Abstract
Objective
Type-2 deiodinase gene (DIO2) polymorphisms have been associated with changes in pituitary-thyroid axis homeostasis. The −258 A/G (SNP rs12885300) polymorphism has been associated with increased enzymatic activity, but data are conflicting. To characterize the effects of the −258 A/G polymorphism on intra-thyroidal T4 to T3 conversion and thyroid hormone secretion pattern we studied the effects of acute, TRH-mediated, TSH stimulation of the thyroid gland.
Design
Retrospective analysis.
Methods
The thyroid hormone secretion in response to 500 mcg iv TRH injection was studied in 45 healthy volunteers.
Results
Twenty-six subjects (16 females, 10 males, 32.8±10.4 years) were homozygous for the ancestral (−258 A/A) allele, 19 (11 females, 8 males, 31.1±10.9 years) were carrier of the (−258 G/x) variant. While no differences in the peak TSH and T3 levels were observed, carriers of the −258G/x allele showed a blunted rise in free T4 (p<0.01). The −258G/x 92Thr/Thr haplotype, compared to the other groups, had lower TSH values at 60' (p<0.03). No differences were observed between genotypes in baseline thyroid hormone levels.
Conclusions
The −258G/x DIO2 polymorphism variant is associated with a decreased rate of acute TSH-stimulated free T4 secretion with a normal T3 release from the thyroid consistent with a shift in the reaction equilibrium toward the product. These data indicate that the −258G DIO2 polymorphism cause changes in the pattern of hormonal secretion. These findings are a proof-of-concept that common polymorphisms in the DIO2 can subtly affect the circulating levels of thyroid hormone and might modulate the thyroid hormone homeostasis.
Keywords: Human Type-2 deiodinase, −258A/G Polymorphism, Thr92Ala Polymorphism, Thyroid hormone, Pharmacogenomics, Thyrotropin-Releasing Hormone, TRH stimulation test
Introduction
The serum levels of thyroid hormones (TH) are tightly regulated throughout the adult life by multiple mechanisms and the intra-individual variability of TH levels is minimal1. The serum levels of T3, which is the biologically active form of TH, are the net results of the secretion from the thyroid gland, its catabolism, and the peripheral conversion of the pro-hormone T4 into T3 by the two 5' deiodinases, D1 and D2, and its catabolism and excretion2, 3. Aside their activity in the peripheral tissues, D1 and D2 are active in the thyroid and play an important role in modulating the release of T3 from the gland both in physiologic and pathologic states4–7. Intrathyroidal D2 activity is positively stimulated by the thyrotropin (Thyroid Stimulating Hormone, TSH) receptor pathway via increasing the intracellular cAMP levels8, 9, ultimately leading to an activation of the T4 to T3 intrathyroidal conversion. This phenomenon is thus contributing to the preferential T3 secretion during states of sustained activation of the TSH receptor pathway7. Thus genetic variants in the deiodinases can affect the pattern of TH secretion. Recently we demonstrated that the common polymorphism of the D2 gene (DIO2) (Thr92Ala DIO2, SNP rs225014) causes a differential response in the TH secretion pattern following the acute rise in serum TSH levels in response to an injection of thyrotropin releasing hormone (TRH)10. In this secondary analysis of the original dataset we characterize the role of the −258A/G (D2-ORFa-Gly3Asp, SNP rs12885300)11 polymorphism in the pattern of TH release, and explore the interaction between the two polymorphisms.
Materials and Methods
Participants and study design
This study is a secondary analysis of a previously described dataset10. Briefly, the study was approved by the NIDDK-NIAMS Institutional Review Board and conducted at the National Institutes of Health Clinical Center in Bethesda, MD. The research protocol was designed as a prospective cohort study (ClinicalTrials.gov identifier number NCT00812149).
Healthy volunteers aged 18–65, with normal baseline thyroid function and negative antithyroid peroxidase antibodies or thyroid-stimulating immunoglobulin, were invited to participate in the study. The study volunteers' accrual was designed to achieve three groups of 15 subjects with the following DIO2 codon 92 genotype: Thr/Thr, Thr/Ala, Ala/Ala.
Study volunteers underwent an outpatient screening visit, at which a medical history was taken and physical examination performed. A blood sample was taken for basic metabolic and thyroid function, and a DNA sample was collected for DIO2 polymorphisms status. Eligible subjects were then invited to undergo a TRH-stimulation test.
Blood sampling and Laboratory testing
Blood samples were drawn under sterile technique through the intravenous catheter using the Vacutainer® system (Becton Dickson and Co.). Screening tests, TSH, FT4, T3, antithyroid antibody panel, prolactin, and urine human chorionic gonadotropin [HCG] in women were performed daily in the NIH Department of Laboratory Medicine. TSH, FT4 and T3 were analyzed daily by the Department of Laboratory Medicine by immunoassay on a Siemens Immulite 2500 analyzer platform. Intra- and inter-assay coefficients of variability were TSH 4.2 and 3.5 %; T3 11.2 and 5.4 %; and free T4 16.43 and 2.58 %. Thyroid- stimulating immunoglobulin testing was performed by Mayo Medical Labs (Rochester, MN). Cell pellets were stored in minus 80 C° and processed in batch for genomic DNA isolation.
DNA isolation and DIO2 restriction fragment polymorphisms analysis
Genomic DNA was isolated from peripheral mononuclear cells from the screening blood samples using the QIAamp® system (Qiagen, Valencia, CA). After isolation, DNA concentration was measured using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Purity was determined by measuring the 260/280-nm ratio. The −258 A/G DIO2 polymorphism status was characterized using established methods12 by PCR restriction fragmente amplified with the following primers: forward 5'-AAAGCTGGCGTACTCGTC-3', and reverse 5'-AAAGAGCATAGAGACAATGAAAG-3'. After purification, PCR products were digested with CviKI-1 (New England BioLabs, Ipswich, MA), restriction enzyme for 4 hours at 37°C. The digestion products were separated on 4.5% agarose gel by electrophoresis and visualized by ethidium bromide staining. The Thr92Ala DIO2 polymorphism status was characterized using established methods13 by PCR restriction fragment–length polymorphism (RFLP) analysis. Ten nanograms of genomic DNA were amplified with the following primers: forward 5'CTCAGGGCTGGCAAAGTCAAG3', and reverse 5'CCACACTCTATTAGAGCCAATTG3'. After purification, PCR products were digested with Bsg-1 (New England BioLabs, Ipswich, MA) and the digestion products were separated on a 1.5% agarose gel by electrophoresis.
TRH test procedure
The TRH test was performed at the NIH Clinical Center day hospital. After an overnight fast, subjects underwent testing while resting supine on a comfortable bed in a room maintained at a temperature of 23–25° C. Women of reproductive age had a repeat pregnancy test at admission. A saline lock IV catheter was inserted 15 minutes before the first blood draw. At time 0, 500 mcg TRH was given intravenously over 1 minute followed by a 10 cc normal saline flush. Blood samples were taken at −15, 0, 5, 10, 15, 20, 30, 60, 120, and 180 minutes for measurement of TSH, free T4 (FT4), and total T3 (TT3). Prolactin levels were measured at −15, 0, 60, and 180 minutes as an independent marker of TRH action. Blood pressure was monitored before administration of TRH, and after the 30, 60, 120, and 180 blood draws.
Statistical analysis
This is a secondary analysis of the original dataset10, which was by design enriched in carriers of the Ala92 DIO2 allele. The −258A/G DIO2 (rs12885300) polymorphism is common in the general population (allele frequency 0.34) and it is not in linkage disequilibrium with the Thr92Ala variant11. Thus we reckoned a priori that approximately 50 % of the study population would carry the DIO2 −258 G/G ancestral allele, and the rest of the population carry the minor allele gene polymorphism either as homozygous or heterozygous (A/A or G/x). TRH-stimulation test data are presented as delta from baseline (average minus 15' and 0'). Where indicated, data were log transformed. A two-tailed Student t test was performed as primary analysis for changes in TH levels following the TRH injection. Non-parametric data were analyzed using Mann-Whitney test. A α error of 0.05 was considered the threshold for statistical significance.
Results
Patient recruitment and characteristics
Forty-six eligible individuals from 83 study participants completed TRH testing. The dataset of one volunteer who underwent the TRH stimulation test was omitted from the analysis because of the presence of anti thyroid peroxidase antibodies. Thus, the data of 45 volunteers who underwent an IV injection of 500 μg of TRH with serial measurements of serum TT3, free fT4 and TSH over 180 minutes were analyzed. The study population was composed of 26 subjects with the −258 A/A genotype, age 32.8±10.4 (16 females, 10 males), and 19 subjects with the −258 G/x genotype, age 31.1±10.9 (11 females, 8 males). The −258/codon 92 haplotypes were as follows: 10 subjects 92Trh/Thr −258A/A, age 31.8±7.5 (6 females, 4 males), 16 subjects 92Ala/x −258A/A, age 33.5±11.5 (10 females, 6 males), 5 subjects 92Thr/Thr −258G/x, age 34.0±14.9 (2 females, 3 males), and 14 subjects 92Ala/x −258G/3, age 30.1±9.5 (9 females, 5 males) (Table 1).
Table. 1.
Study participants' characteristics
| −258 | −258/codon 92 haplotype | |||||
|---|---|---|---|---|---|---|
| Parameter | −258 A/A | −258 G/x | 92Thr/Thr −258 A/A | 92Ala/x −258 A/A | 92Thr/Thr −258 G/x | 92Ala/x −258 G/x |
| n. | 26 | 19 | 10 | 16 | 5 | 14 |
| Sex (M/F) | 16/10 | 11/8 | 6/4 | 10/6 | 2/3 | 9/5 |
| Age (years) | 32.8±10.0 | 31.1±10.9 | 31.8±7.5 | 33.5±11.5 | 34.0±14.9 | 33.1±9.5 |
| BMI (Kg/m2) | 25.9±4.7 | 24.9±4.0 | 25.4±3.7 | 26.2±5.3 | 26.7±4.5 | 24.2±3.7 |
| Baseline TSH (μIU/mL) | 1.4±0.9 | 1.5±0.6 | 1.2±0.6 | 1.6±1.1 | 1.2±0.5 | 1.6±0.6 |
| Baseline T3 (ng/dL) | 107.4±19.8 | 101.8±24.0 | 111.8±24.6 | 103.8±17.6 | 90.6±6.9 | 105.2±24.5 |
| Baseline fT4 (ng/dL) | 1.3±0.1 | 1.3±0.1 | 1.2±0.1 | 1.3±0.2 | 1.3±0.2 | 1.3±0.1 |
| Baseline T3/fT4 (ng/dL) | 84.2±18.7 | 80.0±21.1 | 91.5±21.3 | 79.9±14.3 | 71.7±6.8 | 83.3±23.6 |
| Cholesterol (mg/dL) | 151.0±40.0 | 161.3±29.7 | 144.3±36.3 | 154.8±42.6 | 158.2±18.2 | 162.4±33.4 |
| LDL (mg/dL) | 86.1±29.4 | 96.1±25.5 | 78.6±21.1 | 90.7±33.3 | 94.2±18.7 | 96.8±28.1 |
| HDL (mg/dL) | 52.1±12.8 | 49.2±12.2 | 51.8±17.0 | 52.2±9.9 | 47.8±13.8 | 49.1± 12.4 |
| Triglycerides (mg/dL) | 64.3±38.1 | 83.7±55.9 | 72.6±56.9 | 59.7±23.1 | 77.8±27.1 | 85.8±63.9 |
| Glucose (mg/dL) | 79.3±9.9 | 82.3±7.0 | 80.3±7.2 | 78.6±11.4 | 84.2±4.7 | 81.6±7.7 |
| Insulin (μIU/mL) | 4.1±2.9 | 4.0±2.9 | 3.0±1.4 | 4.8±3.4 | 5.8±5.0 | 3.3±1.5 |
| HOMA | 0.8±0.7 | 0.8±0.6 | 0.6±0.3 | 1.0±0.8 | 1.2±1.0 | 0.7±0.3 |
Clinical data of the study participants were reported either according to the −258 status (columns 1–2), or to the −258/codon 92 haplotype (columns 3–6). No significant differences were observed among the groups.
Baseline parameters
As illustrated in Table 1, no significant difference was found in any of the studied baseline parameters among the genotype groups. Specifically, no significant differences between genotypes were noted in baseline serum TSH, FT4, or TT3 levels, or in fasting glucose, fasting insulin, or HOMA levels.
Response to TRH testing
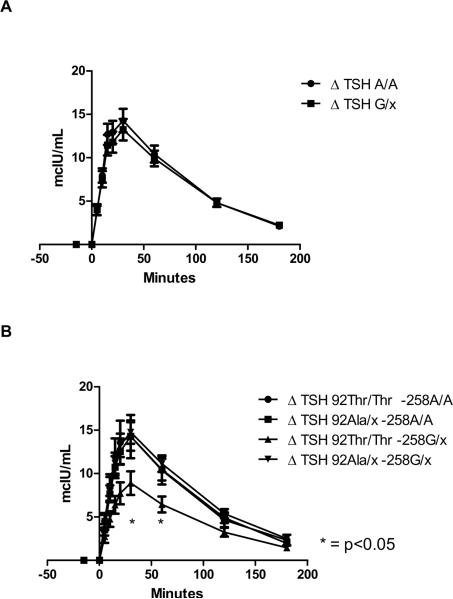
There was no significant difference in maximal (30 minutes) TRH-stimulated levels of serum TSH among the two groups (−258A/A 14.28±6.93 vs. −258G/x 13.23±5.39 mcIU/mL, p=0.845) (Figure 1 A). Similarly, no significant difference between groups were observed in serum TSH level at 60' (−258A/A 10.43±4.99 vs. −258G/x 9.91±3.94 mcIU/mL, p=0.863), 120' (−258A/A 4.80±2.46 vs. −258G/x 4.81±2.07 mcIU/mL, p=0.845), and 180' (−258A/A 2.10±1.57 vs. −258G/x 2.22±1.22 mcIU/mL, p=0.364). When the analysis was performed among −258/ codon 92 haplotypes, the −258G/x 92Thr/Thr showed, compared to the other haplotypes, lower TSH values at times 30' (8.90±3.39 vs. 14.45±6.31 mcIU/mL, p<0.05) 60' (6.44±2.29 vs. 10.68±4.54 mcIU/mL, p=0.03), and non-significant trend at 120' (3.24±1.27 vs. 5.00±2.31 mcIU/mL, p=0.07) (Figure 1 B).
Figure 1.
Changes in serum TSH levels from baseline (Δ) following the intravenous injection of 500 mcg of Thyrotropin-releasing hormone (TRH). Serum levels were measured at −15, 0, 5, 10, 15, 20, 30, 60, 120 and 180 minutes of TRH injection. Error bars represent the standard error of the mean at each time point. A: Data reported according to the −258A/G deiodinase type-2 genotype. No significant differences were observed between genotypes. B: Data reported according to the −258/codon 92 haplotype. As compared to the other haplotypes, the −258G/x 92Thr/Thr showed significant lower TSH values at times 30' and 60' after TRH injection.
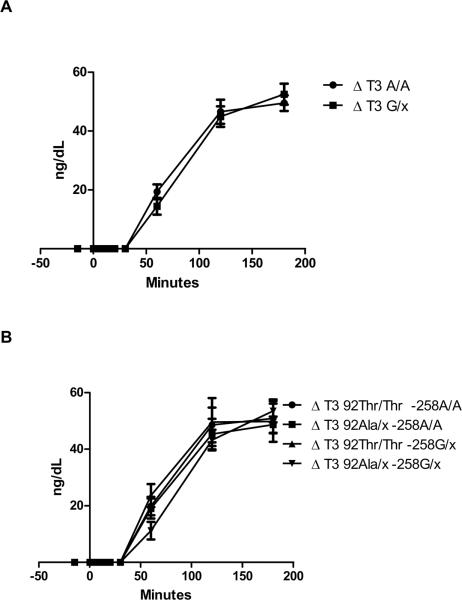
A rise in serum TT3 level was noted in all participants undergoing TRH testing starting at 30 minutes after TRH injection (Fig. 2). No differences between groups were observed in TT3 at 60' (−258A/A 19.35±12.89 vs. −258G/x 14.44±12.44 ng/dL, p=0.206), 120' (−258A/A 46.58±20.91 vs. −258G/x 44.91±15.29 ng/dL, p=0.769) and at 180' (−258A/A 49.51±13.61 vs. −258G/x 52.54±15.54 ng/dL, p=0.490) (Figure g 2 A). No differences were observed among haplotypes (Figure 2 B).
Figure 2.
Changes in serum T3 levels from baseline (Δ) following the intravenous injection of 500 mcg of Thyrotropin-releasing hormone (TRH). Serum levels were measured at −15, 0, 5, 10, 15, 20, 30, 60, 120 and 180 minutes of TRH injection. Error bars represent the standard error of the mean at each time point. A: Data reported according to the −258A/G deiodinase type-2 genotype. No significant differences were observed between genotypes. B: Data reported according to the −258/codon 92 haplotype. No significant differences were observed among haplotypes.
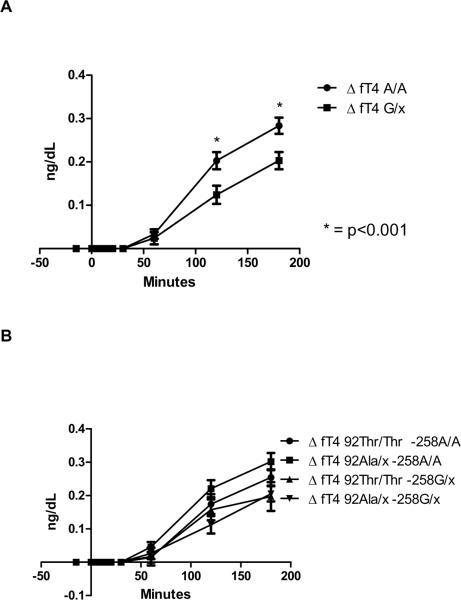
A rise in serum FT4 level was noted in all participants undergoing TRH testing starting at 60 minutes after TRH injection. Compared to −258A/A group, the −258 G/x group showed a significantly blunted TRH-stimulated increase in serum FT4 at 120' (−258A/A 0.20±0.10 vs. −258 G/x 0.12±0.09 ng/dL, p<0.01) and at 180' (−258A/A 0.28±0.09 vs. −258 G/x 0.20±0.09 ng/dL, p<0.01) (Figure 3 A). When the analysis was performed among haplotypes, no significant differences were observed (Figure 3 B).
Figure 3.
Changes in serum free T4 levels from baseline (Δ) following the intravenous injection of 500 mcg of Thyrotropin-releasing hormone (TRH). Serum levels were measured at −15, 0, 5, 10, 15, 20, 30, 60, 120 and 180 minutes of TRH injection. Error bars represent the standard error of the mean at each time point. A: Data reported according to the −258A/G deiodinase type-2 genotype. Compared to −258A/A ancestral allele, carriers of the −258 G/x minor allele showed a significantly blunted TRH-stimulated increase in serum free T4 at 120' and at 180' after TRH injection. B: Data reported according to the −258/codon 92 haplotype. No significant differences were observed among haplotypes.
Discussion
The interaction between the TSH and its receptor on the thyrocyte plays a central role in regulating the development, growth, and function of the thyroid gland. In particular, the interaction with the downstream G-protein signal pathways directly affects the secretion of thyroid hormone from the gland14. Sustained activation of this pathway by congenital or acquired conditions is associated with a shift in the ratio of serum T3/T415 secondary to activation of intrathyroidal conversion of T4 into T3 by both deiodinases type-1 and type-27. While deiodinase type-1 gene transcription is primarily driven by positive feedback of increased intracellular levels of T316, thyroidal transcription of the deiodinase type-2 gene is driven by the TSH-mediated cAMP pathway8, 9. Hence, the latter has a primary role in regulating intrathyroidal TH conversion and the pattern of TH secretion after TSH stimulation.
Genetic variants of deiodinase type-2 have been associated with changes in indices of TH action at various end-organs12, 13, 17–19, and also with changes in circulating levels of TH11, 20, but the results are controversial21, 22. Clinical and in vitro studies suggest that the two most common polymorphisms have opposite effects on enzymatic activity. In fact while the Thr92Ala variant is associated with a decreased activity23 and indices of reduced TH at the end-organ level13, the −258A/G has been associated with increased enzymatic activity11, 24. With respect to the effects of these polymorphisms on circulating levels of TH, the effects of hr92Ala are minimal20, while the −258A/G is associated with a change in the T3/fT4 ratio11, confirming in vitro observations that the minor G allele confers an increase in transcription and ultimately activity24. It is worth noting that the changes in TH levels observed at a steady state are small, and not all studies confirmed the original observations22. Conversely, a secondary analysis of a large L-T3/L-T4 combination therapy trial suggest that the common Ala92 variant is associated with worse baseline indices of quality of life, and an improved response to L-T3/L-T4 combination therapy19. These observations suggest that subtle changes in deiodinase type-2 activity may play an important role in the individual response to replacement therapy.
The injection of TRH allows the dynamic assessment of the hypothalamus-pituitary-thyroid axis, and is a sensitive tool to demonstrate small differences in its pathophysiology; hence we exploited the characteristics of this test to analyze differences secondary to polymorphisms in the deiodinase type-2 gene in acute TSH secretion and in the pattern of TH secretion after TRH injection. Indeed, we previously demonstrated that the Ala92 allele causes a blunted secretion of T3 following a TRH-mediated acute rise in TSH, consistent with a reduced intrathyroidal conversion of T4 into T310.
The results of this study indicate that while the TSH and T3 levels were similar to those observed in the ancestral −258 A/A alleles, carriers of the −258 G minor allele displayed a significantly blunted rise in fT4. These findings are consistent with an increase in enzymatic activity, resulting in a change of T3/fT4 ratio consistent with a shift of the reaction equilibrium toward the product. This is in keeping with the initial observations of Peeters and colleagues 11, as well as with our in vitro data indicating that the −258 G allele causes an increase in transcription of the deiodinase type-2 gene by decreasing the binding of a repressor factor in the 5' untranslated region of the gene24. One can speculate that the −258A/G polymorphism also generates a shifted equilibrium in the thyrotroph, thus preventing an overstimulation of the thyroid gland which would otherwise result in increased T3 levels. Although no differences were found between alleles in the dynamic response of TSH to TRH injection, when the analysis was performed according to the combination of the −258/codon 92 polymorphisms, the −258 G/x-Thr/Thr 92 was associated with a significant decrease in the peak of TSH, consistent with a state of local “hyperthyroidism” in the thyrotroph, in keeping with an overall increase in deiodinase activity due to the additive effects of the activating −258 G polymorphism and the Thr92 “wild type” allele.
The strengths of this pharmacogenomic intervention reside in the study of well-characterized healthy volunteers devoid of thyroid disease and the use of dynamic testing in a controlled environment able to amplify relatively small differences between the genotypes. On the other hand, the major limitation of the study is related to the secondary analysis of a previously collected dataset, hence the inability to perform the analysis in homozygous −258 G/G subjects. Conversely, it is worth noting that the outcomes tested fall within the physiological pathway explored, the results confirm the study hypothesis, and are supported by in vitro and in vivo observations24, 25.
In conclusion, the data indicate that the −258 A/G and Thr92Ala type-2 deiodinase polymorphisms affect the dynamic response of the thyroid after TRH-induced TSH stimulation, and in particular the −258 G allele causes a shift in the substrate/product ratio, consistent with an increased activation of the enzyme. Furthermore, the analysis of the haplotype suggests that the combination of the activating −258 G allele, with the wild type Thr92 allele confer a blunted TSH response to TRH stimulation, in keeping with a “priming” of the thyrotroph26 with T3 derived by an increased state of conversion of T4 into T3.
These findings are a proof of concept that common polymorphisms in the type-2 deiodinase can subtly affect the circulating levels of thyroid hormone and might modulate thyroid hormone homeostasis, particularly in the case of replacement therapy when the entire pool of T3 derives from the deiodinase activity20. Further studies are needed to demonstrate the relevance of these observations in relation to clinically meaningful endpoints.
Acknowledgements
The authors gratefully acknowledge the help and professionalism of the nursing, laboratory, and ancillary personnel of the NIH Clinical Center. This research could have not been accomplished without the selfless participation of the study volunteers. The authors are grateful to Dr. Douglas Forrest (NIDDK) for his invaluable encouragement, comments and suggestions.
Funding: This work was supported by the Intramural Research Program of the National Institute of Diabetes, Digestive, and Kidney Diseases, programs Z01-DK047057-01 and Z01-DK047057-02.
ClinicalTrials.gov identifier: NCT00812149
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 2.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest. 2005;115:2524–2533. doi: 10.1172/JCI25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvatore D, Tu H, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J Clin Invest. 1996;98:962–968. doi: 10.1172/JCI118880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami M, Araki O, Hosoi Y, Kamiya Y, Morimura T, Ogiwara T, Mizuma H, Mori M. Expression and regulation of type II iodothyronine deiodinase in human thyroid gland. Endocrinology. 2001;142:2961–2967. doi: 10.1210/endo.142.7.8280. [DOI] [PubMed] [Google Scholar]
- 6.Brtko J, Bobalova J, Podoba J, Schmutzler C, Kohrle J. Thyroid hormone receptors and type I iodothyronine 5'-deiodinase activity of human thyroid toxic adenomas and benign cold nodules. Exp Clin Endocrinol Diabetes. 2002;110:166–170. doi: 10.1055/s-2002-32147. [DOI] [PubMed] [Google Scholar]
- 7.Celi FS, Coppotelli G, Chidakel A, Kelly M, Brillante BA, Shawker T, Cherman N, Feuillan PP, Collins MT. The role of type 1 and type 2 5'-deiodinase in the pathophysiology of the 3,5,3'-triiodothyronine toxicosis of McCune-Albright syndrome. J Clin Endocrinol Metab. 2008;93:2383–2389. doi: 10.1210/jc.2007-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canettieri G, Celi FS, Baccheschi G, Salvatori L, Andreoli M, Centanni M. Isolation of human type 2 deiodinase gene promoter and characterization of a functional cyclic adenosine monophosphate response element. Endocrinology. 2000;141:1804–1813. doi: 10.1210/endo.141.5.7471. [DOI] [PubMed] [Google Scholar]
- 9.Bartha T, Kim SW, Salvatore D, Gereben B, Tu HM, Harney JW, Rudas P, Larsen PR. Characterization of the 5'-flanking and 5'-untranslated regions of the cyclic adenosine 3',5'-monophosphate-responsive human type 2 iodothyronine deiodinase gene. Endocrinology. 2000;141:229–237. doi: 10.1210/endo.141.1.7282. [DOI] [PubMed] [Google Scholar]
- 10.Butler PW, Smith SM, Linderman JD, Brychta RJ, Alberobello AT, Dubaz OM, Luzon JA, Skarulis MC, Cochran CS, Wesley RA, Pucino F, Celi FS. The Thr92Ala 5' type 2 deiodinase gene polymorphism is associated with a delayed triiodothyronine secretion in response to the thyrotropin-releasing hormone-stimulation test: a pharmacogenomic study. Thyroid. 2010;20:1407–1412. doi: 10.1089/thy.2010.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters RP, van den Beld AW, Attalki H, Toor H, de Rijke YB, Kuiper GG, Lamberts SW, Janssen JA, Uitterlinden AG, Visser TJ. A new polymorphism in the type II deiodinase gene is associated with circulating thyroid hormone parameters. Am J Physiol Endocrinol Metab. 2005;289:E75–81. doi: 10.1152/ajpendo.00571.2004. [DOI] [PubMed] [Google Scholar]
- 12.He B, Li J, Wang G, Ju W, Lu Y, Shi Y, He L, N Zhong. Association of genetic polymorphisms in the type II deiodinase gene with bipolar disorder in a subset of Chinese population. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:986–990. doi: 10.1016/j.pnpbp.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Mentuccia D, Proietti-Pannunzi L, Tanner K, Bacci V, Pollin TI, Poehlman ET, Shuldiner AR, Celi FS. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor. Diabetes. 2002;51:880–883. doi: 10.2337/diabetes.51.3.880. [DOI] [PubMed] [Google Scholar]
- 14.Corvilain B, Laurent E, Lecomte M, Vansande J, Dumont JE. Role of the cyclic adenosine 3',5'-monophosphate and the phosphatidylinositol-Ca2+ cascades in mediating the effects of thyrotropin and iodide on hormone synthesis and secretion in human thyroid slices. J Clin Endocrinol Metab. 1994;79:152–159. doi: 10.1210/jcem.79.1.8027219. [DOI] [PubMed] [Google Scholar]
- 15.Laurberg P. Mechanisms governing the relative proportions of thyroxine and 3,5,3'-triiodothyronine in thyroid secretion. Metabolism. 1984;33:379–392. doi: 10.1016/0026-0495(84)90203-8. [DOI] [PubMed] [Google Scholar]
- 16.Jakobs TC, Schmutzler C, Meissner J, Kohrle J. The promoter of the human type I 5'-deiodinase gene--mapping of the transcription start site and identification of a DR+4 thyroid-hormone-responsive element. Eur J Biochem. 1997;247:288–297. doi: 10.1111/j.1432-1033.1997.00288.x. [DOI] [PubMed] [Google Scholar]
- 17.Dora JM, Machado WE, Rheinheimer J, Crispim D, Maia AL. Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: case-control study and meta-analysis. Eur J Endocrinol. 2010;163:427–434. doi: 10.1530/EJE-10-0419. [DOI] [PubMed] [Google Scholar]
- 18.Meulenbelt I, Min JL, Bos S, Riyazi N, Houwing-Duistermaat JJ, van der Wijk HJ, Kroon HM, Nakajima M, Ikegawa S, Uitterlinden AG, van Meurs JB, van der Deure WM, Visser TJ, Seymour AB, Lakenberg N, van der Breggen R, Kremer D, van Duijn CM, Kloppenburg M, Loughlin J, Slagboom PE. Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis. Hum Mol Genet. 2008;17:1867–1875. doi: 10.1093/hmg/ddn082. [DOI] [PubMed] [Google Scholar]
- 19.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- 20.Torlontano M, Durante C, Torrente I, Crocetti U, Augello G, Ronga G, Montesano T, Travascio L, Verrienti A, Bruno R, Santini S, D'Arcangelo P, Dallapiccola B, Filetti S, Trischitta V. Type 2 deiodinase polymorphism (threonine 92 alanine) predicts L-thyroxine dose to achieve target thyrotropin levels in thyroidectomized patients. J Clin Endocrinol Metab. 2008;93:910–913. doi: 10.1210/jc.2007-1067. [DOI] [PubMed] [Google Scholar]
- 21.Grarup N, Andersen MK, Andreasen CH, Albrechtsen A, Borch-Johnsen K, Jorgensen T, Auwerx J, Schmitz O, Hansen T, Pedersen O. Studies of the common DIO2 Thr92Ala polymorphism and metabolic phenotypes in 7342 Danish white subjects. J Clin Endocrinol Metab. 2007;92:363–366. doi: 10.1210/jc.2006-1958. [DOI] [PubMed] [Google Scholar]
- 22.de Jong FJ, Peeters RP, den Heijer T, van der Deure WM, Hofman A, Uitterlinden AG, Visser TJ, Breteler MM. The association of polymorphisms in the type 1 and 2 deiodinase genes with circulating thyroid hormone parameters and atrophy of the medial temporal lobe. J Clin Endocrinol Metab. 2007;92:636–640. doi: 10.1210/jc.2006-1331. [DOI] [PubMed] [Google Scholar]
- 23.Canani LH, Capp C, Dora JM, Meyer EL, Wagner MS, Harney JW, Larsen PR, Gross JL, Bianco AC, Maia AL. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90:3472–3478. doi: 10.1210/jc.2004-1977. [DOI] [PubMed] [Google Scholar]
- 24.Coppotelli G, Summers A, Chidakel A, Ross JM, Celi FS. Functional Characterization of the 258 A/G (D2-ORFa-Gly3Asp) Human Type-2 Deiodinase Polymorphism: A Naturally Occurring Variant Increases the Enzymatic Activity by Removing a Putative Repressor Site in the 5' UTR of the Gene. Thyroid. 2006;16:625–632. doi: 10.1089/thy.2006.16.625. [DOI] [PubMed] [Google Scholar]
- 25.Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, Visser TJ. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 26.Faglia G. The clinical impact of the thyrotropin-releasing hormone test. Thyroid. 1998;8:903–908. doi: 10.1089/thy.1998.8.903. [DOI] [PubMed] [Google Scholar]