1. Introduction
Since the early 1990s, asthma burden has been recognized as a national public health concern in the United States [1]. The asthma burden has disproportionately affected persons of certain racial/ethnic backgrounds, principally African Americans [2] and those persons living in urban environments [3,4]. Concern about the growing problem of asthma has led to a number of national, state, and local efforts towards improving asthma outcomes and control [5,6,7,8].
No national effort toward asthma control has been more celebrated than the implementation of the National Heart, Lung and Blood Institute's National Asthma Education and Prevention Program (NAEPP). Initiated in 1989, to a large extent in response to the public's concern about the increased asthma prevalence and burden, the NAEPP set its first programmatic effort to the establishment of guidelines to improve asthma care [9]. Since the initial release of these guidelines in 1991, hundreds of thousands of copies have been distributed [10] and there have been countless efforts directed toward moving these guidelines into practice including continuing medical education (CME) programs, disease management programs, clinical performance measures, and research efforts. The NAEPP continues efforts in the establishment of national guidelines through a series of updates to the original guidelines, including the recent release of a major update in November 2007 [11,12].
While there continues to be numerous reports of progress of local implementation and health plan efforts, these reports have focused on changes in asthma processes of care or on outcomes limited primarily to health care utilization among selected, mostly health plan or practice-based samples. To date, there is a rather modest literature on community-wide population-based status of asthma burden and quality of care.
The Chicago Initiative to Raise Asthma Health Equity (CHIRAH) is one of the NHLBI Centers of Excellence in Reducing Asthma Disparities. The core activity of the CHIRAH has been to conduct a community-based cohort study designed to characterize those factors that are contributing to racial/ethnic disparities with the purpose of identifying mutable factors that may provide the basis for new intervention strategies to eliminate these disparities. The CHIRAH project therefore provides a unique opportunity to report on a population-based understanding of the burden of asthma in a large urban environment known to have one of the highest asthma mortality rates in the US [13]. The purpose of this report is to examine the overall burden of asthma morbidity and treatment as seen from the perspective of this community-based study.
2. Methods
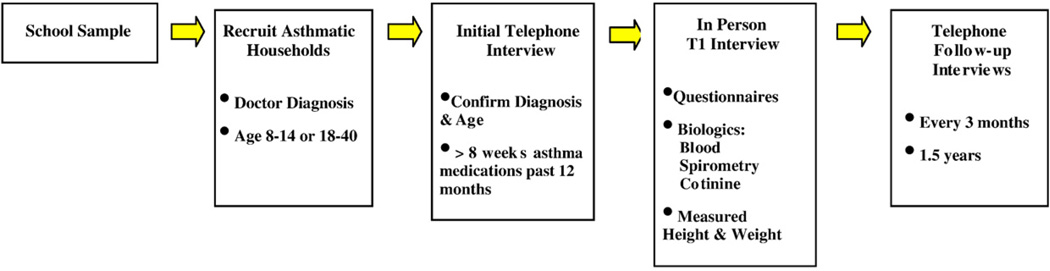
CHIRAH has been designed as a community-based cohort study. As seen in Fig. 1, the first stage of the study was a community-based screening and recruitment which was followed by a second stage of enrollment resulting in an “in person” baseline visit, called T1. Subsequently, there were 6 follow-up telephone contact visits every 3 months (T2–T7). This study was conducted under the approval of the Institutional Review Boards at Northwestern University, the John H. Stroger, Jr. Hospital of Cook County, Rush-Presbyterian-St. Luke'sMedical Center, Children'sMemorial Hospital (Chicago), the Archdiocese of Chicago and the Chicago Public Schools.
Fig. 1.
Design Of The CHIRAH Study.
2.1. Community-wide population-based screening and recruitment
2.1.1. School selection
As we sought to enroll a diverse patient sample, and because of striking differences in the sociodemographic makeup of student populations in the public and private systems in Chicago, CHIRAH recruited from both systems. In 2002, Chicago Public Schools (CPS) consisted of 458 elementary schools with 309,572 students. Overall, CPS students were 52% African American, 36% Hispanic, and 9% white. Eighty-seven percent of CPS students were considered low income, defined as coming from families who are receiving public aid, living in institutions for neglected or delinquent children, being supported in foster homes with public funds, or being eligible to receive free or reduced-price lunches. In 2002, the Archdiocese of Chicago had 41,907 students in 129 elementary schools. Archdiocese students were 25% African American, 26% Hispanic, and 43% white. Forty-one percent of Archdiocese students were low-income.
Schools were eligible if they had not participated in on-site asthma screening within the previous two years. Although schools can only estimate the percent of their enrollment that comes from outside their district, we eliminated schools whose enrollment was reported by school administration to be more than 50% from outside as we wished to be able to explore community-level influences on asthma.
The school selection strategy was based on the ultimate goal of enrolling the longitudinal sample neighborhood by neighborhood (with school districts as proxy). The schools were first stratified by percentage of African American race and then by income group so that 4 school sampling groups were created. The “high African American” (high AA) schools had enrollments of more than 50% African American students. The “low-income” schools had enrollments in which more than 70% of students received subsidized or free school lunch. One hundred and fifty schools were identified by a combination of population proportionate and cluster sampling methods within each of the 4 race-income sampling groups (high AA/low income, high AA/mid-income, low AA/low income and low AA/high income) [14].
Schools were selected in two waves. Initially, five schools in each race-income sampling group were selected with probability proportionate to their size to represent larger neighborhood areas. The two geographically closest cluster schools to each of these schools were also selected, resulting in a total of 40 schools in the first wave of recruitment. In the second year of recruitment, 78 additional schools were selected with population proportionate probabilities from the four race-income sampling groups. Of the 138 selected schools, 28 refused to participate, two were excluded due to status changes since initial screening, and 3 were unused at the end of recruitment, yielding a final sample of 105 schools (78 CPS, 27 ACS) that were widely dispersed throughout the city. Reasons for refusal generally related to competing academic priorities for the principal's attention and unwillingness to distract classes from their lessons. To our knowledge, there were no apparent systematic reasons for non-participation that might bias the sample. All children in grades K through 8 were eligible to be surveyed in the selected schools.
2.1.2. School asthma screening to assess household eligibility
The 1-page survey instrument had 4 primary components: social-demographic characteristics of the child and caregiver, an asthma screening tool, a question to identify asthma among household members, and a question to express the household's interest in being contacted for secondary screening for possible research participation (enrollment into the longitudinal cohort). The survey was produced as a single sheet with identical queries printed in English on the front and Spanish on the back.
The Brief Pediatric Asthma Screen Plus (BPAS+, asthma questions only) was used to assess child asthma status. The BPAS+ is a parent-report questionnaire that identifies children ages 6 to 12 years with diagnosed asthma and children who need evaluation for possible undiagnosed asthma [15,16]. Similar to the National Health Interview Survey, the BPAS+ asks the caregiver, “Has a doctor or nurse ever told you that your child has asthma?” Four respiratory symptom questions follow. In addition to assessment of the individual child, the CHIRAH household asthma screening tool included a question to assess asthma status of other members of the household.
2.1.3. School survey administration
The school surveys were distributed from May 2003 through June 2005. The study team distributed the survey in each classroom on a single day and answered students' questions. Students took the form home for completion by parents/caregivers and brought the completed form back to their teachers. Teachers batched the forms for pickup by the research staff the next week. All classrooms had a party for completing the survey. We distributed 62,005 survey forms and collected 48,917 completed forms for a completion rate of 79%. By school, completion rates ranged from 39.6% to 99.4%. Additional information on the initial school screening has been published elsewhere [17].
2.2. Enrollment and baseline study visit
2.2.1. Enrolling study participants
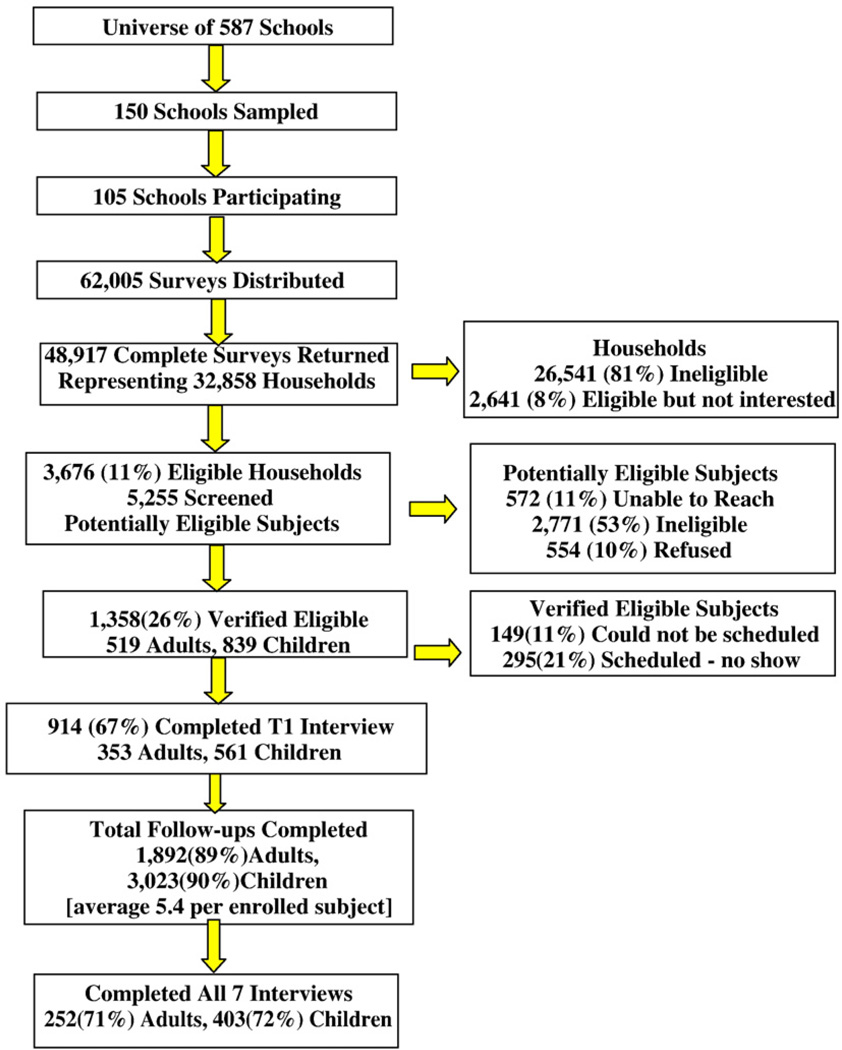
From the completed asthma screening surveys, 6317 households were identified with possible members having asthma. Interest in the study was indicated by 3676 households containing 5255 possibly asthmatic individuals who were selected for telephone screening for eligibility to participate in the longitudinal study.
Children were eligible if they were age 8–14 years old, and adults ages 18 to 40. All eligible study participants were required to have a history of physician or nurse-diagnosed (see screening question) persistent, symptomatic asthma, defined in this screening process as requiring at least 8 weeks of asthma medication over the previous 12 months. Exclusion criteria included: caregiver not fluent in spoken English, no telephone, and not residing in Chicago. Adults were not able to participate both as the caregiver and subjects in their own right. Eight hundred thirty-nine child-caregiver dyads and 519 adults were verified eligible and ultimately 561 child-caregiver dyads, and 353 adults were enrolled and completed the baseline protocol between January 24, 2004 and July 30, 2005. As seen in Fig. 2, major categories for non-participation included no eligible subjects in household, inability to reach eligible subjects and refusals.
Fig. 2.
CHIRAH Study recruitment.
2.2.2. Baseline interview, exam, and biologic testing
Once adults and caregivers were consented (children were assented) and enrolled, each study participant was asked to complete an in-person baseline interview (for adult–child dyads both were interviewed). Anthropometrics (height and weight), spirometry, and blood sampling were completed on participants with asthma only (not caregivers). These were conducted by trained research assistants in convenient community-based settings. Content for the baseline and follow-up interviews consisted of all or parts of several widely-used scales.
2.2.2.1. Demographic and socioeconomic variables
Ethnicity was assigned by self-report (for adults and caregivers) or caregiver report (for children) as per categories defined by the National Census [18]. Subjects could choose more than one ethnicity. To measure socioeconomic status, adults and caregivers were asked to classify themselves into 4 household income categories (<$15,000, $15,000–$30,000, $30,000–$50,000, and >$50,000) and report highest level of education completed (less than HS diploma, HS/GED graduate, some college, 4-year college graduate, or professional/graduate degree) and insurance status. Work status, home ownership, and government assistance also were assessed.
2.2.2.2. Asthma burden, quality of life, medications, and health care use
Measures of asthma burden consisted of two-week recall of asthma daytime symptoms (wheezing, chest tightness, cough, and shortness of breath) and separate recall of nighttime symptoms that resulted in waking up. For children, this was assessed by caregiver report.
For adults, Asthma Quality of Life (AQOL) in the past two weeks was measured using the Mini Asthma Quality of Life Questionnaire (MiniAQLQ) [19]. The 15 items consist of 4 domains: Symptoms, Activity Limitations, Emotional Function, and Environmental Stimuli. For each item, there was a response range of 1 to 7, with higher scores representing higher quality of life. An overall mean score as well as the above mentioned subscale scores were tallied.
Portions of the caregiver-reported responses to the Children's Health Survey for Asthma (CHSA) were used to measure AQOL in children (based on the past two weeks) [20]. Questions from the Child Activities, Family Activities, and Emotional Health subscales were asked in their entirety. For the CHSA, the medication items were not included as these domains were covered elsewhere in the asthma burden section of the interview. In addition, seven items of the 15 item Physical Health subscale (excluding asthma medication questions) were asked for a total of 23 items, each item having a response that ranged from 1 to 5 (higher numbers indicating more positive outcomes). An overall mean score as well as mean subscale scores were calculated from the 23 items. Subscale scores were transformed to a 0–100 scale.
Study participants were asked to bring their medications to the interview. For those who did not, photographs of commonly used asthma medications were available for reference. Frequency of medication use was assessed within a two-week recall period. For children, we assessed medication use by caregiver report. The analysis used dichotomous variables indicating use of beta agonists and/or inhaled corticosteroids in the past 14 days as well as steroid burst use in the past 12 months. Severity of asthma was assigned to each study participant at the baseline and subsequent visits based on symptoms burden and beta agonist use as derived from the NAEPP EPR2 [11].
The baseline interview included history of emergency department, hospitalization and urgent care doctor visits for asthma in the preceding 12 months, as well as a number of questions focused on quality of care, access to care, and experience with health care. Asthma knowledge, attitudes, locus of control, and beliefs were assessed as were perceived barriers to health care.
2.2.2.3. General health status
For both adults and caregivers (in the case of enrolled children) health status was measured using the general health question from the SF-12® Health Survey [21]. Adults and caregivers report general health status on a point scale ranging from 1 to 5 corresponding from excellent to poor. Due to small numbers in the poor category, categories 4 and 5 were combined, resulting in 4 categories.
We measured child health status using the general health question on the parent form of the Child Health Questionnaire (CHQ-PF50), a generic assessment tool specifically developed for children and adolescents five years of age and older [22]. Caregivers rated their child's health in general on a 5-point scale from excellent to poor. We collapsed poor and fair due to a small number of respondents reporting poor child health. The questionnaire overall and the general health item in particular have been shown to have good internal consistency and item discriminant validity in a variety of populations. The Pediatric Symptom Checklist was included to further characterize the child's health status. The Pediatric Symptom Checklist [23] is a 35-item screening instrument designed to assess cognitive, emotional, and behavioral problems in children in order to determine whether further evaluation is needed.
2.2.2.4. Health literacy
Caregiver and adult health literacy was assessed using the Rapid Estimate of Adult Literacy in Medicine (REALM) [24,25]. This measure involves respondents reading aloud a list of 66 medical terms that are scored by a trained research assistant based upon whether or not the word is pronounced correctly. The REALM score equals the number of words that are pronounced correctly with a dichotomous classification of adequate literacy (scores above 60) or inadequate literacy. The REALM was validated against standardized reading tests as well as the Test of Functional Health Literacy in Adults (TOFHLA) [26,27]. The REALM was selected for this study because it can be administered quickly with minimum respondent burden.
2.2.2.5. Symptoms of Depression
Adults and caregivers of enrolled children were assessed using the Center for Epidemiologic Studies-Depression Scale (CES-D) which is a 20 item screen for depression in the general population [28]. Questions are framed in terms of the past week with scores ranging from 0 to 60. A cutoff of 16 or higher is considered to be suggestive of depression in adults. Studies have found this cutoff to have a sensitivity between 0.75 and 0.84 and a specificity between 0.68 and 0.80 for clinical depression [29].
2.2.2.6. Stress and life events
For both caregivers and adults with asthma, stress was assessed using the Perceived Stress Scale (PSS). The PSS is a 10-item self-report measure developed to assess the degree to which life situations are appraised as stressful [30]. The PSS time frame for responses is “the past week.” The PSS can be completed in approximately 5 min. Cronbach alpha reliability coefficient ranged from 0.84 and 0.86 across samples. The PSS has been shown to be sensitive to measuring change in stress levels following intervention [31]. The psychometric properties of the PSS have been evaluated within minority populations and items have been shown to be relatively invariant to sex, race, and education [32,33].
In addition, adults and adult caregivers completed a measure of stressful life experiences. The Crisis in Family Systems (CRISYS) is a 63-item instrument designed to measure life events experienced by adults from diverse backgrounds [34]. The time frame for the responses is the prior six months. Construct validity is supported by its correlation with symptoms of depression (total stressors and CES-D, r=.44; negative stressors and CES-D, r=.58, p<.001). The test–retest reliability for total count of stressors is 0.86 and for total count of negative stressors is 0.93. Three dimensions of stress are assessed: valence of the stress (whether the experience of the stressor was positive, negative or neutral), difficulty, and chronicity. There are 11 content domains of life stressors including: financial, legal, career, relationships, safety in the home, safety in the community, medical issues pertaining to respondent, medical issues pertaining to others, home issues, difficulty with authority, prejudice and two single items (literacy and substance use). The psychometric properties of the CRISYS have been established in African-American and low literacy populations, and the items are written at a third grade reading level.
Additional areas of focus for the baseline interview included adult and caregiver tobacco use, alcohol and drug use, history of discrimination and, for women, any history of violence.
2.2.2.7. Child interview
While most of the baseline interview focused on adult and caregiver report of information, children were also asked to complete a short interviewer administered questionnaire that included a series of questions on medication use, coping with illness (KidCope), [35,36] and the youth report of the pediatric checklist [37].
2.2.2.8. Anthropometrics/biologics
All study subjects (children and adults with asthma) were asked to complete the following clinical tests: spirometry, height, weight, cotinine by saliva, and specific IgE in vitro blood tests. Weight was obtained using a calibrated digital scale, and standing height was measured using a stadiometer.
2.2.2.9. Spirometry
Spirometry was performed during the baseline study visit. Trained research assistants utilized a SpiroPro® spirometer. (Jaeger a subsidiary of VIASYS Health-care/Cardinal Health, Conshohocken, PA, USA) and followed the guidelines of the American Thoracic Society/European Respiratory Society [38]. Following baseline spirometry, participants were instructed to use two puffs of bronchodilator medication as prescribed by their physician or health care provider, using a spacer provided by the research assistant. A repeat spirometry was carried out 20 min after bronchodilator administration. Spirometry data were then uploaded from SpiroPro into Vmax v 12.1, a pulmonary physiology analysis software (SensorMedics, Yorba Linda, CA, USA).
References for spirometry values were determined utilizing age, standing height, gender and ethnicity. For subjects who self-identified as “only” white, AA, or Hispanic/Mexican we used the NHANES III reference standards. For subjects of mixed or undeclared ethnic background, we used the Mexican-American standards, which fall in the middle of those for AAs and whites. This would apply to anyone with anything other than “only” white, AA, or Hispanic/Mexican (including Asians).
Based on biologic understanding of asthma and practical issues, specifically the lack of post-bronchodilator values on many subjects who had normal or near-normal baseline values, this report includes only pre-bronchodilator spirometry values [39,40].
2.2.2.10. Cotinine levels
To determine tobacco exposure through salivary cotinine, saliva specimens were collected by placing a cellulose pad affixed to a polypropylene stem (Quantisal, Immunalysis, Pomona, CA) under the tongue of the study participant until an adequate volume of saliva saturated the cellulose pad, as indicated by coloration in the window on the stem. Assays were performed using a high sensitivity quantitative immunoassay (Salimetrics, Inc, State College, PA), calibrator range 0.8–200 ng/mL, sensitivity 0.05 ng/mL.
Studies using similar methods have suggested that salivary cotinine levels ranging from 1–7 ng/ml may be the result of passive smoking for both adults [41–43] and children [43–45]. One of these studies suggested a level of 8 ng/ml or greater is associated with active smoking for adults and afforded a sensitivity of 0.975 and a specificity of 0.968 [41]. For the purposes of describing the smoking status of our cohort, cotinine levels greater than 7 ng/ml indicated active smoking, 1–7 ng/ml passive exposure, and <1 ng/ml no exposure.
2.2.2.11. ImmunoCAP atopy testing
Each subject was requested to provide a blood specimen for specific IgE testing for two allergens: house dust mite (Dermatophagoides pteronyssinus) and cockroach (Blattella germanica). Levels of specific IgE>0.35 kUA/L were considered positive (ImmunoCAP system, Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden).
2.3. Follow-up study visits
Between May 10, 2004 and March 8, 2007, each enrolled participant (caregiver for the child) was followed-up by a series of six periodic telephone visits at three month intervals. These visits were conducted from a centralized call center staffed by professional interviewers. The duration of telephone contact for each visit varied in mean length from 18 to 37 min. Each interview included repeated assessment of asthma burden (2-week recall on asthma symptoms and medications, and 3 month recall on health care use) and asthma-related quality of life. In addition, participants were asked to complete reassessments of depressive symptoms (CES-D), stress, anxiety, coping, health care access, asthma self-efficacy, information seeking and health insurance. Every six months, follow-up items included stressful life events (CRISYS), asthma knowledge, discrimination based on ancestry, smoking, drug use, and employment. At selected periodic interviews assessments were conducted on perceived stigma associated with asthma, spirituality, barriers to care, perceived control, the home environment, discrimination based on gender, and wealth/income.
3. Analysis
The purpose of this report is to examine the overall burden of asthma morbidity and treatment as seen from the perspective of this community-based study. As such, the data are presented primarily as descriptive findings. ANOVAs and t-tests were used to compare groups and Pearson correlations were used to assess the relationship between continuous variables.
4. Results
4.1. Asthma prevalence in the community—the results of the school screening
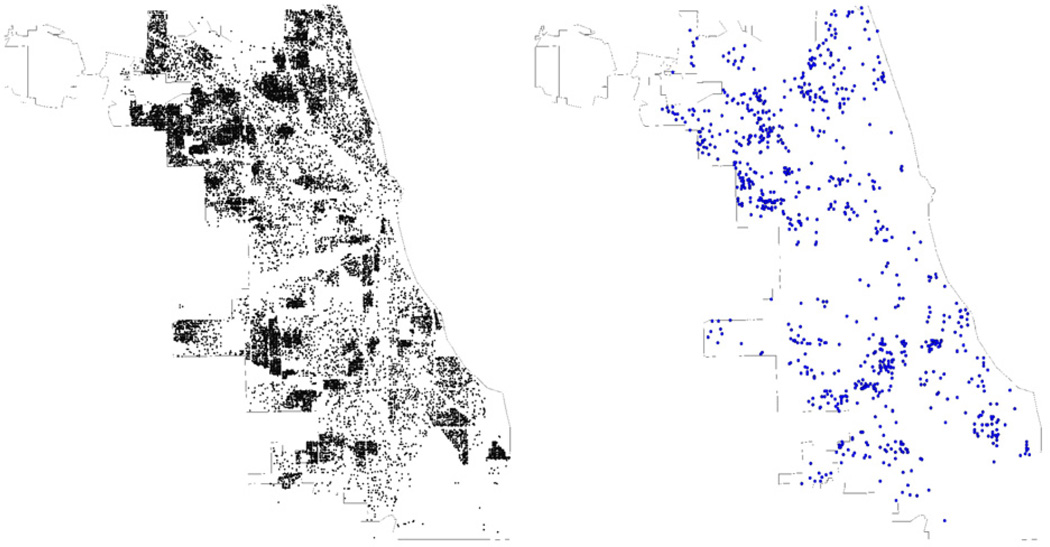
A total of 48,917 children completed the screening process (78.9%) among the 105 schools. Fig. 3 provides a map of Chicago that identifies the distribution of the residences of those children who were screened. The figure suggests that this screening process was successful at achieving a broad sampling of the Chicago community. The overall prevalence of diagnosed asthma in this population was 13.5%. An additional 12.4% of the children had symptoms that could suggest possible undiagnosed asthma. As seen in Table 1, asthma prevalence varied by race/ethnicity. Additional publications provide further detail on the results of this asthma screening [17,46,47].
Fig. 3.
Locations of surveys returned (n=41,255) and T1 cohort (n=914) in Chicago.
Table 1.
Demographic characteristics and asthma status at screening of Chicago public and parochial elementary schools.
| Diagnosed Asthma n=6625, 13.5% |
Possible undiagnosed asthma n=6074, 12.4% |
No asthma n=34,850, 71.2% |
Missing asthma status n=1368, 2.8% |
Total n=48,917 | |
|---|---|---|---|---|---|
| Age mean(SD) | 9.2(2.8) | 8.8(2.9) | 9.1(2.8) | 9.1(2.7) | 9.0(2.8) |
| Gender (% female) | 42.9 | 50.7 | 51.8 | 50.5 | 50.4 |
| Race/ethnicity (%) | |||||
| HA | 34.9 | 41.1 | 42.7 | 47.4 | 41.5 |
| AA/non HA | 43.9 | 32.1 | 25.7 | 24.6 | 28.9 |
| White/non AA, HA | 15.3 | 20.8 | 22.4 | 12.4 | 20.9 |
| Other | 4.8 | 5.1 | 7.8 | 5.4 | 7.0 |
| Language (% Spanish) | 9.3 | 17.5 | 17.5 | 33.9 | 16.8 |
| Cell (%) | |||||
| Low AA, Mid Income | 31.7 | 36.3 | 38.9 | 27.3 | 37.3 |
| High AA, Mid Income | 10.5 | 8.4 | 7.1 | 5.9 | 7.7 |
| Low AA, Low Income | 31.0 | 35.3 | 37.4 | 48.9 | 36.6 |
| High AA, Low Income | 26.9 | 20.1 | 16.6 | 17.9 | 18.5 |
HA=Hispanic American, AA=African American.
4.2. General description of the study cohort
As noted above, a total of 561 child/caregiver dyads, and 353 adults with asthma were enrolled in the study. The study population of adults averaged 30.9 (+/− 6.1) years old and was 77.9% female. For children, the study population averaged 10.6 (+/− 1.8) years old and was 41.4% female. Caregivers averaged 38.3 (+/−.1) years old and were 93.6% female. As seen in Table 2, the distribution of race/ethnicity was similar across both child and adult cohorts, 56.7–57.4% African American (AA) /non-Hispanic, 22.1–28.3% Hispanic, and 13.6–19.2% white/non AA/non Hispanic.
Table 2.
Demographic Characteristics and General Health Status at Baseline.
| Adult (n=353) |
Child/caregiver (n=561) |
||
|---|---|---|---|
| Child | Caregiver | ||
| Age mean(SD) | 30.9(6.1) | 10.6(1.8) | 38.3(8.1) |
| Gender (% female) | 77.9 | 41.4 | 93.6 |
| Race/ethnicity (%) | |||
| HA | 28.3 | 25.1 | 22.1 |
| AA/non HA | 56.7 | 57.4 | 56.7 |
| White/non AA, HA | 13.6 | 15.9 | 19.2 |
| Other | 1.4 | 1.6 | 2.0 |
| General health | |||
| Perceived health status (%) | |||
| Excellent | 6.8 | 13.5 | 9.8 |
| Very good | 24.4 | 37.1 | 26.0 |
| Good | 37.7 | 33.5 | 39.8 |
| Fair | 28.3 | 14.8 | 21.9 |
| Poor | 2.8 | 1.1 | 2.5 |
| BMI percentile for age and gender (median with IQR) |
NA | 89.1 (62.3–97.6) | NA |
| Percent overweight a | 78.4 | 56.2 | NA |
| Percent obese b | 54.3 | 35.9 | NA |
| Cotinine level median (IQR) | 2.5(1.0–153.7) | 1.4(0.9–2.6) | NA |
| % Smoke (cotinine level > =8 ng/ml) |
35.7 | 4.4 | NA |
| Dust mite sensitization (% IgE>.35 /L) |
46.4 | 36.6 | NA |
| Cockroach sensitization (% IgE>0.35 ku A/L) |
46.1 | 26.4 | NA |
HA=Hispanic American, AA=African American, NA=not applicable.
Adults BMI>25, Children>85th BMI percentile for age and gender.
Adults BMI>30, Children>95th BMI percentile for age and gender.
As shown in Table 3, approximately 50% of both adults and child/caregivers reported incomes below $30,000 annually. More than 60% of the entire study cohort (adults and caregivers) reported that they work for pay and more than 85% reported some type of insurance coverage. Health literacy as measured by the REALM was generally low, with 32.5% of adults and 28.4% of caregivers scoring below an eighth grade reading level. Table 3 also provides additional detail on other socio-demographic characteristics of this population.
Table 3.
Socioeconomic characteristics at baseline.
| Adult (n=353) |
Child/caregiver (n=561) |
|
|---|---|---|
| Income (%) | ||
| <$15,000 | 28.3 | 20.7 |
| $15–30,000 | 25.5 | 28.0 |
| $30–50,000 | 18.1 | 18.5 |
| >$50,000 | 28.1 | 32.8 |
| Education (%) | ||
| >High School | 17.0 | 13.0 |
| High School/GED | 32.6 | 26.9 |
| Some College | 34.5 | 41.2 |
| Baccalaureate | 10.8 | 13.0 |
| Prof/Graduate | 5.1 | 5.9 |
| Insurance status medical bills (%) | ||
| Medicaid | 41.5 | 41.2 |
| Private insurance | 45.2 | 48.5 |
| Self pay | 13.3 | 2.8 |
| Kidcare | N/A | 7.5 |
| Insurance status prescriptions (%) | ||
| Medicaid | 34.9 | 42.6 |
| Private insurance | 4.6 | 5.9 |
| Insurance and self pay | 45.2 | 44.6 |
| Self pay | 10.5 | 3.9 |
| Free | 4.8 | 3.0 |
| Living conditions (%) | ||
| Own | 28.9 | 36.0 |
| Rent | 54.4 | 56.3 |
| Live relatives | 16.2 | 7.7 |
| Work for pay (%) | 61.8 | 65.1 |
| Assistance (%) | 57.2 | 54.7 |
| Number of people in home mean (SD) | 4.8(1.8) | 4.7(1.7) |
| Household density mean (SD) | 0.9(0.4) | 0.8(0.3) |
Percentages not adding to 100 reflect missing data.
Fifty-four percent of the adult participants were obese with an average BMI of 32.4 (+/−9.2) kg/m2. More than a third of the child participants were determined to be obese.
Depressive symptoms were common within the adult population and among the caregivers. For the adult participants, 45.5% had CES-D scores that exceeded the cutoff, suggesting possible depression. Total number of life stressors, measured by the CRISYS, was similarly reported by adults 10.2 (+/−6.2) and caregivers 9.2 (+/−6.1). Life stressors were positively correlated with total CES-D scores both in adults (r=0.46, p<.0001) and in caregivers (r=0.43, p<.0001). Caregiver report of psychosocial impairment, defined as >28 on the PSC, occurred in 30.1% of the children.
4.3. Asthma burden
Table 4 provides an overview of asthma burden in this population. The adult study participants reported a median 3 (IQR 2–7) symptom days over the past 14 days and a median of 2 (IQR 0–4) nights awakened with symptoms. Caregivers reported a median 2 (IQR 0–4) symptom days and 1 (IQR 0–4) symptom nights for children.
Table 4.
Burden of asthma, medication and healthcare utilization at baseline.
| Adult (n=353) |
Child (n=561) |
|
|---|---|---|
| Asthma burden | ||
| Symptom days/14 median (IQR) | 3(2–7) | 2(0–4) |
| Symptom nights/14 median (IQR) | 2(0–4.5) | 1(0–3) |
| Symptom days+nights median (IQR) | 6(3–12) | 4(1–8) |
| AQOL (range 1–7) mean (SD) | 4.4(1.2) | NA |
| CHSA (range 1–5) median (IQR) | NA | 4.4(3.6–4.9) |
| Lung function | ||
| Percent predicted FEV1 pre-bronchodilator mean(SD) |
87.0(18.5) | 97.1(15.7) |
| Medication use | ||
| Beta agonist use days/14 median(IQR) | 4(1–12) | 2(0–6) |
| Inhaled steroid use days/14 median(IQR) | 0(0–14) | 0(0–12) |
| Steroid bursts/12 months median (IQR) | 0(0–1) | 0(0–2) |
| Asthma healthcare use | ||
| Hospitalizations/12 months median (IQR) | 0(0–0) | 0(0–0) |
| Emergency room visit/12 months median (IQR) |
0(0–1) | 0(0–1) |
| Same day office visits/12 months median (IQR) |
0(0–1) | 0(0–2) |
| Routine office visit/12 months median (IQR) | 2(0–4) | 2(1–4) |
AQOL=Juniper Asthma Quality of Life (modified, see text), CHSA=Children's Health Status for Asthma (this is a modified version of the CHSA, see text).
In the 314 adults (89.0%) and 399 children (71.1%) with accurate spirometry test readings, the average pre-bronchodilator FEV1 percent predicted was 87.0% [IQR 75.9–98.5 (22.6)] and 97.1% [IQR 87.1–107.9 (20.8)] respectively.
4.4. Asthma quality of life
Overall, the Asthma Quality of Life scores were high. Adult scores averaged 4.4 (1.2 SD) on the MiniAQLQ and caregivers reported a median of 4.4 (IQR 3.6–4.9) on the CHSA items used.
4.5. Medication use
Among the adult and child participants, 78.5% and 65.1% respectively reported beta-agonist use in the past two weeks. Alternatively, inhaled steroid use was infrequent, with only 41.6% of adults and 40.1% of children reporting use in the prior two weeks.
Fig. 4 characterizes controller use in the context of asthma related symptoms as per the NAEPP guidelines. In the 84.1% of adults with symptoms consistent with moderate or severe persistent asthma, only 42.4% were on an inhaled corticosteroid, and 37.8% were on any other controller. Similar low use was seen for the 58.7% of children with symptoms consistent with moderate or severe persistent asthma, only 42.3% were on an inhaled corticosteroid and 38.0% were on any other type of controller.
Fig. 4.
Proportion of adults and children with moderate or severe persistent asthma on medications.
4.6. Asthma-related health care use
Table 4 also characterizes asthma related health care use. Thirteen percent of adults reported an asthma hospitalization in the past year, 40.5% reported an asthma-related emergency department visit, and 28.5% reported one or more urgent visits to their physician for asthma related symptoms. Caregivers reported similar results for the children, with hospitalizations at 10.7% and emergency department visits at 39.5%, but quite a few more children had urgent care visits (48.1%).
4.7. Tobacco exposure and atopy
Of the 291 (82.4%) adults and 504 (95.6%) children who provided saliva samples, 35.7% and 4.4% respectively had a cotinine assay consistent with active smoking; only 28.5% of the adults reported they smoked. Cotinine levels indicated 38.5% of adults and 65.5% of children were exposed to tobacco smoke.
As noted in Table 4, 46.4% of adults had clinically significant IgE to DerP and 46.1% had IgE to cockroach. Similarly, children also had moderate atopy to these two allergens, 36.6% with clinically significant IgE to the major allergen in a house dust mite (DerP1), and 26.3% with IgE to cockroach.
4.8. Asthma outcomes over time
Over the 18 months of the follow up period only one adult and five child/caregiver dyads disenrolled from the study. The 1892 adult and 3021 caregiver interviews represent overall completion rates of 89 and 90% respectively. The interview completion rate varied from 85% to 93% across the specific follow up periods. Seventy-one percent of adults and 72% of caregivers completed the baseline interview and all six follow-up interviews (Table 5 ).
Table 5.
Subject retention.
| Interviews completed |
Adults |
Caregivers |
||
|---|---|---|---|---|
| N | % | N | % | |
| 7 | 252 | 71 | 403 | 72 |
| 6 | 49 | 14 | 75 | 13 |
| 5 | 18 | 5 | 34 | 6 |
| 4 | 13 | 4 | 15 | 3 |
| 3 | 11 | 3 | 19 | 3 |
| 2 | 2 | 1 | 10 | 2 |
| 1 | 8 | 2 | 5 | 1 |
| Total | 353 | 100 | 561 | 100 |
5. Discussion
It has now been more than 15 years since the publication of reports of increasing asthma prevalence and morbidity in the US. Asthma mortality and morbidity have demonstrated some improvements [48] however they have not been uniform across population groups [49]. In response to these disturbing trends in asthma epidemiology, the NIH through the NAEPP responded with an effort to establish national guidelines for the care of persons with asthma. These guidelines have been widely distributed and have been the basis of numerous attempts to implement asthma care quality improvement efforts.
The results of this study suggest that, at least for one large urban environment, there is still a substantive burden of asthma morbidity. This burden appears to be associated with a high degree of inadequate asthma control as compared to NAEPP guideline recommendations regarding appropriate medication use for persons with asthma.
Aside from the CHIRAH project, there are very few other community-based studies of asthma burden in the literature. The largest surveillance initiative is being conducted by the National Center for Health Statistics. Since the late 1990s, the Centers for Disease Control (CDC) have reported small but steady reductions in asthma mortality rates and hospitalization rates [48]. In 1998, GlaxoSmithKline conducted a national telephone survey of asthma morbidity in the US to gain a better understanding of asthma burden [50]. Some of their key findings were a high degree of asthma symptom burden and a general lack of asthma control and use of anti-inflammatory medications [50,51].
It appears that the only published population-based insight to both quality of life and quality of care comes from a CDC sponsored telephone survey in New York conducted in 2002–2003—the National Asthma Survey-New York State (NAS-NYS). The main findings from that survey reported lower asthma symptoms and exacerbations than were found in this CHIRAH population [52].
In 1990, Chicago was recognized as having one of the highest asthma mortality rates in the US [4]. The CHIRAH study suggests that currently, more than decade and a half after the release and broad dissemination of national guidelines, asthma in Chicago is poorly controlled and inadequately clinically managed in the general population. What might be the reasons for this lack of progress? The results of this study suggest that the health care needs for many persons with asthma in this population may require consideration of issues that go beyond asthma in clinical management. For example, this population demonstrated high prevalence of both coincident obesity and depression related symptoms. Further understanding of such co-morbidities and their relationship to asthma may lead to better clinical strategies that are tailored for this population.
Perhaps the high asthma burden in this population is related to inadequate health care. This would be supported by the lack of use of anti-inflammatory therapy for persons that appear to have moderate-severe asthma symptoms. Other results from this study reported elsewhere that some of the factors associated with this burden may be associated with the community in which the participants live [53]. Ultimately, further studies on this issue will likely lead to an understanding that it is some combination of hierarchical relationships between individual, health system, and community factors that are contributing to this continued public health problem.
What amount of the asthma burden is due to personal health behaviors? In subsequent analyses, we will be exploring relationships between symptoms, perceptions of bother, outcomes expectancy, and use of controller medications. We replicate the findings of others [54] who have shown a disturbing and widespread exposure of these adults and children with asthma to tobacco smoke, through direct use or indirect exposure. Tobacco smoke is known to increase the likelihood of persistence in asthma, to increase asthma exacerbations, and to decrease the effectiveness of inhaled and oral corticosteroids, and to hasten the decline of lung function in asthma. Given the modest improvements in use of controller medicines since the publications of the original guidelines, perhaps more efforts should be expended on clinical trials addressing effective methods of reducing tobacco use and tobacco exposure among people with chronic lung disease [55].
In November 2007, the NAEPP released its most recent version of the national guidelines. These guidelines, while up to date on the advances in clinical assessment and treatment of asthma, provide little information or direction to address complex hierarchical relationships between individuals and their communities. Additional community-based research may lead to new insights in asthma morbidity that could inform the development of future clinical guidelines and public health programs to reduce the burden of asthma in high risk areas.
Acknowledgements
We thank those individuals and families in Chicago who participated in our study. We also thank the Chicago Public and Archdiocese Schools for allowing us to conduct asthma screening among their elementary schools. We would like to express our appreciation to our team of research assistants and also thank Robert Cohen, MD for his assistance with interpreting PFTs and Robin Wagner, MHSA for her assistance with manuscript preparation. Finally, we thank the NHLBI for supporting this study and other Centers for Excellence in Reducing Asthma Disparities.
Footnotes
This study was supported by a grant from the National Heart, Lung, and Blood Institute (1-U01 HL 72496-1) sponsor of the Chicago Initiative to Raise Asthma Health Equity (CHIRAH).
References
- 1.Healthy People. Washington, DC: Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services, Government Printing Office; 2000. [PubMed] [Google Scholar]
- 2.Mannino DM, Homa DM, Akinbami LJ, et al. Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 3.Weiss KB, Gergen PJ,Wagener DK. Breathing better or wheezing worse? The changing epidemiology of asthma morbidity and mortality. Annu Rev Public Health. 1993;14:491–513. doi: 10.1146/annurev.pu.14.050193.002423. [DOI] [PubMed] [Google Scholar]
- 4.Weiss KB, Wagener DK. Changing patterns of asthma mortality. Identifying target populations at high risk. JAMA. 1990;264:1683–1687. [PubMed] [Google Scholar]
- 5.Schmidt DK, Fulwood R, Lenfant C. The National Asthma Education and Prevention Program: partnering with local asthma coalitions to implement the guidelines. Chest. 1999;116:235S–236S. doi: 10.1378/chest.116.suppl_2.235s. [DOI] [PubMed] [Google Scholar]
- 6.Shannon JJ, Catrambone CD, Coover L. Targeting improvements in asthma morbidity in Chicago: a 10-year retrospective of community action. Chest. 2007;132:866S–873S. doi: 10.1378/chest.07-1923. [DOI] [PubMed] [Google Scholar]
- 7.Kwon HL, Ortiz B, Swaner R, et al. Harlem Children's Zone Asthma Initiative. Childhood asthma and extreme values of body mass index: the Harlem Children's Zone Asthma Initiative. Urban Health. 2006;83:421–433. doi: 10.1007/s11524-006-9050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark NM, Doctor LJ, Friedman AR, et al. Community coalitions to control chronic disease: Allies against asthma as a model and case study. Health Promot Pract. 2006;7:14S–22S. doi: 10.1177/1524839906287055. [DOI] [PubMed] [Google Scholar]
- 9.National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 1991. NIH publication 91-3642. [Google Scholar]
- 10. [Last accessed June 11, 2008]; http://www.nhlbi.nih.gov/about/naepp/naep_pd.htm.
- 11.National Asthma Education and Prevention Program. Expert panel report 2: guidelines for the diagnosis and management of asthma. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 1997. NIH publication 97-4051. [Google Scholar]
- 12.National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 2007. NIH publication 08-5846. [Google Scholar]
- 13.Thomas S, Whitman S. Asthma hospitalizations and mortality in Chicago: an epidemiologic overview. Chest. 1999;116:135S–141S. doi: 10.1378/chest.116.suppl_2.135s. [DOI] [PubMed] [Google Scholar]
- 14.Kish L, Heeringa S, Kalton G. Leslie Kish: selected papers. Hoboken, NJ: J Wiley; 2003. [Google Scholar]
- 15.Wolf RL, Berry CA, Quinn K. Development and validation of a brief pediatric screen for asthma and allergies among children. Ann Allergy Asthma Immunol. 2003;90:500–507. doi: 10.1016/S1081-1206(10)61843-1. [DOI] [PubMed] [Google Scholar]
- 16.Berry CA, Quinn K, Wolf R, et al. Validation of the Spanish and English versions of the asthma portion of the Brief Pediatric Asthma Screen Plus among Hispanics. Ann Allergy Asthma Immunol. 2005;95:53–60. doi: 10.1016/S1081-1206(10)61188-X. [DOI] [PubMed] [Google Scholar]
- 17.Shalowitz MU, Sadowski LM, Kumar R, Weiss KB, Shannon JJ. Asthma burden in a citywide, diverse sample of elementary schoolchildren in Chicago. Ambul Pediatr. 2007;7:271–7. doi: 10.1016/j.ambp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 18. [Last accessed July 21, 2008]; http://www.census.gov.population/www/socdemo/race/Ombdir15.html.
- 19.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 20.Asmussen L, Olson LM, Grant EN, Fagan J,Weiss KB. Reliability and validity of the Children's Health Survey for Asthma. Pediatrics. 1999;104:e71. doi: 10.1542/peds.104.6.e71. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Kosinski M, Turner-Bowker DM, Gandek B. How to score version 2 of the SF-12™ health survey (with a supplement documenting version 1) Lincoln (RI): QualityMetric Incorporated; 2002. [Google Scholar]
- 22.Landgraf JL, Abetz L, Ware JE. The CHQ user's manual. Boston: The Health Institute, New England Medical Center; 1996. [Google Scholar]
- 23.Jellinek MS, Murphy JM, Robinson J, et al. Pediatric symptom checklist: screening school-age children for psychosocial dysfunction. J Pediatr. 1988;112:201–209. doi: 10.1016/s0022-3476(88)80056-8. [DOI] [PubMed] [Google Scholar]
- 24.Davis TC, Crouch MA, Long S, et al. Rapid assessment of literacy levels of adult primary care patients. Fam Med. 1991;23:433–435. [PubMed] [Google Scholar]
- 25.Davis TC, Michielutte R, Askov EN, Williams MV, Weiss BD. Practical assessment of adult literacy in healthcare. Health Educ Behav. 1998;25:613–624. doi: 10.1177/109019819802500508. [DOI] [PubMed] [Google Scholar]
- 26.Davis TC, et al. Literacy testing in health care research in understanding health literacy: implications for medicine, public health. In: Schwartzberg JG, VanGeest JB, Wang CC, editors. Chicago, IL: AMA Press; 2004. pp. 157–179. [Google Scholar]
- 27.Parker RM, Baker DW, Williams MV, Nurss JR. The test of functional health literacy in adults: a new instrument for measuring patients' literacy skills. J Gen Intern Med. 1995;10:537–541. doi: 10.1007/BF02640361. [DOI] [PubMed] [Google Scholar]
- 28.Radliff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–410. [Google Scholar]
- 29.Mulrow CK, Williams JW, Jr, Gerety MB, et al. Case-finding instruments for depression in primary care settings. Ann Intern Med. 1995;122:913–921. doi: 10.7326/0003-4819-122-12-199506150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soci Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 31.Johnson SK, Frederick J, Kaufman M, Mountjoy B. A controlled investigation of bodywork in multiple sclerosis. J Altern Complement Med. 1999;5(3):237–243. doi: 10.1089/acm.1999.5.237. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Williamson G. Perceived stress in a probability sample in the US. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 33.Cole S. Assessment of the differential item functioning in the perceived stress scale-10. J Epidemiol Community Health. 1999;53:319–320. doi: 10.1136/jech.53.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33(5 Pt 1):1381–1402. [PMC free article] [PubMed] [Google Scholar]
- 35.Knapp LG, Stark LJ, Kurkjian JA, Spirito A. Assessing coping in children and adolescents. Educ Psychol Rev. 1991;3:309–334. [Google Scholar]
- 36.Spirito A, Stark LJ, Williams C. Development of a brief coping checklist for use with pediatric populations. J Pediatr Psychol. 1988;13:555–574. doi: 10.1093/jpepsy/13.4.555. [DOI] [PubMed] [Google Scholar]
- 37.Jellinek M, Murphy J, Robinson J, et al. Pediatric Symptom Checklist: screening school-age children for psychosocial dysfunction. J Pediatr. 1988;112:201–209. doi: 10.1016/s0022-3476(88)80056-8. [DOI] [PubMed] [Google Scholar]
- 38.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 39.Pellegrino R, Viegi G, Brusasco V, et al. Interpretive strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 40.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Nishida N, Tanaka M, et al. Association between passive and active smoking evaluated by salivary cotinine and periodontitis. J Clin Periodontol. 2005;32:1041–1046. doi: 10.1111/j.1600-051X.2005.00819.x. [DOI] [PubMed] [Google Scholar]
- 42.Binnie V, McHugh S, Macpherson L, et al. The validation of self-reported smoking status by analyzing cotinine levels in stimulated and unstimulated saliva, serum and urine. Oral Dis. 2004;10:287–293. doi: 10.1111/j.1601-0825.2004.01018.x. [DOI] [PubMed] [Google Scholar]
- 43.Scherer G, Meger-Kossien I, Riedel K, Renner T, Meger M. Assessment of the exposure of children to environmental tobacco smoke (ETS) by different methods. Hum Exp Toxicol. 1999;18:297–301. doi: 10.1191/096032799678840075. [DOI] [PubMed] [Google Scholar]
- 44.Langone JJ, Cook G, Bjercke RJ, Lifschitz MH. Monoclonal antibody ELISA for cotinine in saliva and urine of active and passive smokers. J Immunol Methods. 1988;114:73–78. doi: 10.1016/0022-1759(88)90156-1. [DOI] [PubMed] [Google Scholar]
- 45.Kuo HW, Yang JS, Chiu MC. Determination of urinary and salivary cotinine using gas and liquid chromatography and enzyme-linked immunosorbent assay. J Chromotogr B: Anal Technol Biomed Life Sci. 2002;768:297–303. doi: 10.1016/s1570-0232(01)00613-4. [DOI] [PubMed] [Google Scholar]
- 46.Gupta RS, Zhang X, Sharp LK, Shannon JJ, Weiss KB. Geographic variability in childhood asthma prevalence in Chicago. J Allergy Clin Immunol. 2008;121:639–645. doi: 10.1016/j.jaci.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 47.Mosnaim GS, Sadowski LS, Durazo-Arvizu RA, et al. Parental language and asthma among urban Hispanic children. J Allergy Clin Immunol. 2007;120:1160–1165. doi: 10.1016/j.jaci.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 48.Moorman JE, Rudd RA, Johnson CA, et al. Centers for Disease Control and Prevention (CDC). National surveillance for asthma—United States, 1980—2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 49.Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol. 2006;117:351–358. doi: 10.1016/j.jaci.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 50.Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the national asthma education and prevention program guidelines. Am J Respir Crit Care Med. 2002;166:1044–1049. doi: 10.1164/rccm.2107057. [DOI] [PubMed] [Google Scholar]
- 51.Adams RJ, Fuhlbrigge A, Guilbert T, Lozano P, Martinez F. Inadequate use of asthma medication in the United States: results of the asthma in America national population survey. J Allergy Clin Immunol. 2002;110:58–64. doi: 10.1067/mai.2002.125489. [DOI] [PubMed] [Google Scholar]
- 52. [Last accessed June 11, 2008];National Asthma Survey-New York State Summary Report. http://www.health.state.ny.us/statistics/ny_asthma/pdf/national_asthma_survey_nys.pdf.
- 53.Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132:757S–769S. doi: 10.1378/chest.07-1904. [DOI] [PubMed] [Google Scholar]
- 54.Morgan WJ, Crain EF, Gruchalla RS, et al. Inner-city asthma study group: results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 55.Chaudhuri R, Livingstone, McMahon AD, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174:127–133. doi: 10.1164/rccm.200510-1589OC. [DOI] [PubMed] [Google Scholar]