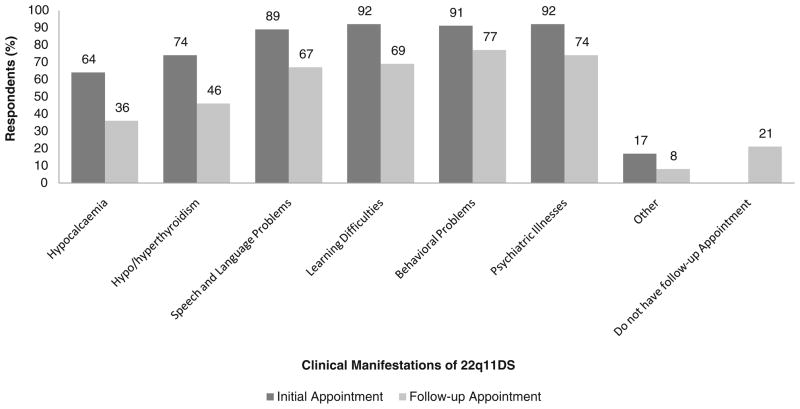
When a diagnosis of 22q11.2DS was made in adolescence/early adulthood, the majority of genetic counselors indicated that they would discuss all manifestations in the initial session after molecular confirmation. The proportion who would discuss psychiatric disorders was very similar to the proportion who would discuss learning difficulties and behavioral problems, while hypocalcemia and thyroid conditions were the least likely to be discussed (Fig. 3). There were 15 (28 %) non-respondents when genetic counselors were asked to describe what information they would discuss in a follow-up session.
Fig. 3.
Responses of 54 genetic counselors to survey questions regarding disclosure of the clinical manifestations of 22q11.2DS to parents with an offspring diagnosed in adolescence/early adulthood
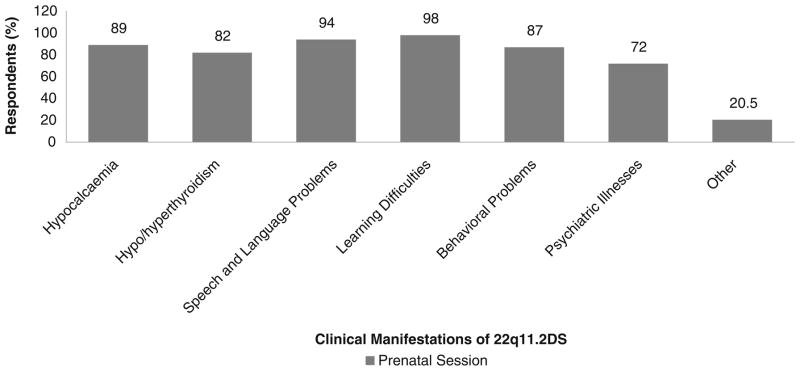
In the provision of prenatal genetic counseling, the majority of genetic counselors reported they would discuss all of the clinical manifestations of 22q11.2DS. Again, the proportion who would discuss psychiatric disorders was lower (72 %) than that for other clinical manifestations (82 %–98 %), although not significantly so (Fig. 4).
Fig. 4.
Responses of 54 genetic counselors to survey questions regarding disclosure of the clinical manifestations of 22q11.2DS to parents with an offspring diagnosed in a prenatal session
Comparing the responses of genetic counselors who had seen 5 or more 22q11.2DS patients (n=20) with those who had seen fewer than 5 patients (n=34) identified some interesting trends. Counselors who had seen fewer patients with 22q11.2DS were significantly more likely to discuss psychiatric problems with families in a prenatal session than counselors who had seen 5 or more patients (p=0.0463). There was a similar but non-significant trend for discussing hypocalcemia and hypo/hyperthyroidism (p=0.091 and p= 0.0974, respectively). At an initial session for patients in adolescence/early adulthood genetic counselors who had seen fewer 22q11.2DS patients were also more likely to inform them (and their parents) about hypocalcemia and hypo/hyperthyroidism (p=0.0651 and p=0.0463, respectively). Neither of the variables “number of years in the profession” nor “type of patients seen” significantly affected results, however numbers for the categories in these subgroup analyses were small.
Survey—Genetic Counselors’ Attitudes and Experiences—Qualitative and Selected Quantitative Results
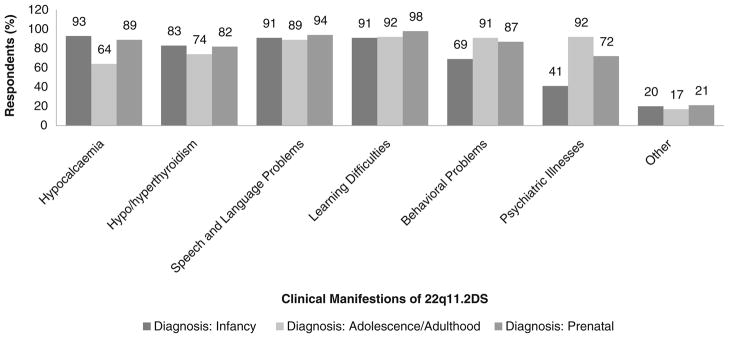
Genetic counselors were invited to comment on reasons why their responses regarding the disclosure process of the various manifestations of 22q11.2DS may have differed based on the patient’s age at the time of diagnosis (Fig. 5). For example, there were proportionately fewer counselors who would disclose information about hypocalcemia to families in the initial session if the patient was diagnosed in adolescence/early adulthood (64 %) compared to a diagnosis in infancy (93 %; 0.00005<P<0.0005) or prenatal diagnosis (89 %; 0.0005<P<0.005) (Fig. 5). The rationale provided by several counselors was that hypocalcemia is a more important medical concern in infancy than in adulthood. There were also proportionately fewer counselors who would discuss behavioral and psychiatric problems in an initial session in infancy (69 % and 41 %, respectively) compared to either a prenatal (87 %; 0.01<P<0.25 and 72 %; 0.005<P<0.01, respectively) or an initial adolescence/early adulthood session (91 %; 0.01<P<0.025 and 92 %; P<0.00005, respectively) (Fig. 5). Qualitative analysis showed several factors that may influence a genetic counselors’ practice towards the timing of disclosure of the increased risk to develop a psychiatric illness in 22q11.2DS, including:
Fig. 5.
Summary of responses of 54 genetic counselors to survey questions comparing disclosure of the six main clinical manifestations of 22q11.2DS to parents of an affected offspring with regard to a diagnosis made in the three life stages (infancy, adolescence/adulthood, prenatal)
Disclosure of information at adequate intervals is necessary so as to not overwhelm parents.
Disclosure is dependent on the age, timing of diagnosis and the age of onset of the clinical manifestations of 22q11.2DS.
Disclosure is dependent on the parents’ emotional state and whether they wish to hear all the information at once or only discuss the immediate concerns.
Disclosure of all information in the initial session is necessary due to the institution not having regular follow-up sessions.
Full disclosure is necessary in a prenatal session for parents to make well informed decisions.
Disclosure in the initial session may be important to make parents aware of the information they may read on the internet or support group literature.
Nearly all respondents (98 %) indicated that geneticists/genetic counselors should be one source of information for parents obtaining information about the increased risk for their child with 22q11.2DS to develop a psychiatric illness. Other possible sources included health care professionals such as cardiologists and family physicians (69 %), support group literature (69 %) and parents with children with 22q11.2DS (41 %). Very few chose the internet (15 %) as a recommended source of information.
Reluctance to Disclose Risk for Psychiatric Illnesses
A potential reason for the reluctance to disclose the psychiatric risk associated with 22q11.2DS reported by a substantial majority of genetic counselors (74 %) pertained to the stigma that surrounds the diagnosis of a psychiatric illness. The stigma appeared to be related not only to societal perceptions of psychiatric illness but also to the possibility that parents may unconsciously treat their child differently. One counselor wrote, “Are we creating a monster by letting parents over-read behavioral changes, which might be within a normal spectrum?” Genetic counselors also reported that the reluctance to disclose this information may be related to the severity of medical issues a child with 22q11.2DS may face in the first few years of life, a concern about overwhelming parents (n= 30; 56 %). Nearly half (n=26; 48 %) of the genetic counselors reported that they had personal discomfort in discussing psychiatric illnesses. A minority of genetic counselors stated their discomfort was related to their own limited knowledge about the early signs, treatment and prevention of psychiatric illnesses, or to their perception that there was insufficient literature on the increased risk of serious psychiatric illnesses in 22q11.2DS (n=17; 32 % and n=13; 24 %, respectively).
Genetic Counselor Perception of Parental Reactions
Lastly, we asked genetic counselors who had disclosed information about the risks for psychosis in 22q11.2DS to share their opinions regarding their perception of parental reactions. Responses from 30 genetic counselors ranged from extreme anxiety, fear, shock and denial to relief or parents who were not overly alarmed. Genetic counselors reported several factors that appeared to be associated with more positive parental responses including the parents’ previous experience with psychiatric illnesses, their understanding and appreciation that there is treatment and that earlier detection improves long-term outcome, realization that this may be an explanation for their child’s behavior, and the parents’ approach of focusing on the immediate problems and addressing the issue of psychiatric illness only if it appears.
Discussion
This study assessed genetic counselors’ experiences with, and attitudes toward, disclosing features associated with 22q11.2DS, with a specific focus on the elevated risk of schizophrenia. Most genetic counselors agreed that the elevated risk of developing a psychiatric illness for patients with 22q11.2DS should be disclosed to parents. However, the findings from our study indicate that a significantly lower proportion of genetic counselors would disclose this information compared to other health related information associated with 22q11.2DS in an initial session. Results for disclosure of risk for thyroid disease suggested that deferral or non-disclosure in regard to psychiatric illness did not appear to be due solely to later onset of expression. Consistent with our hypothesis, stigma associated with psychiatric illness did appear to play a role. The results showed that concern about stigma related to psychiatric illness is prevalent among genetic counselors and likely to affect counseling for 22q11.2DS. Other studies have reported that stigma in general is a significant concern for parents of children with 22q11.2DS (Hercher and Bruenner 2008). Although stigmatizing attitudes towards psychiatric illness persist to the current day in the general population and among health care professionals, including genetic counselors, these may be diminished with increasing awareness and more positive attitudes toward treatment (Feret et al. 2011; Hodgkinson et al. 2001; Mann and Himelein 2004; Reavley and Jorm 2011).
Consistent with several studies (Hasnat and Graves 2000; Hercher and Bruenner 2008; Pain 1999), and despite the small sample interviewed, the parents in our study believed it was important to receive information about the clinical manifestations of 22q11.2DS, including risks for a psychiatric illness, prior to the possibility of their child developing symptoms. A study by Hinkson et al. (unpublished) concluded that parents with a child with another multisystem condition, Cornelia De Lange Syndrome, wanted all information at the time of diagnosis, even if their child did not present with the particular medical illness. The results from the parental component of our study further supported findings from other studies, which indicate that timely information assists parents to adjust emotionally to their child’s disabilities and enables parents to access services and be proactive in the management of their child’s condition (Costain et al. 2011a; Hasnat and Graves 2000; Hercher and Bruenner 2008; Pain 1999).
Genetic counselors expressed concerns however about the emotional readiness of the parents and uncertainty about what information they would like to receive at the time of diagnosis. Parents in our study concurred with respect to the anxiety engendered about hearing of the risk for psychiatric illness but proposed some potential strategies to reduce such anxiety. These included discussion regarding the early warning signs and treatment of psychiatric illnesses. This was consistent with literature suggesting that individuals with 22q11.2DS should be followed for early signs of psychotic illness because early diagnosis and effective treatment for schizophrenia can lead to a better prognosis and improved functioning (Bassett and Chow 2008; Bassett et al. 2011; Hodgkinson et al. 2001). A pilot study reported that genetic counselors felt unprepared to raise the issue of psychiatric illness with patients or to answer questions regarding psychiatric illness due to their lack of understanding of a psychiatric diagnosis and their knowledge about issues involved in psychiatric genetics (Peay and McInerney 2002). Providing information regarding changes in behavior, thinking, emotions and/or sleep, energy level or appetite that should prompt psychiatric assessment as well as the treatable nature of psychiatric illness would not only help parents but may also help to reduce genetic counselors’ discomfort in discussing the increased prevalence of psychiatric illness in 22q11.2DS (Bassett and Chow 2008, Bassett et al. 2008a). Notably, there are no clinical tests or individual features, including childhood conditions such as attention deficit or learning difficulties, that are useful for predicting later onset of psychotic illness, although there is much research interest in this area for 22q11.2DS (Bassett and Chow 2008; Philip and Bassett 2011). In the context of reassurance about the inability to predict or control the future, it may be helpful to discuss general factors that may help with resilience to both the stress of daily living (Feder et al. 2009) and the chronic and acute adversity faced by individuals with 22q11.2DS. These would include a close relationship with a caring adult, a sense of purpose, practicing coping strategies with manageable stressors, a positive social support network, environmental enrichment and physical exercise (Feder et al. 2009). Good nutrition and avoidance of street drugs and alcohol would also be broadly helpful recommendations (Bassett and Chow 2008; Philip and Bassett 2011). A matter-of-fact approach, emphasizing availability of multiple effective treatments for psychiatric illnesses, that standard treatments are effective in 22q11.2DS, and advice about consultation options if changes or symptoms of concern arise, will be of further practical assistance (Bassett and Chow 2008; Bassett et al. 2011; Hodgkinson et al. 2001).
Given the multi-system nature of 22q11.2DS, parents in our study also stated their belief about the importance of health care professionals providing information in a multi-disciplinary team approach, with follow-up appointments. A study examining parental attitudes towards the disclosure process of children with a severe disability also concluded that it is not only immediately after diagnosis that information is required; parents also wanted information at least two years after diagnosis (Sloper and Turner 1993). A study of parents/caregivers of children with 22q11.2DS reported that not only was information about the association of 22q11.2DS and psychiatric illness often omitted at initial diagnosis this topic was also not adequately addressed at follow-up (Hercher and Bruenner 2008). This study reported that 61.5 % of the 41 families they surveyed learned about the association with psychiatric disease from the internet and other non-medical sources (Hercher and Bruenner 2008), consistent with the counselors’ concerns in our study. Our study found that not all institutions book follow-up appointments for 22q11.2DS after initial diagnosis. Recent clinical practice guidelines (Bassett et al. 2011) recommend routine follow-up and may help to change patterns of practice. Information about 22q11.2DS is fast evolving and follow-up appointments may increase the likelihood that counselors and families would get updated on the latest findings for 22q11.2DS.
Supporting the possibility that lack of updated information may have played a role in the results of our study of counseling decisions for 22q11.2DS, is the fact that there was a significantly lower proportion of genetic counselors providing information about the risk to develop hypocalcemia when the diagnosis of 22q11.2DS was established in adolescence/early adulthood compared to prenatally or in infancy. Symptomatic hypocalcemia, with seizures, tremors and/or rigidity, in the first 3 months of life is observed in a minority of patients (Thomas and Graham 1997). Previously, treatment would be discontinued by 1 year of age with no subsequent monitoring. However, a high prevalence (~60 %) and serious associated symptoms of hypocalcemia in adolescents and adults (Bassett et al. 2005; Goldberg et al. 1993; Sykes et al. 1997) have lead to recommendations for continued surveillance of calcium levels, especially during times of biological stress such as surgeries, and for ongoing treatment after initial diagnosis (Bassett et al. 2011).
Limitations
A small number of parents were interviewed and may have held biased views towards the disclosure process of the increased risk to develop a psychiatric illness in this population because their offspring had developed such conditions. Although the information obtained from these interviews was helpful in interpreting the survey results and was consistent with other literature, it may not be representative of the entire population of parents with offspring with 22q11.2DS. Future studies should also survey adults with 22q11.2DS for their opinions. Analyses of the survey results from genetic counselors were also limited due to a relatively small sample size. However, we received responses from over 35 centers across Canada, the United States and internationally, limiting the potential for skewed responses based on common institutional beliefs. Also, this study was limited to genetic counselors, who may not always be the initial health care professionals to discuss the phenotypic manifestations. The study involved patterns of practice related to counseling parents and did not include issues related to directly counseling adolescents and adults with 22q11.2DS. There were no detailed demographic data available on patients, nor about whether the individuals with 22q11.2DS had a psychiatric diagnosis at the time of counseling. A minority of respondents reported on what they would do; the actual experience of the majority of genetic counselors sampled is likely to provide more reliable results. It is possible that results of this study may be different if repeated in the next few years with expanding literature on later onset features of 22q11.2DS. Anecdotal reports from large cohorts of patients with 22q11.2DS and their families and clinicians however suggest disclosure about psychiatric illnesses remains an important issue to address. The principal conclusions of the study however would likely apply to any professionals involved with families of individuals with 22q11.2DS.
Conclusion
Genetic counseling is an important component in the comprehensive management of patients with 22q11.2DS and genetic counselors play a significant role in the process of disclosing and explaining clinically relevant information. The findings from this study suggest that genetic counselors may feel reluctant to discuss the risk of psychiatric illness at the initial counseling session, despite evidence that parents may want this information. Reasons for this reluctance include stigma associated with psychiatric illness, personal discomfort in discussing psychiatric illness and limited knowledge about the manifestations and treatment of psychiatric disorders. There is an elevated prevalence of schizophrenia and related psychotic disorders as well as anxiety and other non-psychotic illnesses in individuals with 22q11.2DS. Early diagnosis and effective management of these treatable conditions is likely to improve outcome. Thus, information regarding risks, warning signs and intervention should be discussed with families prior to the onset of symptoms, which may occur as early as childhood. This study reveals that additional efforts are needed to improve awareness and understanding of psychiatric illness in 22q11.2DS in order to facilitate open communication between genetic counselors and parents regarding this important topic.
Acknowledgments
The authors thank all the participants in the study, and Monica Torsan and Gladys Wong for assistance with preparing the manuscript. This work was supported by a Canadian Institutes of Health Research grant (MOP-97800) a Canada Research Chair in Schizophrenia Genetics and Genomic Disorders (ASB), and Restracomp, Hospital for Sick Children.
Contributor Information
Nicole Martin, Prenatal Diagnosis and Medical Genetics Program, Mount Sinai Hospital, Toronto, ON, Canada.
Marina Mikhaelian, Signature Genomics Laboratories, Spokane, WA, USA.
Cheryl Cytrynbaum, Clinical and Metabolic Genetics, Hospital for Sick Children, Toronto, ON, Canada.
Cheryl Shuman, Clinical and Metabolic Genetics, Hospital for Sick Children, Toronto, ON, Canada, University of Toronto, Toronto, ON, Canada.
David A. Chitayat, University of Toronto, Toronto, ON, Canada, Department of Obstetrics and Gynecology, The Prenatal Diagnosis and Medical Genetics Program, Mount Sinai Hospital, Toronto, ON, Canada, Department of Pediatrics, Division of Clinical and Metabolic, Genetics, Department of Clinical and Metabolic Genetics, Hospital for Sick Children, Toronto, ON, Canada
Rosanna Weksberg, Clinical and Metabolic Genetics, Hospital for Sick Children, Toronto, ON, Canada, University of Toronto, Toronto, ON, Canada.
Anne S. Bassett, University of Toronto, Toronto, ON, Canada, Clinical Genetics Research Program, Centre for Addiction and Mental Health, Toronto, ON, Canada, 33 Russell Street, 1/F, Toronto, ON, Canada M5S 2S1
References
- Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:333–344. [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD, Gatzoulis MA. Clinical features of 78 adults with 22q11 Deletion Syndrome. American Journal of Medical Genetics Part A. 2005;138:307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC. Schizophrenia and 22q11.2 deletion syndrome. Current Psychiatry Reports. 2008;10:148–157. doi: 10.1007/s11920-008-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Hodgkinson KA. Genetics of schizophrenia and psychotic disorders. In: Smoller JW, Sheidley BR, Tsuang MT, editors. Psychiatric genetics: Applications in clinical practice. Washington, DC: American Psychiatric Publishing; 2008a. pp. 99–130. [Google Scholar]
- Bassett AS, Marshall CR, Lionel AC, Chow EW, Scherer SW. Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Human Molecular Genetics. 2008b;17:4045–4053. doi: 10.1093/hmg/ddn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel, et al. Practical guidelines for managing patients with 22q11.2 deletion syndrome. Journal of Pediatrics. 2011;159:332–339. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Costain G, Chow EW, Ray PN, Bassett AS. Care-giver and adult patient perspectives on the importance of a diagnosis of 22q11.2 deletion syndrome. Journal of Intellectual Disability Resesearch. 2011a doi: 10.1111/j.1365-2788.2011.01510.x. (e published) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G, Chow EWC, Silversides CK, Bassett AS. Sex differences in reproductive fitness contribute to preferential maternal transmission of 22q11.2 deletions. Journal of Medical Genetics. 2011b;48:819–824. doi: 10.1136/jmedgenet-2011-100440. [DOI] [PubMed] [Google Scholar]
- Diekelmann N. Learning-As-Testing: a Heideggerian Hereneutical analysis of the lived experiences of students and teachers in nursing. Advances in Nursing Science. 1992;14:72–83. doi: 10.1097/00012272-199203000-00010. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, et al. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. International Journal of Developmental Neuroscience. 2011;29:259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel BS. Molecular mechanisms and diagnosis of chromosome 22q11.2 rearrangements. Developmental Disabilities Research Reviews. 2008;14:11–18. doi: 10.1002/ddrr.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feret H, Conway L, Austin JC. Genetic counselors’ attitudes towards individuals with schizophrenia: desire for social distance and endorsement of stereotypes. Patient Education and Counseling. 2011;82:69–73. doi: 10.1016/j.pec.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung WLA, McEvilly R, Fong J, Silversides C, Chow E, Bassett AS. Elevated prevalence of generalized anxiety disorder in adults with 22q11.2 deletion syndrome. American Journal of Psychiatry. 2010;167:998. doi: 10.1176/appi.ajp.2010.09101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R, Motzkin B, Marion R, Scambler PJ, Shprintzen RJ. Velo-cardio-facial syndrome: a review of 120 patients. American Journal of Medical Genetics. 1993;45:313–319. doi: 10.1002/ajmg.1320450307. [DOI] [PubMed] [Google Scholar]
- Hasnat MJ, Graves P. Disclosure of developmental disability: a study of parent satisfaction and the determinants of satisfaction. Journal of Paediatrics and Child Health. 2000;36:32–35. doi: 10.1046/j.1440-1754.2000.00463.x. [DOI] [PubMed] [Google Scholar]
- Hercher L, Bruenner G. Living with a child at risk for psychotic illness: the experience of parents coping with 22q11 deletion syndrome: an exploratory study. American Journal of Medical Geneics Part A. 2008;146A:2355–2360. doi: 10.1002/ajmg.a.32466. [DOI] [PubMed] [Google Scholar]
- Hodgkinson KA, Murphy J, O’Neill S, Brzustowicz L, Bassett AS. Genetic counselling for schizophrenia in the era of molecular genetics. Canadian Journal of Psychiatry. 2001;46:123–130. doi: 10.1177/070674370104600202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370:1443–1452. doi: 10.1016/S0140-6736(07)61601-8. [DOI] [PubMed] [Google Scholar]
- Mann CE, Himelein MJ. Factors associated with stigmatization of persons with mental illness. Psychiatric Services. 2004;55:185–187. doi: 10.1176/appi.ps.55.2.185. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Tonnesen MK, Laufer-Cahana A, Finucane B, Driscoll DA, Emanuel BS, et al. Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: cast a wide FISHing net! Genetics in Medicine. 2001;3:23–29. doi: 10.1097/00125817-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Archives of General Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Peay H, McInerney J. A pilot study on psychiatric genetic counselling: counselor’s needs. Journal of Genetic Counseling. 2002;11:485. (Abstract) [Google Scholar]
- Pain H. Coping with a child with disabilities from the parents’ perspective: the function of information. Child: Care, Health and Development. 1999;25:299–312. doi: 10.1046/j.1365-2214.1999.00132.x. [DOI] [PubMed] [Google Scholar]
- Philip N, Bassett AS. Cognitive, behavioural and psychiatric phenotype in 22q11.2 deletion syndrome. Behavior Genetics. 2011;41:403–412. doi: 10.1007/s10519-011-9468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. Journal of Nervous and Mental Disorders. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Reavley NJ, Jorm AF. Stigmatizing attitudes towards people with mental disorders: findings from an Australian National Survey of Mental Health Literacy and Stigma. Australian and New Zealand Journal of Psychiatry. 2011;45:1086–1093. doi: 10.3109/00048674.2011.621061. [DOI] [PubMed] [Google Scholar]
- Sloper P, Turner S. Determinants of parental satisfaction with disclosure of disability. Developmental Medicine and Child Neurology. 1993;35:816–825. doi: 10.1111/j.1469-8749.1993.tb11733.x. [DOI] [PubMed] [Google Scholar]
- Sykes KS, Bachrach LK, Siegel-Bartelt J, Ipp M, Kooh SW, Cytrynbaum C. Velocardiofacial syndrome presenting as hypocalcemia in early adolescence. Archives of Pediatrics & Adolescent Medicine. 1997;151:745–747. doi: 10.1001/archpedi.1997.02170440107021. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Graham JMJ. Chromosome 22q11 Deletion Syndrome: an update and review for the primary pedicatrician. Clinical Pediatrics. 1997;36:253–266. doi: 10.1177/000992289703600502. [DOI] [PubMed] [Google Scholar]