Summary
Maternal smoking during pregnancy is associated with increased risk of childhood overweight body mass index (BMI). Less is known about the association between prenatal secondhand tobacco smoke (SHS) exposure and childhood BMI. We followed 292 mother-child dyads from early pregnancy to 3 years of age. Prenatal tobacco smoke exposure during pregnancy was quantified using self-report and serum cotinine biomarkers. We used linear mixed models to estimate the association between tobacco smoke exposure and BMI at birth, 4 weeks, and 1, 2, and 3 years. During pregnancy, 15% of women reported SHS exposure and 12% reported active smoking, but 51% of women had cotinine levels consistent with SHS exposure and 10% had cotinine concentrations indicative of active smoking. After adjustment for confounders, children born to active smokers had higher BMI at 2 and 3 years of age (self-report or serum cotinine), compared to unexposed children. Children born to women with prenatal serum cotinine concentrations indicative of SHS exposure had higher BMI at 2 (Mean Difference [MD]:0.3; 95% confidence interval [CI]:−0.1, 0.7) and 3 (MD:0.4; [0, 0.8]) years compared to unexposed children. Using self-reported prenatal exposure resulted in non-differential exposure misclassification of SHS exposures that attenuated the association between SHS exposure and BMI compared to serum cotinine concentrations. These findings suggest active and secondhand prenatal tobacco smoke exposure may be related to an important public health problem in childhood and later life. In addition, accurate quantification of prenatal secondhand tobacco smoke exposures is essential to obtaining valid estimates.
Keywords: Body Mass Index, Children, Cotinine, Growth, Prenatal, Tobacco Smoke
Introduction
Between 15 and 20% of United States children have overweight body mass index (BMI) and the proportion of children with overweight BMI is increasing in other countries.1, 2 Elevated childhood BMI is associated with increased BMI and adiposity in adolescence and adulthood.3, 4 Increased childhood BMI may also be a risk factor for metabolic disorders in early adulthood and coronary heart disease in later adulthood.5, 6
Several studies have observed excess adiposity and increased risk of overweight or obese BMI among children born to women who actively smoke during pregnancy compared to children born to non-smokers.7–10 A recent meta-analysis of 14 studies indicates that children born to active smokers have 1.5-times the risk of being overweight or obese as children born to non-smokers.11 Increases in BMI and adiposity among children born to active smokers may be due to decreased weight and length at birth, rapid weight gain after birth, and decrements in height during childhood.10, 12–17
Less is known about the association between prenatal SHS exposure and childhood BMI. We are aware of only two studies examining the association between prenatal secondhand tobacco smoke (SHS) exposure and childhood BMI. Leary et al. reported elevated BMI, total body fat, and truncal fat among 10-year old children whose mothers had a partner who smoked during pregnancy.9 Oken et al. did not report any association between self-reported prenatal SHS exposure and BMI at 3 years of age.8
Prior studies examining the association between prenatal active and SHS exposure and childhood BMI have relied on self-reported exposures. Self-reported SHS exposures may result in exposure misclassification that could bias the association between prenatal exposure and childhood BMI.18–20 A prospectively collected, valid biomarker of exposure might provide a more accurate estimate of the dose of tobacco smoke constituents received by the mother and fetus.
Given widespread exposure to secondhand tobacco smoke,21 especially in newly industrialized countries,22, 23 and the potential adverse health consequences of increased childhood BMI, we investigated the association between prenatal tobacco smoke exposure and early childhood BMI. In addition, we estimated and compared the associations between self-report and serum cotinine biomarkers of prenatal tobacco smoke exposure and early childhood BMI.
Methods
Study Sample
We used data collected from mothers and their children participating in the Health Outcomes and Measures of the Environment Study, an ongoing prospective birth cohort in the Cincinnati metropolitan area designed to examine low-level environmental toxicant exposure and the efficacy of injury and lead hazard controls in the home.24 Between March of 2003 and January of 2006, women were identified from seven prenatal clinics associated with three hospitals. We mailed letters to 5,512 women ≥ 18 years of age who were living in a home built before 1978 to see if they were eligible and interested in participating in our study. Additional eligibility criteria included: ≤ 19 weeks gestation upon enrollment; living in Brown, Butler, Clermont, Hamilton, or Warren counties in Ohio; intention to continue prenatal care and deliver at collaborating obstetric practices; HIV negative; and not receiving seizure, thyroid, or chemotherapy/radiation medications. Of the 1,263 eligible women, 468 enrolled in our study. The current analyses were further restricted to singleton children. We did not collect additional demographic information from women who refused to participate.
Tobacco Smoke Measurements
Self-Reported Exposure
Women were interviewed twice about secondhand and active tobacco smoke exposures for the periods between conception and 20 weeks (measured at 20 week home visit) and 20 weeks and birth (measured at 4 week postpartum visit) by trained research staff. Trained interviewers asked the women the average number of cigarettes they smoked per day (if any), the number of smokers living in the home, and the number of cigarettes smoked per day in the home for each time period. Women were classified as unexposed if they reported no exposure for all of pregnancy. Women with active exposure during one or both periods were classified as active smokers. All others were classified as having SHS exposure.
Serum and Meconium Biomarkers of Exposure
Women provided blood samples around 16 weeks gestation, 26 weeks gestation, and within 24 hours of birth. All samples were stored at −20° C until they were transported to the Centers for Disease Control and Prevention (CDC) laboratory for analysis, where they were stored at or below −20° C. Serum from each sample was analyzed for cotinine, a biomarker of nicotine exposure, using high performance liquid chromatography-tandem mass spectroscopy (HPLC-MS/MS).25 The limit of detection (LOD) for this method was 0.015 ng/mL.
Prenatal serum cotinine concentrations were categorized as no exposure (<LOD), SHS exposure (LOD to 3 ng/mL), and active exposure (>3 ng/mL). The threshold of 3 ng/mL for active smoking was chosen based on results from the 1999–2004 National Health and Nutrition Examination Survey using self-reported smoking status and serum cotinine levels among a representative sample of the U.S. population.26 These results showed that using a higher cutpoint of 15 ng/mL as the threshold for active smoking underestimates the proportion of active smokers. Prenatal serum cotinine concentrations were also analyzed as a continuous log10-transformed variable. In addition, we estimated the association between continuous prenatal serum cotinine concentrations and BMI among women with no prenatal active smoking (serum cotinine concentrations ≤3 ng/mL at each measurement).
Infant and Childhood Weight and Height Measurements
Birthweight and length were abstracted from the child’s medical records. Subsequent weight and height measurements were obtained by study staff in the child’s home or at our study clinic at 4 weeks and 1, 2, and 3 years of age. Each measurement was taken three times and the mean was used in the analysis. Weight was measured at 4 weeks and 1 year with an infant scale and at 2 and 3 years with a pediatric scale. Length at 4 weeks and 1 year was taken using a length board. At 2 and 3 years, height was obtained using a stadiometer; however, if the child was uncooperative at 2 years, we used a length board. We did not record whether children’s length or height was measured at 2 years of age. BMI was calculated by dividing weight in kilograms by height in meters squared. While BMI may not be an ideal measure of excess adiposity before 2 years of age, the World Health Organization advocates using BMI as a measure of adiposity in the first two years of life.27
BMI was modeled as a continuous and dichotomous outcome. BMI was dichotomized to classify children as overweight at 2 or 3 years of age. We defined children as overweight if they had age and sex specific BMI ≥ the 85th percentile at 2 or 3 years of age. 28
Confounders
We used directed acyclic graphs (DAGs) to examine the role of confounding by sociodemographic, perinatal, and childhood nutrition factors in our exposure-outcome relation.29 Sociodemographic covariates were collected at the baseline clinic visit and included maternal age, race, education marital status, and household income. Perinatal variables were maternal depression, maternal BMI at 16 weeks gestation, and parity. Depression was measured at 20 weeks gestation with the Beck Depression Inventory (BDI-II).30 Maternal BMI was modeled as a continuous variable from weight and height measurements collected at the 16-week prenatal clinic visit. Parity was abstracted from medical records. Breastfeeding was collected quarterly during telephone interviews and included as a proxy of infant/childhood nutrition. We modeled breastfeeding as a time-varying covariate coded as the length of time (in fractions of years) in which the child received any breast milk since the last study visit. At birth, children were given a value of 0 years of breastfeeding.
The HOME Inventory, a semi-structured interview that assesses the quality of the caregiving environment, was administered by trained interviewers during a home visit when the child was 1 year old.31 The HOME score, as a continuous variable, was evaluated as an additional proxy for socioeconomic status in a secondary analysis that examined the potential for residual confounding after controlling for household income and maternal education. We did not adjust for gestational age at birth since our DAG placed it on a causal pathway between prenatal tobacco smoke exposure and childhood growth.
Statistical Analysis
We descriptively compared the demographic characteristics of mothers and their children who had complete and missing covariate or exposure data. We also calculated the Pearson correlation coefficients between log10-transformed prenatal serum cotinine concentrations at each pair of time points.
We examined the shape of the relationship between log10-transformed prenatal serum cotinine concentrations and children’s BMI at each measurement using Locally Weighted Scatter Plot Smoothing (LOESS).32 We observed an approximately linear relationship between log10-transformed prenatal serum cotinine concentration and BMI at each time.
Since our data involved repeated measurements on individuals, we used linear mixed models to examine the association between prenatal tobacco smoke exposure and BMI over the first 3 years of life.33 Models were fit with time since birth (i.e., age in years), time-squared, and time-cubed terms. We allowed the association between prenatal tobacco smoke exposure and childhood size to vary over time by including interaction terms between tobacco smoke exposure and time variables. Tobacco smoke parameter estimates from our categorical models represent the mean change in BMI from the reference category, while estimates from models using continuous cotinine concentrations represent the mean change for each unit increase in log10-transformed (i.e., 10-fold increase) serum cotinine concentrations.
We used logistic regression with generalized estimating equations to estimate the association between secondhand and active prenatal tobacco smoke exposure and being overweight (BMI ≥ 85th percentile) at 2 or 3 years of age.34 We generated odds ratios (OR) for being overweight given prenatal exposure to secondhand or active tobacco smoke compared to no exposure. We assessed the precision of our difference and ratio estimates using the confidence limit difference (CLD) and confidence limit ratio (CLR), respectively.35
Secondary Analyses
We examined which component of BMI was responsible for associations between prenatal tobacco smoke exposure and BMI by constructing linear mixed models using categorical prenatal serum cotinine concentrations as the predictor variable and continuous weight or height z-scores at birth, 4 weeks, and 1, 2, and 3 years as the outcome. We calculated weight and height z-scores using publicly available software from the National Center for Health Statistics.36 We repeated our analyses using data from participants who had BMI measurements from all four follow-up visits to determine if children with incomplete data had excessive influence on our results. Finally, we constructed linear mixed models to analyze the association between individual serum cotinine concentrations (16 week, 26 week, and birth) and BMI over the first 3 years of life.
Ethical Considerations
The Institutional Review Boards (IRB) of the University of North Carolina-Chapel Hill and Cincinnati Children’s Hospital Medical Center approved this study. The Cincinnati Children’s Hospital Medical Center IRB was involved in the oversight of this study. All mothers provided written informed consent for themselves and their children prior to enrollment in the study.
Results
Of the 468 women who initially enrolled in our study, 67 dropped out before delivery. We excluded 9 non-singleton and 3 still-born children. Of the remaining 389 women and infants, at least two prenatal serum cotinine samples were collected and assayed from 384 (99%) women and self-reported prenatal tobacco smoke exposure was collected from 356 (92%) women. A higher proportion of women with complete self-report, serum cotinine, and covariate data at birth (N=292, 75%) were non-Hispanic white, better educated, wealthier, married, multiparous, and 25–34 years of age than women with missing data (Table 1). Of these women and infants, 202 (52%) had complete covariate and follow up data at 3 years of age.
Table 1.
Distribution of demographic, perinatal, anthropometric, and tobacco smoke exposure variables among mothers in Health Outcomes and Measures of the Environment study
| Variable | All Mothers and Infants N =389 (%) |
Mothers and Infants with Complete Data N=292 (%) |
Mothers and Infants with Missing Data N=97 (%) |
|---|---|---|---|
| Maternal Race | |||
| Non-Hispanic White | 237 (62) | 191 (65) | 46 (50) |
| Non-Hispanic Black | 121 (31) | 84 (29) | 37 (40) |
| Other | 26 (7) | 17 (6) | 9 (10) |
| Missing | 5 | 0 | 5 |
| Maternal Education (years) | |||
| <12 | 41 (11) | 27 (9) | 14 (15) |
| 12 | 54 (14) | 36 (12) | 18 (20) |
| >12 | 289 (75) | 229 (78) | 60 (65) |
| Missing | 5 | 0 | 5 |
| Marital Status | |||
| Married | 249 (65) | 197 (67) | 52 (57) |
| Single | 135 (35) | 95 (33) | 40 (43) |
| Missing | 5 | 0 | 5 |
| Maternal Age Category (years) | |||
| <25 | 96 (25) | 60 (20) | 35 (37) |
| 25–34 | 231 (59) | 181 (62) | 50 (52) |
| 35+ | 62 (16) | 51 (18) | 11 (11) |
| Missing | 0 | 0 | 0 |
| BDI at 20 Weeks | |||
| Minimal Depression (0–13) | 291 (78) | 232 (79) | 59 (71) |
| Mild Depression (14–19) | 54 (14) | 35 (12) | 19 (23) |
| Moderate or Severe Depression (20–28) | 30 (8) | 25 (9) | 5 (6) |
| Missing | 14 | 0 | 14 |
| Parity | |||
| 0 | 171 (44) | 122 (42) | 49 (52) |
| 1 | 124 (32) | 91 (31) | 33 (35) |
| >1 | 92 (24) | 79 (27) | 13 (14) |
| Missing | 2 | 0 | 2 |
| Prenatal Serum Cotinine Category | |||
| <LOD | 140 (37) | 114 (39) | 26 (28) |
| LOD to 3 ng/mL | 199 (52) | 147 (50) | 52 (57) |
| >3 ng/mL | 45 (12) | 31 (11) | 14 (15) |
| Missing | 5 | 0 | 5 |
| Mean Maternal Weight at Baseline (SD) | 70 (61, 84) | 70 (61, 82) | 72 (60, 87) |
| Missing | 5 | 0 | 5 |
| Mean Maternal Height at Baseline (SD) | 165 (160, 169) | 165 (160, 169) | 165 (160, 170) |
| Missing | 20 | 0 | 20 |
| Mean BMI at Baseline (SD) | 26 (23, 30) | 26 (23, 30) | 27 (23, 31) |
| Missing | 26 | 0 | 26 |
| Median Income in Thousands of Dollars (IQR) | 55 (25, 85) | 55 (25, 85) | 35 (15, 75) |
| Missing | 13 | 0 | 13 |
| Median Total Weeks of Breast Feeding (IQR) | 14 (1, 40) | 15 (2, 41) | 9 (1, 32) |
| Missing | 58 | 0 | 58 |
Nearly half of women (48%) with no self-report of prenatal tobacco smoke exposure had serum cotinine concentrations indicative of secondhand exposure (Table 2). Almost 84% of women who self-reported active smoking had corresponding serum cotinine concentrations. Among women with prenatal serum cotinine concentrations indicative of SHS exposure, geometric mean (GM) serum cotinine concentrations were higher among women with self-reported prenatal SHS exposure (GM: 0.19) compared to women without self-reported prenatal SHS exposure (GM: 0.06). Pairs of prenatal serum cotinine concentrations taken at different times during pregnancy were highly correlated (Pearson R=0.7–0.8).
Table 2.
Joint distribution of prenatal self-reported tobacco smoke exposure and serum cotinine concentration categories.
| No Self-Reported Exposure N=214 (%) | Self-Reported Secondhand Exposure N=42 (%) | Self-Reported Active Exposure N=36 (%) | |
|---|---|---|---|
| Unexposed (<LOD) | 108 (50) | 5 (12) | 1 (3) |
| SHS Exposure (LOD – 3ng/mL) | 103 (48) | 35 (83) | 9 (25) |
| Active Exposure (> 3ng/mL) | 3 (1) | 2 (5) | 26 (72) |
| Median Serum Cotinine Concentration (Min, Max) | 0.015 (<LOD, 14.9) | 0.134 (<LOD, 3.92) | 35.3 (<LOD, 355.5) |
-Limited to women with complete covariate data.
After adjustment for confounders, the point estimates of the mean BMI difference between actively exposed and unexposed children rose as children grew older (Table 3). The estimates were imprecise and became even more so over time with participant attrition. For self-reported active exposure, the 95% CI was wider at 3 years of age (CLD: 1.3 BMI units) than it was at birth (CLD: 1.0 BMI units). The point estimates and pattern of estimates over time was similar using self-report or serum cotinine concentrations to classify prenatal active tobacco smoke exposure. The HOME inventory score did not improve adjustment for socioeconomic confounding over maternal education and household income and was not retained in our models.
Table 3.
Adjusted mean and difference in BMI by change in prenatal tobacco smoke exposure*
| Self-Reported Prenatal Tobacco Smoke Category | N† | Birth, N=293 (SD) [95% CI] | 4-Week, N=297 (SD) [95% CI] | 1 Year, N=266 (SD) [95% CI] | 2 Year, N=229 (SD) [95% CI] | 3 Years, N=202 (SD) [95% CI] |
|---|---|---|---|---|---|---|
| Mean among unexposed | 214 | 13.0 (1.5) | 14.7 (1.4) | 16.9 (1.6) | 16.6 (1.3) | 15.9 (1.3) |
| SHS | 43 | 0.0 [−0.4, 0.5] | 0.0 [−0.4, 0.4] | −0.1 [−0.7, 0.4] | −0.1 [−0.6, 0.5] | 0.0 [−0.5, 0.6] |
| Active | 36 | 0.3 [−0.2, 0.8] | 0.3 [−0.2, 0.8] | 0.2 [−0.4, 0.8] | 0.6 [0.0, 1.2] | 1.1 [0.4, 1.7] |
|
| ||||||
| Categorical Prenatal Serum Cotinine Concentrations | N† | Birth, N=300 (SD) [95% CI] | 4-Week, N=303 (SD) [95% CI] | 1 Year, N=273 (SD) [95% CI] | 2 Years, N=236 (SD) [95% CI] | 3 Years, N=209 (SD) [95% CI] |
| Mean among unexposed (<0.015 ng/mL) | 116 | 13.2 (1.4) | 14.9 (1.3) | 17.0 (1.7) | 16.4 (1.2) | 15.7 (1.2) |
| SHS (0.015–3 ng/mL) | 153 | −0.2 [−0.5, 0.2] | −0.2 [−0.5, 0.1] | −0.1 [−0.6, 0.3] | 0.3 [−0.1, 0.7] | 0.4 [0, 0.8] |
| Active (>3 ng/mL) | 31 | 0.0 [−0.6, 0.6] | 0.1 [−0.5, 0.6] | 0.4 [−0.4, 1.1] | 0.6 [−0.1, 1.4] | 1.0 [0.3, 1.8] |
|
| ||||||
| Continuous Prenatal Serum Cotinine Concentrations | ||||||
| Log10-transformed mean cotinine | 300 | 0.0 [−0.2, 0.2] | 0.0 [−0.2, 0.2] | 0.1 [−0.1, 0.3] | 0.2 [0.0, 0.4] | 0.3 [0.1, 0.5] |
| Log10-transformed mean cotinine (SHS exposed only)‡ | 269 | −0.1 [−0.4, 0.2] | −0.2 [−0.5, 0.1] | −0.2 [−0.6, 0.2] | 0.2 [−0.2, 0.5] | 0.3 [−0.1, 0.6] |
-Adjusted for child age (in years), child age squared, child age cubed, maternal age (<25, 25–34, and >34 years), education (<12, 12, and >12 years), race (non-Hispanic white, non-Hispanic black, and other), marital status (married and non-married), depression at baseline home visit (moderately or severely depressed and mildly or minimally depressed), breast feeding duration since last visit (years), parity (0, 1, and >1), income ($10,000 increments), and maternal BMI (kg/m2) at 16 weeks gestation.
-Sample size at birth
-Women could not have cotinine concentration > 3 ng/mL in any serum measurement. SHS-Secondhand smoke
The associations between self-reported prenatal SHS exposure and BMI were very close to the null value at every time point (Table 3). However, point estimates between serum cotinine concentrations indicative of SHS exposure and BMI rose as children grew older. Again, these estimates became less precise because of participant attrition over time.
Odds of overweight BMI among children born to smokers were similar when exposure was classified with self-report or serum cotinine concentrations (Table 4). Estimates using self-report were more precise than estimates using serum cotinine concentrations (CLR: 16 vs. 10). The magnitude of association between SHS exposure and overweight BMI was greater and more precise when exposure was quantified using prenatal serum cotinine concentrations (OR: 1.9; [0.8, 4.4], CLR: 5.5) compared to self-report (OR: 1.0; [0.3, 3.0], CLR: 10).
Table 4.
Unadjusted and adjusted association between categorical prenatal tobacco smoke exposure and overweight BMI at 2 or 3 years of age*
| Total | >85th Cases (%) | >85th Unadjusted OR [95% CI] | >85th Adjusted OR [95% CI] | |
|---|---|---|---|---|
| Self-Reported Prenatal Tobacco Smoke Category | ||||
| Unexposed | 307 | 43 (14) | Reference | Reference |
| SHS | 56 | 9 (16) | 1.2 [0.5, 3.1] | 1.0 [0.3, 3.0] |
| Active | 48 | 14 (29) | 2.5 [1.0, 6.1] | 1.9 [0.6, 6.1] |
| Categorical Prenatal Serum Cotinine Concentrations | ||||
| Unexposed (<0.015 ng/mL) | 176 | 18 (10) | Reference | Reference |
| SHS (0.015–3 ng/mL) | 209 | 40 (19) | 2.1 [1.0, 4.5] | 1.9 [0.8, 4.4] |
| Active (>3 ng/mL) | 40 | 10 (25) | 3.3 [1.1, 10.0] | 2.4 [0.6, 9.7] |
-Adjusted for maternal age (<25, 25–34, and >34 years), education (<12, 12, and >12 years), race (non-Hispanic white, non-Hispanic black, and other), marital status (married and non-married), depression at baseline home visit (moderately or severely depressed and mildly or minimally depressed), breast feeding duration (years), parity (0, 1, and >1), income ($10,000 increments), and maternal BMI (kg/m2) at 16 weeks gestation.
Secondary Analyses
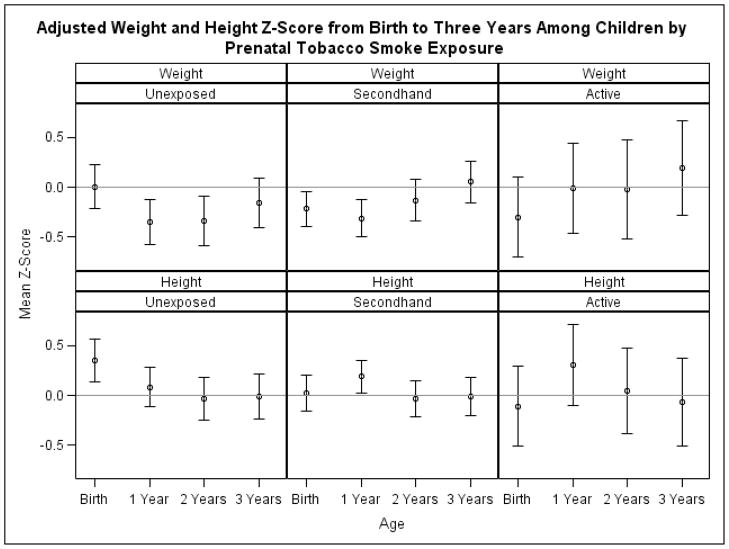
Associations between prenatal secondhand and active tobacco smoke exposure and birth weight were below the null and imprecise (Mean Difference [MD] SHS vs. unexposed: −112, [−268, 33], CLD: 302; MD active vs. unexposed: −136; [−405, 132], CLD: 537). Children born to exposed women were shorter in length than unexposed children (MD SHS vs. unexposed: −0.8, [−1.5, −0.1]; MD active vs. unexposed: −1.2; [−2.4, 0]). Increased BMI at 2 and 3 years of age was the result of increased weight among prenatally exposed children at 2 and 3 years of age compared to unexposed children (Figure 1). In contrast, height at 1, 2, or 3 years of age was similar across categories of prenatal tobacco smoke exposure using self-report or serum cotinine concentrations (results for serum cotinine shown in Figure 1).
Figure 1.
Mean weight and height z-score from birth to 3 years of age by prenatal serum cotinine category.* †
*-Adjusted for child age (in years), child age squared, child age cubed, maternal age (<25, 25–34, and >34 years), education (<12, 12, and >12 years), race (non-Hispanic white, non-Hispanic black, and other), marital status (married and non-married), depression at baseline home visit (moderately or severely depressed and mildly or minimally depressed), breast feeding duration since last visit (years), parity (0, 1, and >1), income ($10,000 increments), and maternal BMI at 16 weeks gestation.
†-Bars represent 95% confidence intervals
Our associations between prenatal serum cotinine concentrations and early childhood BMI were strengthened and less precise when we restricted analyses to the 194 children who completed all 4 follow-up visits. For example, at 3 years of age children with prenatal secondhand (MD: 0.5; [0.1, 1.0]; CLD: 0.9) and active (MD: 1.4; [0.5, 2.3], CLD: 1.8) serum cotinine concentrations had higher BMI at 3 years compared to unexposed children.
Results from models separately examining 16 week, 26 week, and birth serum cotinine concentrations in relation to BMI were relatively similar to results using mean prenatal serum cotinine concentrations. Point estimates using individual or mean serum cotinine were similar in magnitude and precision for prenatal active tobacco smoke exposure. The point estimates for SHS were somewhat higher from serum cotinine concentrations taken at 26 weeks and birth compared to 16 week serum concentration estimates, but similar to mean prenatal serum cotinine concentration estimates.
Discussion
Active prenatal tobacco smoke exposure, classified using self-report or serum cotinine concentrations, was imprecisely associated with higher BMI and odds of overweight BMI at 2 or 3 years of age compared to unexposed children. Our results using serum cotinine concentrations were more suggestive of increased BMI among children with prenatal SHS exposure than the results using self-reported exposures. Self-reported secondhand tobacco smoke exposures were misclassified to a greater extent than self-reported active smoking, and accordingly, the point estimates were attenuated when compared to estimates based on serum cotinine concentrations.
Prior studies of prenatal tobacco smoke exposure and BMI have relied on self-reported tobacco smoke exposures and many studies have compared actively smoking women to non-smokers, without considering the impact SHS exposure. This misclassification may have contributed to an underestimate of the impact of active prenatal tobacco smoke exposure on childhood BMI. Our results show that self-reported tobacco smoke measures fail to accurately quantify secondhand tobacco smoke exposures and may result in biased estimates of association.
Several investigators have reported increased BMI and risk of overweight BMI among 2–4 year old children born to active smokers.7, 8, 10, 11, 37 ORs between prenatal active smoking and overweight BMI in prior studies range from 1.2 to 2.2.7, 8, 10, 37 Our estimated association between active smoking and BMI were less precise than prior estimates, similar to Oken et al. and Adams et al. (OR: 2.2),7, 8 and larger than Chen et al. and Whitaker et al. (OR: 1.2–1.5).10, 37
Consistent with our findings, Oken and colleagues did not report increased BMI among 3 year old children born to women with self-reported prenatal secondhand tobacco smoke exposure.8 However, Leary et al. reported that 10 year old children born to women whose partners smoked regularly during pregnancy had BMI 0.1 standard deviations higher than unexposed children.9 The discrepancy in results could be due to differences in the tobacco smoke exposure questionnaire, temporal or geographic variations in secondhand exposure, or the age of follow-up.
Increased weight at 2 or 3 years of age was responsible for increases in BMI among children born to women with serum cotinine concentrations consistent with secondhand and active tobacco smoke exposures. Children with prenatal tobacco smoke exposures were born lighter and shorter and grew heavier over the first 3 years of life compared to unexposed children. Attained height at 1, 2, or 3 years of age was similar between categories of prenatal tobacco smoke exposure.
Prenatal tobacco smoke exposure may influence childhood BMI by restricting fetal growth as a result of vascocontriction and hypoxemia.14–16, 38 Restricted fetal growth may lead to rapid weight gain, resulting in increased BMI and adiposity during early childhood.16, 39 In addition, tobacco smoke constituents may act on various hormonal systems that change metabolic programming.40, 41 There has also been some suggestion that prenatal tobacco smoke exposure may alter infant appetite and leptin concentrations.42–44
The results of this study should be considered in light of several limitations. Confounding due to dietary, lifestyle, or socioeconomic factors may eliminate the observed elevation in BMI among children born to women with serum cotinine concentrations indicative of SHS exposure. Women who smoke or are exposed to higher levels of SHS during pregnancy are less likely to provide optimal diets and exercise for their children.45, 46 Residual confounding may eliminate the positive association between prenatal secondhand tobacco smoke exposure and BMI at 2 and 3 years of age. We attempted to indirectly control for confounding due to dietary or lifestyle factors by including breast feeding duration and maternal BMI in our models. In addition, we controlled for other markers of socioeconomic status, including household income and maternal education which are associated with diet and exercise.47, 48 We also used HOME Inventory scores as a proxy for socioeconomic, dietary, and exercise factors not captured by other variables. Our results were not substantially different when we included HOME Inventory scores in our models.
While serum cotinine is considered an ideal biomarker for tobacco smoke exposure, it only reflects exposure over the last 2 to 3 days, which introduces the potential for exposure misclassification.49, 50 Women could have been exposed intermittently (e.g., only on weekends). However, we do not believe this is a substantial source of bias since maternal serial serum cotinine concentrations were highly correlated and we used 2 or more prenatal serum samples from each woman to quantify prenatal exposure.
Increased BMI can result from increases in fat mass or fat-free mass. Other measures of body composition, including densitometry, bioelectric impedance, dual energy x-ray absorptiometry (DXA), and isotope dilution can derive these separate components of body composition and provide more accurate quantification of excess adiposity.51 In addition, BMI is differentially correlated with direct measures of adiposity, with higher correlation among children ≥ 85th BMI percentile.52 While there are limitations to using BMI as a marker of early childhood adiposity, BMI is endorsed by the World Health Organization to measure and track body composition across the life-course.27 In addition, prior studies report that BMI measurements before 2 years of age are correlated with other direct measures of adiposity.53 Future research should examine the association between prenatal tobacco smoke exposures using direct measures of fat and fat-free mass and study children in later childhood when BMI measurements become more reflective of adult adiposity.4
A substantial proportion of women and their children dropped out of the study by 3 years of age. Attrition in our study was associated with socioeconomic factors, which are related to health and caregiving behaviors that influence childhood adiposity and tobacco smoke exposures. The association between prenatal serum cotinine concentrations and BMI was slightly strengthened when we restricted to children who completed all four follow-up visits.
Women in this study were required to be living in housing built before 1978. We do not believe this limits the generalizability of our study, since almost 70% of US housing units were built prior to 1978.54 However, we analyzed a relatively homogenous sample of mothers who come from higher socioeconomic backgrounds which may reduce the generalizability of these results to samples drawn from populations with lower SES.
These results suggest that prenatal serum cotinine concentrations may be associated with increased BMI and odds of overweight BMI at 2 and 3 years of age. Given the high prevalence of tobacco smoke exposure and detrimental health consequences of increased BMI, additional research should be conducted examining the relationship between prenatal SHS exposures and childhood BMI and adiposity. Specifically, future studies should use validated biomarkers of tobacco smoke exposure to reduce misclassification of SHS exposure and examine this association at later ages. The presented findings add to a growing body of literature suggesting that prenatal environmental insults, including secondhand tobacco smoke exposure, may impact the public’s health by influencing the risk of later life health outcomes through excess childhood BMI.55, 56
Acknowledgments
Funding: This study was supported by the NICHD Reproductive, Perinatal, and Pediatric Epidemiology Training Grant (T32-HD052468-01), a grant from the National Institute of Environmental Health Sciences (P30ES10126), and from a Children’s Environmental Health Center Grant from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (PO1 ES11261).
We would like to acknowledge the mothers, children, and study staff that made the HOME study possible. We are grateful for Dr. Amy Kalkbrenner’s feedback on this manuscript.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Competing Financial Interests: The authors have no competing financial interests.
References
- 1.De Onis M, Blossner M. Prevalence and trends of overweight among preschool chidlren in developing countries. Am J Clin Nutr. 2000;72:1032–1039. doi: 10.1093/ajcn/72.4.1032. [DOI] [PubMed] [Google Scholar]
- 2.Prevalence of overweight among chidlren and adolescents: United States, 2003–2004. National Center for Health Statistics; 2009. [updated 2009; cited 2010 Jan 5]; Available from: http://www.cdc.gov/nchs/data/hestat/overweight/overwght_child_03.htm. [Google Scholar]
- 3.Sachdev HS, Fall CH, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 4.Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Body mass index during childhood and adult body composition in men and women aged 56–70 y. Am J Clin Nutr. 2008;87:1769–1775. doi: 10.1093/ajcn/87.6.1769. [DOI] [PubMed] [Google Scholar]
- 5.Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Arch Dis Child. 2003;88:748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams AK, Harvey HE, Prince RJ. Association of maternal smoking with overweight at age 3 y in American Indian children. Am J Clin Nutr. 2005;82:393–398. doi: 10.1093/ajcn.82.2.393. [DOI] [PubMed] [Google Scholar]
- 8.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–2028. doi: 10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leary SD, Smith GD, Rogers IS, Reilly JJ, Wells JC, Ness AR. Smoking during pregnancy and offspring fat and lean mass in childhood. Obesity (Silver Spring) 2006;14:2284–2293. doi: 10.1038/oby.2006.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int J Epidemiol. 2006;35:121–130. doi: 10.1093/ije/dyi218. [DOI] [PubMed] [Google Scholar]
- 11.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32:201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The health consequences of smoking: A report of the Surgeon General. Washington, D.C: Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2004. Contract No.: Document Number|. [Google Scholar]
- 13.Hamilton BH. Estimating treatment effects in randomized clinical trials with non-compliance: the impact of maternal smoking on birthweight. Health Econ. 2001;10:399–410. doi: 10.1002/hec.629. [DOI] [PubMed] [Google Scholar]
- 14.Williams SM. Weight and height growth rate and the timing of adiposity rebound. Obes Res. 2005;13:1123–1130. doi: 10.1038/oby.2005.131. [DOI] [PubMed] [Google Scholar]
- 15.Williams SM, Goulding A. Early adiposity rebound is an important predictor of later obesity. Obesity (Silver Spring) 2009;17:1310. doi: 10.1038/oby.2009.104. [DOI] [PubMed] [Google Scholar]
- 16.Hui LL, Schooling CM, Leung SS, Mak KH, Ho LM, Lam TH, et al. Birth weight, infant growth, and childhood body mass index: Hong Kong’s children of 1997 birth cohort. Arch Pediatr Adolesc Med. 2008;162:212–218. doi: 10.1001/archpediatrics.2007.62. [DOI] [PubMed] [Google Scholar]
- 17.Williams SM, Goulding A. Patterns of growth associated with the timing of adiposity rebound. Obesity (Silver Spring) 2009;17:335–341. doi: 10.1038/oby.2008.547. [DOI] [PubMed] [Google Scholar]
- 18.DeLorenze GN, Kharrazi M, Kaufman FL, Eskenazi B, Bernert JT. Exposure to environmental tobacco smoke in pregnant women: the association between self-report and serum cotinine. Environ Res. 2002;90:21–32. doi: 10.1006/enrs.2001.4380. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman FL, Kharrazi M, Delorenze GN, Eskenazi B, Bernert JT. Estimation of environmental tobacco smoke exposure during pregnancy using a single question on household smokers versus serum cotinine. J Expo Anal Environ Epidemiol. 2002;12:286–295. doi: 10.1038/sj.jea.7500224. [DOI] [PubMed] [Google Scholar]
- 20.George L, Granath F, Johansson AL, Cnattingius S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet Gynecol Scand. 2006;85:1331–1337. doi: 10.1080/00016340600935433. [DOI] [PubMed] [Google Scholar]
- 21.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, et al. Smoking in China: findings of the 1996 National Prevalence Survey. Jama. 1999;282:1247–1253. doi: 10.1001/jama.282.13.1247. [DOI] [PubMed] [Google Scholar]
- 23.Gupta D, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Jindal SK, et al. Household environmental tobacco smoke exposure, respiratory symptoms and asthma in non-smoker adults: a multicentric population study from India. Indian J Chest Dis Allied Sci. 2006;48:31–36. [PubMed] [Google Scholar]
- 24.Dietrich KN, Eskenazi B, Schantz S, Yolton K, Rauh VA, Johnson CB, et al. Principles and practices of neurodevelopmental assessment in children: lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113:1437–1446. doi: 10.1289/ehp.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24:333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 27.WHO. WHO Child Growth Standards: Methods and development: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-forage. Geneva: World Health Organization; 2006. Contract No.: Document Number|. [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 29.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 30.Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2. San Antonio: The Psychological Corporation; 1996. (BDI-II) [Google Scholar]
- 31.Caldwell B, Bradley R. HOME Inventory Administration Manual. Little Rock, AK: University of Arkansas at Little Rock; 2003. [Google Scholar]
- 32.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. Journal of the American Statistical Association. 1979;74:829–836. [Google Scholar]
- 33.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Data Analysis. 2. Hoboken: Wiley; 2004. [Google Scholar]
- 34.CDC. 2000 CDC Growth Charts: United States. Hyattsville, MD: 2009. [updated 2009; cited 2009 July 1]; Available from: http://www.cdc.gov/GrowthCharts/ [Google Scholar]
- 35.Poole C. Low P-values or narrow confidence intervals: which are more durable? Epidemiology. 2001;12:291–294. doi: 10.1097/00001648-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 36.A SAS Program for the CDC Growth Charts. Centers for Disease Control and Prevention: National Center for Health Statistics; 2009. [updated 2009; cited 2009 November 1]; Available from: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 37.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 38.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 39.Ong KK, Preece MA, Emmett PM, Ahmed ML, Dunger DB. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr Res. 2002;52:863–867. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Levin ED. Fetal nicotine overload, blunted sympathetic responsivity, and obesity. Birth Defects Res A Clin Mol Teratol. 2005 doi: 10.1002/bdra.20162. [DOI] [PubMed] [Google Scholar]
- 41.Oncken CA, Henry KM, Campbell WA, Kuhn CM, Slotkin TA, Kranzler HR. Effect of maternal smoking on fetal catecholamine concentrations at birth. Pediatr Res. 2003;53:119–124. doi: 10.1203/00006450-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Helland IB, Reseland JE, Saugstad OD, Drevon CA. Smoking related to plasma leptin concentration in pregnant women and their newborn infants. Acta Paediatr. 2001;90:282–287. [PubMed] [Google Scholar]
- 43.Pardo IM, Geloneze B, Tambascia MA, Barros-Filho AA. Does maternal smoking influence leptin levels in term, appropriate-for-gestational-age newborns? J Matern Fetal Neonatal Med. 2004;15:408–410. doi: 10.1080/14767050410001680046. [DOI] [PubMed] [Google Scholar]
- 44.Toschke AM, Ehlin AG, von Kries R, Ekbom A, Montgomery SM. Maternal smoking during pregnancy and appetite control in offspring. J Perinat Med. 2003;31:251–256. doi: 10.1515/JPM.2003.034. [DOI] [PubMed] [Google Scholar]
- 45.Burke V, Gracey MP, Milligan RA, Thompson C, Taggart AC, Beilin LJ. Parental smoking and risk factors for cardiovascular disease in 10- to 12-year-old children. J Pediatr. 1998;133:206–213. doi: 10.1016/s0022-3476(98)70221-5. [DOI] [PubMed] [Google Scholar]
- 46.Rogers I, Emmett P. The effect of maternal smoking status, educational level and age on food and nutrient intakes in preschool children: results from the Avon Longitudinal Study of Parents and Children. Eur J Clin Nutr. 2003;57:854–864. doi: 10.1038/sj.ejcn.1601619. [DOI] [PubMed] [Google Scholar]
- 47.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23 (Suppl 8):S1–107. [PubMed] [Google Scholar]
- 48.Power C, Parsons T. Nutritional and other influences in childhood as predictors of adult obesity. Proc Nutr Soc. 2000;59:267–272. doi: 10.1017/s002966510000029x. [DOI] [PubMed] [Google Scholar]
- 49.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 50.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107 (Suppl 2):349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wells JC. A critique of the expression of paediatric body composition data. Arch Dis Child. 2001;85:67–72. doi: 10.1136/adc.85.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 53.Rolland-Cachera MF, Sempe M, Guilloud-Bataille M, Patois E, Pequignot-Guggenbuhl F, Fautrad V. Adiposity indices in children. Am J Clin Nutr. 1982;36:178–184. doi: 10.1093/ajcn/36.1.178. [DOI] [PubMed] [Google Scholar]
- 54.Jacobs DE, Clickner RP, Zhou JY, Viet SM, Marker DA, Rogers JW, et al. The prevalence of lead-based paint hazards in U.S. housing. Environ Health Perspect. 2002;110:A599–606. doi: 10.1289/ehp.021100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring) 2008;16:1651–1656. doi: 10.1038/oby.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]