Abstract
Epicuticular wax production was evaluated along the length of expanding leek (Allium porrum L.) leaves to gain insight into the regulation of wax production. Leaf segments from the bottom to the top were analyzed for (a) wax composition and load; (b) microsomal fatty acid elongase, plastidial fatty acid synthase, and acyl-acyl carrier protein (ACP) thioesterase activities; and (c) tissue and cellular morphological changes. The level of total wax, which was low at the bottom, increased 23-fold along the length of the leaf, whereas accumulation of the hentriacontan-16-one increased more than 1000-fold. The onset of wax accumulation was not linked to cell elongation but, rather, occurred several centimeters above the leaf base. Peak microsomal fatty acid elongation activity preceded the onset of wax accumulation, and the maximum fatty acid synthase activity was coincident with the onset. The C16:0- and C18:0-ACP-hydrolyzing activities changed relatively little along the leaf, whereas C18:1-ACP-hydrolyzing activity increased slightly prior to the peak elongase activity. Electron micrographic analyses revealed that wax crystal formation was asynchronous among cells in the initial stages of wax deposition, and morphological changes in the cuticle and cell wall preceded the appearance of wax crystals. These studies demonstrated that wax production and microsomal fatty acid elongation activities were induced within a defined and identifiable region of the expanding leek leaf and provide the foundation for future molecular studies.
The ubiquitous presence of surface waxes among terrestrial plant species is a strong testament that they are essential for life in an aerial environment (Gülz, 1994). Cuticular waxes provide the hydrophobic barrier of the plant surface, and as such they function primarily to shed water and prevent nonstomatal water loss. In addition, they provide a first line of defense against bacterial and fungal pathogens and against abiotic stresses such as drought and UV damage (Kolattukudy, 1980). Epicuticular waxes also play a role in plant-insect communication, by either attracting or deterring insects (Eigenbrode and Espelie, 1995). Waxes differ widely among plant species and among the organs and tissues of a single plant, attesting to the genetic diversity and developmental influences (for recent reviews, see von Wettstein-Knowles, 1995; Lemieux, 1996; Post-Beittenmiller, 1996). In addition, wax content and composition are affected by environmental conditions. Relatively high-humidity conditions, such as in tissue culture, suppress wax production (Sutter and Langhans, 1979, 1982), and the photoperiod affects the chain length of wax components (von Wettstein-Knowles et al., 1980). Despite their vital importance to plant survival and protection, and extensive studies of wax composition, very little is known about the initiation of epicuticular wax production and how production may be influenced by developmental and environmental factors.
Plant waxes are a complex heterogeneous mixture of very-long-chain (C20-C34) fatty acids and their derivatives. The fatty acid primers used for elongation are derived from plastidial de novo fatty acid biosynthesis and are exported to the cytoplasm after hydrolysis by acyl-ACP thioesterases. These fatty acids are then partitioned among membrane glycerolipid, cutin, and wax biosynthetic pathways. Elongation of long-chain fatty acids occurs in the microsomal membranes of epidermal cells (Kolattukudy and Buckner, 1972; Cassagne and Lessire, 1978) and proceeds in a series of enzymatic reactions similar to the reactions of de novo fatty acid biosynthesis (Fehling and Mukherjee, 1991). The enzymes that catalyze these reactions are collectively referred to as elongases. During wax biosynthesis very-long-chain fatty acids are further modified to aldehydes, alkanes, and ketones, which are the major components of mature leek (Allium porrum L.) leaf epicuticular waxes. Thus, elongases are important for wax production and as such would be expected to be the target for environmental and developmental controls. In addition, because plant epicuticular waxes predominantly contain components that are derived from saturated fatty acids, it follows that C16:0-ACP, C18:0-ACP, and C18:1-ACP thioesterase activities may be differentially regulated to provide the required increase in the pool of saturated fatty acids.
In leek epidermal cells that are actively synthesizing wax, the demand for fatty acids can be quite high compared with the other tissues of the leaf. For example, in leek leaf, hentriacontan-16-one, a single component of epicuticular wax, makes up more than 15% of the total leaf lipid, but is synthesized only by epidermal cells that constitute less than 4% of the leaf fresh weight (calculated from Liu and Post-Beittenmiller, 1995). Therefore, to provide sufficient pools of fatty acid precursors for wax production the expression of plastidial FAS, acyl-ACP thioesterases and microsomal fatty acid elongases may be coordinately regulated. To begin to elucidate the controls on epicuticular wax production, we have examined the relationships between wax production, FAS, microsomal fatty acid elongase, and acyl-ACP thioesterase activities and the changes in the epidermal cellular morphology in the region where wax is being actively deposited.
MATERIALS AND METHODS
Plant Material and Wax Analyses
Leeks (Allium porrum L.) were regrown for 14 d from commercially produced plants as described previously (Evenson and Post-Beittenmiller, 1995; Liu and Post-Beittenmiller, 1995). The third interior leaf, counting from the outermost leaf that expanded after cutting and replanting, was used for all studies. The third leaf was cut in transverse segments serially 3, 5, 7, 9, 14, 19, and 24 cm from the leaf base and segments were designated I to VII. The surface areas of each segment were measured (surface area calculations included both adaxial [inner] and abaxial [outer] surfaces), and then the epicuticular wax from each segment was extracted by immersion in CHCl3 for 60 s with gentle agitation. Triacontane was added as an internal standard and used to calculate the amount of individual components. Total wax load was reported as the sum of the identified components only and was therefore a conservative measure of the total wax load. Complete wax composition and content analyses were carried out on segments from six individual leaves. Aliquots of some samples were also methylated as previously described (Liu and Post-Beittenmiller, 1995).
Samples were analyzed using a gas chromatograph (model 5890) and a mass spectrometer (model 5971, Hewlett-Packard) and separated using a 30-m × 250-μm capillary column (model 5MS, Hewlett-Packard). The injector temperature was 250°C and the detector temperature was 280°C. The oven was held at 150°C for 10 min and then increased to 300°C at 10°C/min and held for 10 min. Peaks were identified by retention times and by comparison of mass spectra to standards and to the NBS75K library (Hewlett-Packard). Where indicated, only the level of hentriacontan-16-one was monitored. The surface area was measured as described above, and the epidermis was peeled from the segments, frozen in liquid nitrogen, and ground to a powder in a chilled mortar and pestle. Microscopic examination of these peels indicated that they were free of underlying parenchymal cells over most of the length of the leaf. However, epidermal peels from the lower portion (segments I and II) of the leaf had variable but small amounts of contamination from underlying tissue. Contaminating tissue would not contribute to the hentriacontan-16-one levels or to microsomal fatty acid elongase activities (K.J. Evenson and D. Post-Beittenmiller, unpublished data) but might contribute to FAS and thioesterase activities. An aliquot (2–10 mg) of frozen, powdered tissue was added to CHCl3 (triacontane was added as an internal standard), vortexed briefly, and filtered through CHCl3-washed glass wool to remove cell debris. The collected CHCl3 extract was reduced under nitrogen, and the residue was resuspended in 100 μL of CHCl3 and analyzed by GC-MS as described above. The hentriacontan-16-one levels were determined for samples used for all microscopy by measuring the levels from adjacent leaf segments (1 cm in length).
Enzyme Assays
Specific enzyme activities were expressed per unit of surface area to relate them to the accumulation of surface waxes and to each other. This was necessary because each enzyme activity was assayed from a different fraction (see below) and therefore the protein contents differed for each enzyme assay. Furthermore, the surface area is a more appropriate unit for comparing wax loads than either protein concentration or fresh weight, which may vary considerably with the developmental stage of the leek leaf segment. We found substantial changes in protein and fresh weight along the length of the leaf that did not correlate with the production of surface waxes.
Segments for enzyme assays were prepared as described above with one exception. The first segment that we used for analysis of enzyme activities represented the first 5 cm above the base of the stem, combining segments I and II, as shown in Figure 1. Epidermis peeled from individual segments was frozen in liquid nitrogen, ground to powder, and stored at −75°C until used. Enzyme assays were carried out using fractions prepared from segments of four to seven individual leaves. Each segment was analyzed for hentriacontan-16-one levels. Samples were added to chilled homogenization buffer (80 mm Hepes, pH 7.2, 2 mm EDTA, 320 mm Suc, 2 mm DTT, and 0.3 mm PMSF) at a ratio of 1:6 (w/v) and homogenized with a micropestle in a chilled microfuge tube. Samples were centrifuged (15 min, 5000 rpm, 4°C) and supernatants were transferred to airfuge tubes. Microsomal membranes were collected by centrifugation in an airfuge (15 min, 22 psi, 4°C, Beckman) and pellets were resuspended in buffer (80 mm Hepes, pH 7.2, with KOH, 15% glycerol, and 2 mm DTT). Membranes were frozen in liquid nitrogen stored at −75°C until used for elongase assays. Elongase assays and product analyses were conducted according to the method of Evenson and Post-Beittenmiller (1995).
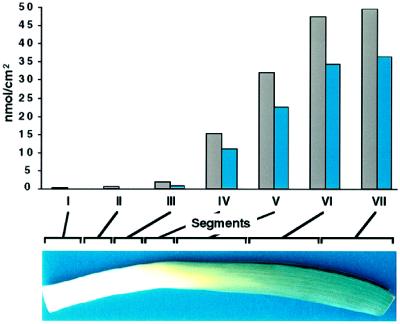
Figure 1.
Accumulation of total wax (gray bars) and hentriacontan-16-one (blue bars) from segments (I–VII) of a single leaf. The leaf segments are indicated by brackets along the top of the leaf pictured below the graph. The brackets are proportional to the segment distance as described in Methods. Data from a representative sample are shown, and the segment with ≥ 2 nmol/cm2 of hentriacontan-16-one was segment III. This experiment was carried out six times. In different leaf samples the maximum levels of total wax and hentriacontan-16-one ranged from 50 to 68 and 27 to 60 nmol/cm2, respectively.
Supernatants from microsomal membrane preparations were used for the FAS and acyl-ACP thioesterase assays. Supernatants were collected, glycerol was added to 5% (v/v), and they were stored at −75°C until used. Thioesterase assays were performed using the supernatant without further preparation, according to the method of Ohlrogge et al. (1978). FAS assays, however, required a concentration of supernatant proteins before the activity could be assayed under initial velocity conditions. Aliquots (200–500 μL) of each supernatant fraction were adjusted to 70% ammonium sulfate (percentage saturation) using saturated ammonium sulfate. The proteins were allowed to precipitate on ice for 30 min and were then collected by centrifugation at 18,000g for 30 min at 4°C. The pellet was resuspended in one-tenth volume of 10 mm Tris, pH 8.0, and desalted using a Sephadex G-50 spun column (9:1, bed volume/sample volume) that had been equilibrated with 10 mm Tris, pH 8.0. The sample was collected by centrifugation at 3,000g for 4 min at 4°C. The samples were adjusted to 5% glycerol (v/v) and used directly in FAS assays, as described by Clough et al. (1992) except that the concentration of [14C]malonyl-CoA was 60 μm, and assays were run for 30 min. All enzyme assays were performed under initial velocity conditions. Proteins were measured using a Bio-Rad protein assay reagent according to the manufacturer's recommendation.
Microscopic Examinations
For SEM examination, 1- × 0.5-cm leaf segments were prepared from the appropriate region of leek leaf as indicated. The samples were air dried for 72 h at room temperature, then mounted onto a copper holder with double-adhesive carbon tape, sputter coated with gold for 3 min, and examined under an electron microscope (model JSM 880, JEOL). Secondary electron images were collected at 15 kV of accelerating voltage.
Leaf segments (5 × 0.5 cm) were cut starting from 5 cm above the base of the leaf for TEM examination. The tissue was fixed with 3% (v/v) paraformaldehyde and 1% glutaraldehyde (v/v) in 0.1 m sodium cacodylate buffer, pH 7.4, at room temperature for approximately 1 h. During the initial fixation a vacuum was applied for approximately 15 min to facilitate fixative penetration. Fixation continued overnight at 4°C. The tissue was rinsed three times in 0.1 m sodium cacodylate buffer, pH 7.4, and then fixed in 2% (w/v) osmium tetroxide in 0.1 m sodium cacodylate buffer, pH 7.4, at room temperature for 2 h. The samples were washed three times in 0.1 m sodium cacodylate buffer and then dehydrated in a graded ethanol series of 10, 30, 50, 70, 95, 100, 100, and 100% for 30 min for each step. The dehydrated leaf segments were infiltrated with Embed 812 (EM Sciences, Fort Washington, PA) at room temperature under the following schedule: Embed 812:ethanol (1:2, v/v) for 4 h; Embed 812:ethanol (2:1, v/v) overnight; and Embed 812 without ethanol for 8 to 12 h three times. Finally, the samples were polymerized overnight in an oven at 70°C. Ultrathin sections were viewed using TEM (with either a model 10C microscope [Zeiss] at 80 kV or a model JEM2000FX microscope [JEOL] at 100 kV).
Epidermal peelings were made from 0.5-cm segments and the unstained peels were examined using bright-field microscopy (Nikon FX). Cell-length measurements were made using a calibrated ocular micrometer. In the bottommost tissue, including the first three tiers of cells, the tier organization was not distinct; therefore, 10 cells were measured randomly through this region without regard to tier association. In tiers 4 through 10, 10 cells were measured for each tier. In the segments from 0.5 to 17 cm, 10 cells were counted in the intact tier closest to the end most distal to the base of the leaf. To ascertain whether distortion was created while preparing the epidermal peels that might lead to erroneous measurements, negative cellulose acetate replicas were also made from contiguous segments. The cellular dimensions measured from the replicas showed similar cell dimensions as the epidermal peels. Data are reported as tiers (below 0.5 cm from the leaf base), single segments (at 0.5–1 cm above the leaf base), or two combined segments, where there were no significant differences between two segments (above 1 cm from the leaf base).
RESULTS
Total Wax Accumulation and Composition
As a monocot, leek leaves expand in a simple, linear fashion from the base of the leaf, in contrast to dicots, which expand in all directions of the leaf plane. To establish whether epicuticular waxes are deposited uniformly on the expanding leaf surface, or in a manner consistent with a developmental influence, the total wax load and composition were determined along the length of individual rapidly expanding leek leaves. The wax load from the bottom to the top of one of six individual leaves examined is shown in Figure 1. Very little CHCl3-extractable lipid was detected from the base of the leaf to approximately 7 cm above the leaf base. At 7 to 9 cm the onset of a dramatic increase (more than 30-fold in most samples) in the wax load was observed (Fig. 1). The increase continued for most of the length of the leaf, and then in segments VI and VII the amount of wax per surface area was similar. The accumulation of hentriacontan-16-one reflected that of the total wax. Although comparable trends were observed for all six leaves examined, the onset (segment III or IV) and duration (through segment VI or VII) of wax accumulation, as well as the absolute levels of wax, varied among the leaves of different plants. Therefore, it was essential for later biochemical studies to be able to identify the onset of wax accumulation.
The wax composition of each segment was also determined from the six leaves and the averages with ses are shown in Table I. Leek leaf waxes consisted primarily of fatty acids (C16–22) and longer-chain (C26–31) derivatives (aldehydes, alkanes, and ketones). In this study samples were not derivatized with silylating reagents and therefore alcohols, which are a minor component (D. Huhman and D. Post-Beittenmiller, unpublished data), were not analyzed. Other unidentified but minor components were also not included in these analyses. CHCl3-extractable lipids from the lower leaf segments were primarily hexadecanoic (C16) and octadecanoic (C18) acids. Smaller amounts of eicosanoic (C20) and docosanoic (C22) acids were also detected. The level of hexadecanoic and octadecanoic acids increased almost 4- and 2-fold, respectively, from the bottom (segment I) to the top of the leaf (segment VII). Even though C16 and C18 fatty acids were a major component (78–92%) of the CHCl3-extractable surface lipids in the bottom 5 cm, they were minor components (12–13%) in the top 5 cm. Undetectable or very low levels (< 5% of the total wax load) of very-long-chain alkanes, aldehydes, and ketones were also observed in the bottom segments. These components, however, increased dramatically in the middle region of the leaf to more than 75% in the top 5 cm.
Table I.
Composition of epicuticular wax on segments of leek leaves
| Fatty Acid and Derivative | Ia
|
II
|
III
|
IV
|
V
|
VI
|
VII
|
|---|---|---|---|---|---|---|---|
| 0–3 cmb | 3–5 cm | 5–7 cm | 7–9 cm | 9–14 cm | 14–19 cm | 19–24 cm | |
| nmol/cm2 area | |||||||
| Hexadecanoic acid | 1.6 (0.6) | 0.67 (0.2) | 1.1 (1) | 2.5 (2) | 3.3 (1) | 5.3 (1) | 6.0 (1) |
| Octadecanoic acid | 0.78 (0.4) | 0.10 (0.1) | 0.23 (0.2) | 0.21 (0.1) | 1.0 (0.4) | 1.6 (0.7) | 1.7 (0.4) |
| Eicosanoic acid | 0.00 (0.0) | 0.18 (0.2) | 1.2 (1) | 2.1 (1) | 2.8 (0.9) | 3.2 (0.3) | 3.5 (0.9) |
| Docosanoic acid | 0.18 (0.1) | 0.00 (0.0) | 0.54 (0.5) | 1.0 (0.5) | 1.4 (0.5) | 1.7 (0.3) | 2.0 (0.3) |
| Hexacosanal | 0.00 (0.0) | 0.00 (0.0) | 0.01 (0.0) | 0.2 (0.0) | 0.09 (0.1) | 0.51 (0.2) | 0.66 (0.2) |
| Octacosanal | 0.00 (0.0) | 0.00 (0.0) | 0.05 (0.0) | 0.17 (0.1) | 0.49 (0.2) | 1.7 (0.5) | 1.8 (0.5) |
| n-Nonacosane | 0.02 (0.0) | 0.01 (0.0) | 0.07 (0.0) | 0.13 (0.1) | 0.38 (0.1) | 1.0 (0.2) | 1.1 (0.3) |
| Triacontanal | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.0) | 0.07 (0.1) | 0.25 (0.1) | 1.1 (0.3) | 0.93 (0.3) |
| n-Hentriacontane | 0.00 (0.0) | 0.01 (0.0) | 0.31 (0.2) | 0.86 (0.3) | 2.7 (0.7) | 6.3 (2) | 5.5 (1) |
| Hentriacontan-16-one | 0.00 (0.0) | 0.03 (0.0) | 4.8 (2) | 18 (6) | 31 (6) | 36 (4) | 38 (3) |
| Total | 2.6 | 1.0 | 8.3 | 25 | 43 | 58 | 61 |
Values are presented as averages with ses in parentheses (n = 3 for fatty acid; n = 6 for other components). Distance measurements are from the leaf base.
Segment number.
Distance.
Hentriacontan-16-one represented 58 to 72% of the total wax load in the middle of the leaf (segments III–V). By comparison, aldehydes and alkanes represented substantially smaller proportions (5–9%) of the total wax content in the same segments. They appeared similarly with hentriacontan-16-one, but their rapid accumulation (beginning in segment V) lagged behind the accumulation of hentriacontan-16-one (beginning in segment III), becoming relatively constant in the topmost leaf segments (compare segments VI and VII). Because the appearance of hentriacontan-16-one began essentially with the rapid increase in total wax load, hentriacontan-16-one levels were monitored and used as a marker to identify the onset of the wax accumulation in all subsequent studies. We defined the segment in which the level of hentriacontan-16-one was ≥ 2 nmol/cm2 as the onset of wax accumulation for the ease of discussion in this paper. Whether this was segment III (5–7 cm above the leaf base), as shown in Figure 1 and Table I, or segment V, as shown in Figure 3 (see below), it was variable among the plants used in these studies. Identification of the onset was of particular interest, as the region preceding the onset of accumulation is presumably where genes and enzymes involved in wax production would be induced.
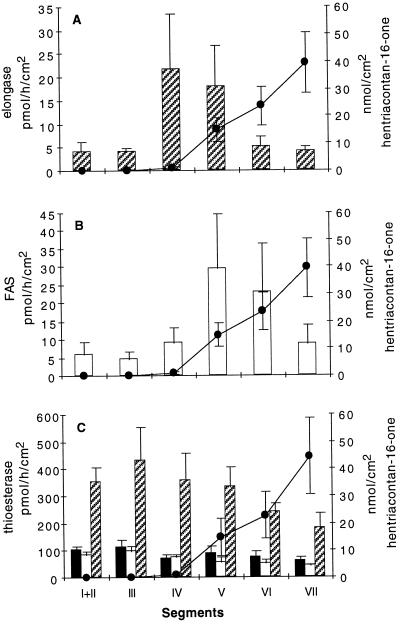
Figure 3.
Assay of enzyme activities in each segment of leek epidermis compared with hentriacontan-16-one accumulation. Segments indicated along x axis are as described in Figure 1. A, Fatty acid elongase activity. B, FAS activity. C, Acyl-ACP-hydrolyzing activity. Solid bars, 16:0-ACP hydrolysis; open bars, 18:0-ACP hydrolysis; and hatched bars, 18:1-ACP hydrolysis. Hentriacontan-16-one (•) was used as a marker for wax load in each experiment. The segment containing ≥ 2 nmol/cm2 hentriacontan-16-one was segment V. Mean values with ses are presented. In A and B, n = 7, and in C, n = 2 to 4.
Wax Accumulation Began after Cell Division and Elongation Ceased
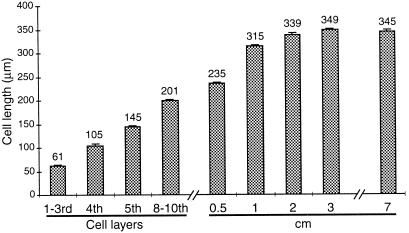
To determine whether wax biosynthesis was concomitant with cell division and elongation in expanding leek leaf, we measured the length of cells from near the base of the leaf to 17 cm above. As indicated in the previous section, the level of hentriacontan-16-one was used to identify the onset of wax accumulation. The results are shown in Figure 2. Substantial increases in the length of the epidermal cells were observed up to the 10th cell layer. Above the 10th cell layer increases in the cell length slowed, and above 1 cm, further increases in the cell length were less than 8%. Analyses of hentriacontan-16-one levels indicated that the onset of wax accumulation (at 9–10 cm from the leaf base in this sample, data not shown) was well above the region of epidermal cell elongation.
Figure 2.
Measurement of cell length to determine cessation of cell elongation in the abaxial epidermal cells of leek leaves. The numbers above the bars indicate the mean cell lengths. se bars are indicated (n = 10). For each segment 10 fields were counted. Cell layers were noted below 0.5 cm. Cell lengths were measured in 0.5-cm segments and data are shown as the lengths in a 1-cm segment; the two 0.5-cm segments did not vary significantly. Hentriacontan-16-one was monitored in 1-cm segments at the same position from the leaf base as the cell-length segments. The onset of wax accumulation began 9 to 10 cm from the leaf base on this leaf. Cell lengths did not change substantially above 7 cm and therefore data are not shown for segments above 7 cm.
Enzyme Activities Associated with Fatty Acid Synthesis and Elongation
The increasing wax accumulation on the leaf surface suggested that there may be a corresponding increase in the enzyme activities associated with the biosynthesis of wax components. One of the key activities leading to the production of hentriacontan-16-one is microsomal elongation of fatty acyl primers. Therefore, we examined the activities of microsomal elongases and FAS in each leaf segment. These experiments were conducted on seven individual leaves. Each leaf was segmented, the epidermis was peeled, and microsomal and supernatant fractions from each segment were assayed separately for elongase and FAS activities, respectively, and hentriacontan-16-one levels were monitored. All individual leaves showed similar trends, although maximum levels of in vitro activities varied. The averages of seven experiments are shown in Figure 3. The maximal elongase (Fig. 3A) and FAS (Fig. 3B) activities were 5- and 6-fold greater, respectively, compared with the activities in the lower segments. Surprisingly, peak elongase and FAS activities occurred in different segments. The peak of fatty acid elongase activity (segment IV) preceded the onset of hentriacontan-16-one accumulation (segment V), as was predicted. However, FAS activity peaked later, in segment V, which was above the segment with peak elongase activity and which coincided with the onset of hentriacontan-16-one accumulation. This relationship between fatty acid elongase and FAS activities was seen in all leaves assayed with the exception of one. In the exceptional case the peak elongase activity was observed in the later segment (V) simultaneously with peak FAS activity.
The termination of FAS and the initiation of microsomal fatty acid elongation is a point where partitioning between extraplastidial glycerolipid and wax biosynthetic pathways might be controlled. The enzymes responsible in part for FAS termination and export of fatty acids from the plastid are the acyl-ACP thioesterases. These enzymes have three substrates available in epidermal leucoplasts: C16:0-ACP, C18:0-ACP, and C18:1-ACP. Extracts from the epidermal peels of segmented leaves were assayed for hydrolyzing activity with each acyl-ACP substrate. The averages of two to four of the same seven leaves used for the FAS and elongase assays are shown in Figure 3C. Essentially, the levels of C16:0-ACP- and C18:0-ACP-hydrolyzing activities did not change along the length of the developing leaf. Except for a small (20%) but consistent increase in C18:1-ACP-hydrolyzing activity in the segment preceding the increased fatty acid elongase activity, the level of C18:1-ACP-hydrolyzing activity gradually decreased to 42% (from 430 to 182 pmol h−1 cm−2) along the length of the leaf.
Cellular Morphology Changed prior to the Appearance of Wax Crystals
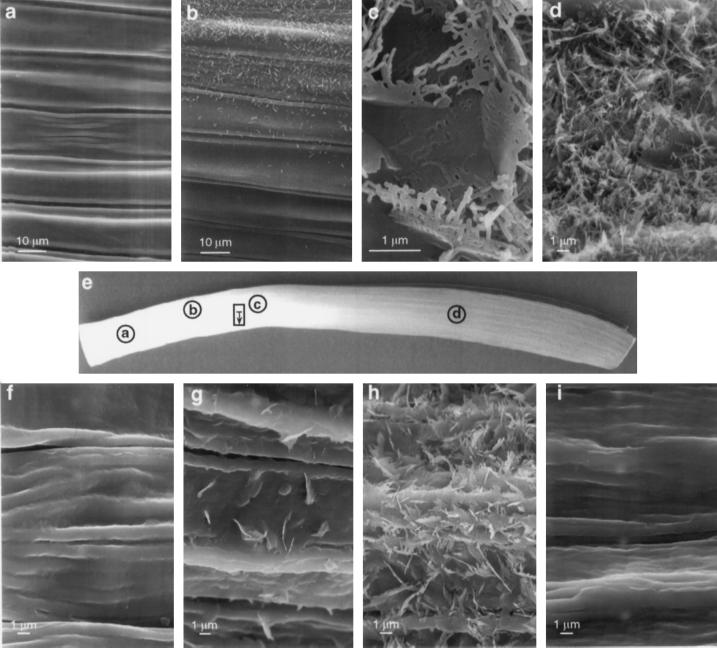
We examined the leaf surfaces by SEM and the cellular morphology of leaf epidermal cells by TEM to identify changes that may correlate with the onset of wax production. Figure 4 is a composite of scanning electron micrographs taken from various sections along the length of two different leek leaves. Figure 4e is a picture of the abaxial (outer) side of a leek leaf with circles drawn to indicate the areas of the leaf that were examined by SEM. No crystals were observed on either side close to the base of the leaf, well below the onset of wax accumulation (Fig. 4a). On one leaf crystals began to appear in the region that was identified by GC-MS as the onset of wax accumulation (> 2 nmol/cm2, Fig. 4b). It was evident that the formation of crystals (i.e. wax deposition) did not occur uniformly across a given latitude of the leaf, because individual cells within the region differed in the abundance of surface wax crystals. The rectangle marked on Figure 4e is a region of the second leaf that was below the onset of wax accumulation (< 2 nmol/cm2) in which a survey of the surface crystals was conducted along a single latitudinal line, indicated by the unidirectional arrow. The four SEM fields shown in Figure 4, f to i, were taken from the survey and represented a progression from the top to the bottom of the rectangle. The crystalline deposits were found to be sparse (Fig. 4, f), moderate (Fig. 4, g), dense (Fig. 4, h), and then sparse again (Fig. 4, i) as scanning proceeded latitudinally across the leek leaf. Approximately 1 cm above the region shown in Figure 4, f to i, but in a region where the levels of hentriacontan-16-one were still < 2 nmol/cm2, the abaxial leaf surface was densely covered with wax crystals (Fig. 4c). Solid plates were seen near the leaf surface, with branched rods projecting out from the plates, creating waffle-shaped crystals. Wax crystals were not observed on the adaxial (inner) surface in this same region. Crystals, primarily branched rods, were heavily distributed on the abaxial surface in a region well above the onset of wax accumulation by GC-MS analysis (Fig. 4d). Densely packed crystals were also seen on the adaxial surface in this region (data not shown). Wax crystals were more abundant around the stomata in all regions (data not shown), where they presumably provided extra protection against water loss.
Figure 4.
Scanning electron micrographs of the abaxial surface of a leek leaf. a, Region below the onset of wax accumulation, where no hentriacontan-16-one was detected by GC-MS and no wax crystals were observed. b, Region within the segment showing the onset of wax accumulation as determined by the presence of hentriacontan-16-one ≥ 2 nmol/cm2. c, Waffle-like structures of wax crystals were observed below the onset of wax accumulation. d, Region well above the onset of wax accumulation showing numerous branched rods and plates. e, Photograph of a leek leaf showing the abaxial surface. All electron micrographs are shown in the same orientation as the pictured leaf. Circles indicate areas examined for a through d, and the rectangle indicates the area examined for f through i. The unidirectional arrow within the rectangle indicates the latitudinal survey covered between 10.5 and 11.5 cm above the leaf base, which was identified as below the onset of wax accumulation by GC-MS analysis on this leaf. Areas shown in f through i were taken along the same lateral line as illustrated in the rectangle, showing the uneven deposition of crystals in adjacent regions below the segment identified as the onset of wax accumulation. a and b are from the third leaf of one plant, and c through i are from the third leaf of a second plant.
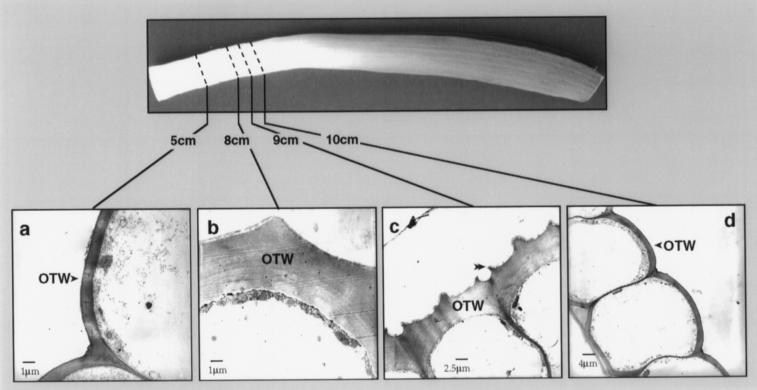
Morphological changes in the epidermis were also observed by TEM of transverse sections. The most conspicuous changes occurred in the OTWs. In the epidermal layer, approximately 5 cm above the base of the leaf, the OTWs (Fig. 5a) of the abaxial epidermal cells were smooth and similar in thickness (1 μm) to the inner tangential cell walls (not shown), and the cuticle was a thin line. Farther up the leaf, at approximately 8 cm, the OTW had thickened approximately 4-fold, and in each epidermal cell, a single central ridge was apparent (Fig. 5b). The cuticle had also thickened, but because it was quite thin at 5 cm above the leaf base, the increase at 8 cm could not be determined. The changes in the cuticular surface of the OTW progressed rapidly and at 9 cm had become distinctly irregular (Fig. 5c). The appearance of wax crystals observed by SEM (Fig. 4b) was accompanied by the increased OTW thickness on the abaxial surface, as observed by TEM (Fig. 5b). GC-MS analysis of the region represented by Figure 5b detected 3.5 nmol/cm2 hentriacontan-16-one. The changes observed in the OTW of the abaxial surface apparently occurred developmentally earlier than the cell wall changes of the adaxial surface. For example, the thickening of the OTW (from 1 to 4 μm) the increased cuticle thickness, and the appearance of the central ridge on the cells of the abaxial surface were observed at 8 cm, whereas at 10 cm, the OTW on the adaxial surface was only 2 μm, the cuticular surface was smooth, and the cuticle was thin (Fig. 5, compare c and d).
Figure 5.
Transmission electron micrographs of epidermal cells at various positions along the length of the leaf. a, Abaxial epidermal cells at 5 cm above the base of the leaf showing the thin OTW and smooth cuticular surface. The cuticle is a thin line on the surface of the OTW. b, Abaxial (outer) epidermal cells at 8 cm showing the thickened OTW and a single central ridge as the beginning of cuticular surface irregularities. The single ridge was observed on the cuticular surface of all abaxial epidermal cells examined in this region. c, Abaxial epidermal cells at 9 cm showing the increasing irregular cuticular surface and the pair of developing ridges over the radial cell walls (double arrowhead). d, Adaxial (inner) epidermal cells at 10 cm showing the thin cuticle and the OTW, which was thin and smooth at the cuticular surface. The epidermal cells on the abaxial surface at 9 cm had already undergone more extensive morphological changes (shown in c) and had a heavy deposit of wax crystals. The adaxial surface did not have wax crystals in this region, although dense wax crystals were evident in segments from higher up on the leaf. Scale bars are indicated.
DISCUSSION
Leeks have been used to study epicuticular wax production for more than 25 years, but the emphasis has been limited primarily to fatty acid elongases and not to pathway regulation. In the present study we sought to determine whether wax production in leeks was induced along the length of the expanding leaf, and if so, whether the region could be readily identified among commercially produced plants for studies of induction of wax biosynthesis using biochemical and molecular approaches. Commercially produced leeks are readily available year-round and the plants are considerably larger (2–4 cm in diameter) than leek seedlings grown in greenhouses or growth chambers, which require more than 6 months to reach a diameter of 1 to 2 cm. Space becomes limiting when growing even moderate numbers of plants to sizes approaching commercially produced plants. A distinct advantage is that commercially produced leeks can be regrown from the cut and replanted false stem. In 10 to 14 d the inner leaves grow 20 to 25 cm and are actively producing epicuticular wax. The rapidly expanding epidermal tissue is easily separated from the underlying parenchyma and is an excellent source of stable and active microsomal fatty acid elongases (Evenson and Post-Beittenmiller, 1995) and acyl-ACP thioesterases (Liu and Post-Beittenmiller, 1995). The epidermis from such regrown plants has been used to construct a cDNA library from which a 3-ketoacyl-ACP synthase III cDNA (Chen and Post-Beittenmiller, 1996) and several cDNAs encoding acyl-ACP thioesterases (D. Liu, D. Hawkins, L. Yuan, J. Kridl, and D. Post-Beittenmiller, unpublished data) have been isolated and characterized. The regrown plants are also the source of our explant material (flower stalk) for leek regeneration and transformation studies (C. Maier and D. Post-Beittenmiller, unpublished data).
As a monocot, the leek leaf expands from the intercalary meristem (basipetal development), representing a developmental progression from the leaf base to the leaf tip (Hay and Brown, 1988). We have used the developing leaf as a means to study the induction of wax production. GC-MS analyses demonstrated that accumulation of epicuticular waxes began in a region 5 to 9 cm above the base of the leaf (Table I), but in some leaves the onset was as high as 9 to 14 cm from the leaf base. Because of the variability of the onset of wax accumulation among regrown leek plants, and because of the small amounts of epidermal tissue available for analysis from segmented samples, it was necessary to identify a reliable, sensitive, and easy to measure marker for wax production that would allow us to compare results from different leaves. In all of these studies the levels of hentriacontan-16-one were used to establish the onset of wax accumulation along the length of the leaf.
Wax Biosynthetic Pathway May Be under Global Regulation
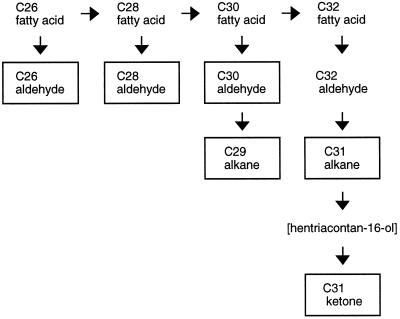
Fatty acids (C16–C22) were the predominant components in the lowest leaf segments, making up 95 to 98% of the total wax. Although the total CHCl3-extractable lipids from the leaf surface in this region were relatively low, total wax accumulation increased 23-fold from the bottom (segment I) to the leaf tip (segment VII) during the regrowth of the leek leaf, and accumulation of hentriacontan-16-one increased more than 1000-fold from segments II to VII. As the total wax load increased, very-long-chain aldehydes and alkanes were also detected, although the accumulation of aldehydes and alkanes lagged behind the accumulation of hentriacontan-16-one. The rate of accumulation of all components frequently slowed in the topmost segments. Figure 6 is a simplified schematic of the biosynthetic pathway leading to the production of hentriacontan-16-one. A recent report has shown that in pea, aldehyde components are produced by a fatty acid reductase that is different from the fatty acid reductase leading to primary alcohol production (Vioque and Kolattukudy, 1997). The alcohol-producing reductase catalyzes the two-step reduction without the release of an aldehyde intermediate. If this is also the case for leeks, all aldehydes in leek epicuticular wax are derived via the decarbonylation pathway. The boxed components are products that accumulated in leek leaf epicuticular wax. Although we assume that the ketone was derived from the corresponding secondary alcohol, the lack of hentriacontan-16-ol accumulation suggested that oxidation to the ketone was very rapid. Similarly, the lack of accumulation of very-long-chain fatty acids (C26–C32) indicated that reduction to the corresponding aldehydes was also very fast. Alternatively, the alcohol and fatty acid precursors for the ketone and aldehydes, respectively, may not be available for transport to the surface. The fact that n-hentriacontane (C31) accumulation lagged behind that of hentriacontan-16-one (Table I) suggested that there may be a decline in the rate of alkane hydroxylation in the upper segments that allowed the precursor alkane to accumulate. Similarly, the increased levels of shorter-chain aldehydes (C26–C30) and alkanes (C29) in the upper segments suggested that the rate of C30 to C32 elongation may decrease somewhat earlier than the other fatty acids. These variations in the accumulation patterns of the individual components were minor, however, and probably do not reflect substantial differences in the expression of any single enzyme activity. Rather, the coincidental increased accumulation of all of the components during wax accumulation, as well as the generally slowed accumulation of all components in the upper segments, implies that the pathway is globally regulated.
Figure 6.
Schematic of the terminal steps of wax biosynthesis in leek leaves leading to the production of hentriacontan-16-one. The boxed components were found in the surface wax. The brackets around hentriacontan-16-ol indicate that although it is assumed to be the immediate precursor to hentriacontan-16-one, this aspect of the pathway has not been characterized in leeks. The synthesis of C26 to C32 fatty acids is evident from in vitro assays of fatty acid elongases. The synthesis of the C32 aldehyde is deduced from the presence of the C26 to C30 aldehydes and the C31 alkane. Similar deductions were not made for hentriacontan-16-ol, since there is only indirect evidence of secondary alcohol synthesis in leeks.
Wax Accumulation Is Not Linked to Cell Elongation
During plant growth the epidermis and cuticle necessarily expand to provide a continuous protective layer. Expansion of the epidermis has been proposed to be a limiting factor to organ growth (Kutschera, 1989). The cuticle is composed of polymerized cutin and embedded hydrophobic compounds, such as fatty acids and other wax components (Walton, 1990). Cutin has been shown to be synthesized in the rapidly expanding internodal region of deepwater rice (Hoffmann-Benning and Kende, 1994) and the bottom third of Clivia miniata leaves (Lendzian and Schönherr, 1983). Similarly, the waterproofing CHCl3-extractable lipids would be expected to be synthesized at a developmental stage similar to cutin biosynthesis. Our studies showed that in the region of cell elongation (< 1 cm above the leaf base) free fatty acids were present, but the major production of epicuticular waxes occurred later. Similarly, the cuticle thickened in segments well above the cessation of cell elongation. Since cell division occurs in the intercalary meristem, below the region of cell elongation, wax accumulation and cuticle thickening were also not strictly coordinated with cell division. This result is in contrast to studies of two genes involved in cuticular wax production in Arabidopsis. CER2 expression is associated with elongating tissues such as developing siliques (Xia et al., 1996) and CER3 expression is associated with meristematic regions (Hannoufa et al., 1996). It is possible that the somewhat delayed induction of wax biosynthesis in leeks differs from that in dicots because of the nature of tissue exposure and tissue expansion in leeks. The telescoped stem discs of the young leek leaves are protected from the environment by the outer, more mature leaves until the young leaf expands beyond the outer leaves, and therefore the meristematic region of the young leaf is protected from the drying effects of an aerial environment. CHCl3-extractable fatty acids on the lowest leaf segments may provide the minimal waterproofing necessary on or in the cuticle to protect the young, developing leek leaves. In dicots the young leaves are exposed to an aerial environment soon after they emerge from buds, and therefore a heavier or more complex mixture of wax components may be required to protect the young dicot tissues from more arid conditions compared with the conditions surrounding the young leek tissues.
Elongase Activity Was Induced before the Onset of Wax Accumulation
The low level of the CHCl3-extractable lipids in the bottom segments (I and II) of the leaf followed by an increase in accumulation of total wax load implied that leaf epicuticular wax production may be due to an induction of the wax biosynthetic enzymes. The increase in elongase activity (segment IV) prior to the onset of wax accumulation supports this hypothesis (Fig. 3A). FAS activity increased in segments developmentally older than segments with peak fatty acid elongase activity. This was observed in six of seven leaves examined. The relative developmental difference between the activity peaks suggests that fatty acid elongation and de novo fatty acid synthesis may be under different developmental regulation. The reason for the delay in peak FAS activity is not known, but the delay suggests that a precursor pool is available for fatty acid elongation prior to the stimulation of FAS activity. The constitutive expression and the comparatively high levels of thioesterase activities suggests that acyl-ACP thioesterases were not induced and may not be limiting. In addition to wax and glycerolipid biosynthesis, fatty acids are precursors for cutin biosynthesis. The increase in cuticle thickness suggests that cutin was being synthesized in the same regions as wax production, and, therefore, the thioesterase activities may reflect the combined needs of glycerolipid, wax, and cutin biosynthesis.
Cellular and Morphological Changes
SEM is more sensitive than GC-MS for detecting the onset of wax production, since SEM analyses can detect a few crystals on a single cell, whereas considerably greater levels of wax are required for detection by GC-MS. However, SEM is not practical for the rapid and routine detection required for many biochemical and molecular analyses. SEM analyses showed that wax crystals were first evident in the segment prior to the onset of wax accumulation, as identified by GC-MS analyses of CHCl3 extracts. In addition, within this segment there were areas in which some cells had a dense layer of crystals and adjacent cells were almost devoid of crystals, indicating that wax production was not strictly synchronous in cells of the same latitude.
TEM analyses provided some insights regarding the changes in cellular morphology that occurred in the segment preceding the onset of wax accumulation. First, no wax crystals were observed in the lower leaf segment, where the OTW and cuticle were thin. Second, the OTW thickening was observed only in the epidermal cells that synthesize and transport wax and cutin, not in the underlying parenchymal cells, and it was only in the OTW and not on the inner tangential or radial cell walls. Third, surface wax crystals were observed by SEM where the irregular cuticular surface of the OTW also occurred. Fourth, these morphological changes and the presence of wax crystals both occurred developmentally earlier in cells of the abaxial surface than in cells of the adaxial surface. Together, these observations suggest that the thickening and the irregular cuticular surface may be related to the deposition of cuticular wax. Additional studies are in progress to address this issue.
Although environmental factors such as light and humidity have been shown to affect wax production in some plants (von Wettstein-Knowles, 1995; Post-Beittenmiller, 1996), we suggest that the induction of wax accumulation we observed in leek leaves was more likely to be controlled primarily by developmental factors, but was potentially influenced by environmental factors. In this context it should be noted that the greening of the leaf lamina, which is indicative of light-induced chloroplast biogenesis in the underlying parenchymal cells, did not correlate with the onset of wax accumulation. The presence of wax crystals, the induction of microsomal fatty acid elongation, and increased levels of hentriacontan-16-one (> 2 nmol/cm2) were found in the nongreen regions of the leaf sheath, deep within the layered leaves of the false stem. These leaf segments were relatively protected from strong light and the potential drying effects of an arid environment. In addition, if humidity and light play a dominant role in inducing wax accumulation, then both the adaxial and abaxial surfaces of the leek leaf would be expected to have similar responses when observed by SEM and TEM. However, whereas wax crystals were abundant on the abaxial epidermis 11.5 to 12.5 cm above the leaf base, they were absent from the adaxial epidermis in the same region. Furthermore, wax crystals did accumulate to high levels on the adaxial surface of the upper segments, suggesting that wax production on the adaxial surface lagged behind that on the abaxial surface. Detailed comparisons of the wax composition of the two surfaces by GC-MS is beyond the scope of these studies but may lead to further insights into the developmental aspects of wax production. In addition to the observed differences in wax crystal formation, the abaxial side had more stomata per surface area than the adaxial side, further evidence of different developmental timing for these two surfaces.
Although these results imply that light and humidity are not involved in the induction of wax biosynthesis in leeks, light has been implicated in influencing fatty acid chain length in maize (von Wettstein-Knowles et al., 1980), and high RH has been implicated in suppression of wax accumulation in in vitro-grown cabbage and carnation leaves (Sutter and Langhans, 1979; Sutter, 1984). Similarly, environmental cues such as light or humidity may modulate the developmental programming of cuticular wax production in leeks.
In these studies we have identified a region in the developing leek leaf where wax accumulation increases dramatically. Fatty acid-elongation activity was induced in the region preceding the onset of wax accumulation. The appearance of wax crystals in this region is associated with morphological changes suggestive of active wax deposition. The onset of wax accumulation can be identified by monitoring the levels of hentriacontan-16-one, thus facilitating the cloning of differentially expressed genes involved in wax production (Liang and Pardee, 1992; Liang et al., 1995). These studies are currently in progress.
ACKNOWLEDGMENTS
We thank Drs. Richard Dixon, John Hipskind, and Jan Jaworski for critical reading of the manuscript and Drs. Grattan Roughan and Jan Jaworski for helpful discussions. We are grateful to Ms. Yuling Sun for harvesting leek epidermis and to Mr. David Huhman for his assistance in GC-MS analyses. We wish to extend our thanks to Mr. Bill Chissoe and Mr. Greg Strout at the University of Oklahoma for their help in SEM and TEM analyses.
Abbreviations:
- ACP
acyl carrier protein
- Cx:y
fatty acid notation where x = number of carbon atoms and y = number of double bonds
- FAS
fatty acid synthase
- OTW
outer tangential cell wall
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
Footnotes
This research was supported by the Samuel Roberts Noble Foundation, Ardmore, OK 73402.
LITERATURE CITED
- Cassagne C, Lessire R. Biosynthesis of saturated very long chain fatty acids by purified membrane fractions from leek epidermal cells. Arch Biochem Biophys. 1978;191:146–152. doi: 10.1016/0003-9861(78)90076-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Post-Beittenmiller D. Molecular cloning of a cDNA encoding 3-ketoacyl-acyl carrier protein synthase III from leek. Gene. 1996;182:45–52. doi: 10.1016/s0378-1119(96)00472-6. [DOI] [PubMed] [Google Scholar]
- Clough RC, Matthis AL, Barnum SR, Jaworski JG. Purification and characterization of 3-ketoacyl-acyl carrier protein synthase III from spinach: a condensing enzyme utilizing acetyl-CoA to initiate fatty acid synthesis. J Biol Chem. 1992;267:20992–20998. [PubMed] [Google Scholar]
- Eigenbrode SD, Espelie KE. Effects of plant epicuticular lipids on insect herbivores. Annu Rev Entomol. 1995;40:171–194. [Google Scholar]
- Evenson KJ, Post-Beittenmiller D. Fatty acid-elongating activity in rapidly expanding leek epidermis. Plant Physiol. 1995;109:707–716. doi: 10.1104/pp.109.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling E, Mukherjee KD. Acyl-CoA elongase from a higher plant (Lunaria annua): metabolic intermediates of very-long-chain acyl-CoA products and substrate specificity. Biochim Biophys Acta. 1991;1082:239–246. doi: 10.1016/0005-2760(91)90198-q. [DOI] [PubMed] [Google Scholar]
- Gülz P-G. Epicuticular leaf waxes in the evolution of the plant kingdom. J Plant Physiol. 1994;143:453–464. [Google Scholar]
- Hannoufa A, Negruk V, Eisner G, Lemieux B. The CER3 gene of Arabidopsis thaliana is expressed in leaves, stems, roots, flowers and apical meristems. Plant J. 1996;10:459–467. doi: 10.1046/j.1365-313x.1996.10030459.x. [DOI] [PubMed] [Google Scholar]
- Hay RKM, Brown JR. Field studies of leaf development and expansion in the leek (Allium porrum) Ann Appl Biol. 1988;113:617–625. [Google Scholar]
- Hoffmann-Benning S, Kende H. Cuticle biosynthesis in rapidly growing internodes of deepwater rice. Plant Physiol. 1994;104:719–723. doi: 10.1104/pp.104.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE (1980) Cutin, suberin, and waxes. In P Stumpf, E Conn, eds, The Biochemistry of Plants. Academic Press, pp 571–645
- Kolattukudy PE, Buckner JS. Chain elongation of fatty acids by cell-free extracts of epidermis from pea leaves (Pisum sativum) Biochem Biophys Res Commun. 1972;46:801–807. doi: 10.1016/s0006-291x(72)80212-2. [DOI] [PubMed] [Google Scholar]
- Kutschera U. Tissue stresses in growing plant organs. Physiol Plant. 1989;77:157–163. [Google Scholar]
- Lemieux B. Molecular genetics of epicuticular wax biosynthesis. Trends Plant Sci. 1996;1:312–318. [Google Scholar]
- Lendzian KJ, Schönherr J. In-vivo study of cutin synthesis in leaves of Clivia miniata reg. Planta. 1983;158:70–75. doi: 10.1007/BF00395405. [DOI] [PubMed] [Google Scholar]
- Liang P, Bauer D, Averboukh L, Warthoe P, Rohrwild M, Muller H, Strauss M, Ardee AB. Analysis of altered gene expression by differential display. Methods Enzymol. 1995;254:304–321. doi: 10.1016/0076-6879(95)54022-9. [DOI] [PubMed] [Google Scholar]
- Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–970. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Liu D, Post-Beittenmiller D. Discovery of an epidermal stearoyl-acyl carrier protein thioesterase: its potential role in wax biosynthesis. J Biol Chem. 1995;270:16962–16969. doi: 10.1074/jbc.270.28.16962. [DOI] [PubMed] [Google Scholar]
- Ohlrogge JB, Shine WE, Stumpf PK. Fat metabolism in higher plants: characterization of plant acyl-ACP and acyl-CoA hydrolases. Arch Biochem Biophys. 1978;189:382–391. doi: 10.1016/0003-9861(78)90225-4. [DOI] [PubMed] [Google Scholar]
- Post-Beittenmiller D. Biochemistry and molecular biology of wax production in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:405–430. doi: 10.1146/annurev.arplant.47.1.405. [DOI] [PubMed] [Google Scholar]
- Sutter E. Chemical composition of epicuticular wax in cabbage plants grown in vitro. Can J Bot. 1984;62:74–77. [Google Scholar]
- Sutter E, Langhans RW. Epicuticular wax formation on carnation plantlets regenerated from shoot tip culture. J Am Soc Hortic Sci. 1979;104:493–496. [Google Scholar]
- Sutter E, Langhans RW. Formation of epicuticular wax and its effect on water loss in cabbage plants regenerated from shoot-tip culture. Can J Bot. 1982;60:2896–2902. [Google Scholar]
- Vioque J, Kolattukudy PE. Resolution and purification of an aldehyde-generating and an alcohol-generating fatty acyl-CoA reductase from pea leaves (Pisum sativum L.) Arch Biochem Biophys. 1997;340:64–72. doi: 10.1006/abbi.1997.9932. [DOI] [PubMed] [Google Scholar]
- von Wettstein-Knowles P (1995) Biosynthesis and genetics of waxes. In RJ Hamilton, eds, Waxes: Chemistry, Molecular Biology and Functions. Oily Press, Dundee, Scotland, pp 91–130
- von Wettstein-Knowles P, Avato P, Mikkelsen JD (1980) Light promotes synthesis of the very long fatty acyl chains in maize wax. In P Mazliak, P Benveniste, C Costes, R Douce, eds, Biogenesis and Function of Plant Lipids, Elsevier/North-Holland Biomedical Press, pp 271–274
- Walton TJ. Waxes, cutin and suberin. In: Harwood JL, Bowyer JR, editors. Methods in Plant Biochemistry: Lipids, Membranes and Aspects of Photobiology. San Diego, CA: Academic Press; 1990. pp. 105–158. [Google Scholar]
- Xia Y, Nikolau BJ, Schnable PS. Cloning and characterization of CER2, an Arabidopsis gene that affects cuticular wax accumulation. Plant Cell. 1996;8:1291–1304. doi: 10.1105/tpc.8.8.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]