Abstract
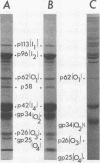
Antisera to SA11 virus proteins were prepared by immunizing rabbits with individual polypeptides separated by polyacrylamide gel electrophoresis under reducing or nonreducing conditions; the resulting antisera were characterized by four immunological methods. Results of complement fixation tests with double-shelled rotavirus particles and sera raised against reduced or unreduced proteins of the outer shell of the virus suggested the presence of common antigenic determinants in the outer capsid layers of SA11 and the Northern Ireland strain of calf rotavirus. In this test, antisera to outer shell polypeptides gp34 (O2) and gp25 (O4) cross-reacted with calf rotavirions, whereas those to p62 (O1) and p26 (O3) reacted only with the homologous virus. Antisera to the reduced outer shell proteins of the virus did not neutralize viral infectivity, nor did they possess hemagglutination inhibition activity. Evidence suggesting the presence of type-specific antigenic determinant(s) in the major inner protein p42 (I4) of SA11 virus, capable of inducing neutralizing antibody, is presented and discussed. Antisera produced against unreduced gp34 and p26 polypeptides of the virus contained type-specific neutralizing antibodies. Polypeptide gp34 was also capable of inducing hemagglutination inhibiting antibody. All of the antisera to unreduced polypeptides had agglutinating activity against double-shelled particles of homologous and heterologous rotaviruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastardo J. W., Holmes I. H. Attachment of SA-11 rotavirus to erythrocyte receptors. Infect Immun. 1980 Sep;29(3):1134–1140. doi: 10.1128/iai.29.3.1134-1140.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M., Pilfold J. N., Thouless M. E., Flewett T. H. Rotavirus serotypes by serum neutralisation. J Med Virol. 1980;5(3):231–237. doi: 10.1002/jmv.1890050307. [DOI] [PubMed] [Google Scholar]
- Bridger J. C. Location of type-specific antigens in calf rotaviruses. J Clin Microbiol. 1978 Dec;8(6):625–628. doi: 10.1128/jcm.8.6.625-628.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Gene-coding assignments of rotavirus double-stranded RNA segments 10 and 11. J Virol. 1981 Jun;38(3):1099–1103. doi: 10.1128/jvi.38.3.1099-1103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Els H. J., Lecatsas G. Morphological studies on simian virus S.A. 11 and the 'related' O agent. J Gen Virol. 1972 Oct;17(1):129–132. doi: 10.1099/0022-1317-17-1-129. [DOI] [PubMed] [Google Scholar]
- Esparza J., Gil F. A study on the ultrastructure of human rotavirus. Virology. 1978 Nov;91(1):141–150. doi: 10.1016/0042-6822(78)90362-8. [DOI] [PubMed] [Google Scholar]
- Fauvel M., Spence L., Babiuk L. A., Petro R., Bloch S. Hemagglutination and hemagglutination-inhibition studies with a strain of Nebraska calf diarrhea virus (bovine rotavirus). Intervirology. 1978;9(2):95–105. doi: 10.1159/000148927. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Thouless M. E., Pilfold J. N., Bryden A. S., Candeias J. A. More serotypes of human rotavirus. Lancet. 1978 Sep 16;2(8090):632–632. doi: 10.1016/s0140-6736(78)92854-4. [DOI] [PubMed] [Google Scholar]
- Hartman B. K., Udenfriend S. A method for immediate visualization of proteins in acrylamide gels and its use for preparation of antibodies to enzymes. Anal Biochem. 1969 Sep;30(3):391–394. doi: 10.1016/0003-2697(69)90132-8. [DOI] [PubMed] [Google Scholar]
- Infantile enteritis viruses: morphogenesis and morphology. J Virol. 1975 Oct;16(4):937–943. doi: 10.1128/jvi.16.4.937-943.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G., Pauli G. The influence of intramolecular disulfide bonds on the structure and function of Semliki forest virus membrane glycoproteins. Virology. 1980 Apr 30;102(2):300–309. doi: 10.1016/0042-6822(80)90097-5. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Kim H. W., Kalica A. R., Wyatt R. G., Vankirk D. H., Chanock R. M., James H. D., Jr, Vaughn A. L. Antigenic relationships among five reovirus-like (RVL) agents by complement fixation (CF) and development of new substitute CF antigens for the human RVL agent of infantile gastroenteritis. Proc Soc Exp Biol Med. 1976 Sep;152(4):535–539. doi: 10.3181/00379727-152-39434. [DOI] [PubMed] [Google Scholar]
- Kelen A. E., Hathaway A. E., McLeod D. A. Rapid detection of Australia-SH antigen and antibody by a simple and sensitive technique of immunoelectronmicroscopy. Can J Microbiol. 1971 Jul;17(7):993–1000. doi: 10.1139/m71-157. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Robbins A. K. An immunochemical investigation of SV40 T antigens. 1. Production properties and specificity of rabbit antibody to purified simian virus 40 large-T antigen. Virology. 1978 Jun 1;87(1):182–193. doi: 10.1016/0042-6822(78)90170-8. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Downie J. C., Webster R. G. Studies on antigenic variation in influenza virus. Evidence for multiple antigenic determinants on the hemagglutinin subunits of A-Hong Kong-68 (H3 N2) virus and the A-England-72 strains. Virology. 1974 May;59(1):230–244. doi: 10.1016/0042-6822(74)90218-9. [DOI] [PubMed] [Google Scholar]
- Malherbe H. H., Strickland-Cholmley M. Simian virus SA11 and the related O agent. Arch Gesamte Virusforsch. 1967;22(1):235–245. doi: 10.1007/BF01240518. [DOI] [PubMed] [Google Scholar]
- Malik N., Berrie A. New stain fixative for proteins separated by gel isoelectric focusing based on Coomassie Brilliant Blue. Anal Biochem. 1972 Sep;49(1):173–176. doi: 10.1016/0003-2697(72)90255-2. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Inouye S., Kono R. Plaque assay of neonatal calf diarrhea virus and the neutralizing antibody in human sera. J Clin Microbiol. 1977 Jan;5(1):1–4. doi: 10.1128/jcm.5.1.1-4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramia S., Sattar S. A. Simian rotavirus SA-11 plaque formation in the presence of trypsin. J Clin Microbiol. 1979 Nov;10(5):609–614. doi: 10.1128/jcm.10.5.609-614.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Killington R. A., Watson D. H. Glycoproteins with type common and type specific antigenic sites excreted from cells infected with herpes simplex virus types 1 and 2. J Gen Virol. 1980 Jun;48(Pt 2):297–310. doi: 10.1099/0022-1317-48-2-297. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E., Bryden A. S., Flewett T. H., Woode G. N., Bridger J. C., Snodgrass D. R., Herring J. A. Serological relationships between rotaviruses from different species as studied by complement fixation and neutralization. Arch Virol. 1977;53(4):287–294. doi: 10.1007/BF01315627. [DOI] [PubMed] [Google Scholar]
- Thouless M. E. Rotavirus polypeptides. J Gen Virol. 1979 Jul;44(1):187–197. doi: 10.1099/0022-1317-44-1-187. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C., Schild G. C. Antibody responses to antigenic determinants of influenza virus hemagglutinin. II. Original antigenic sin: a bone marrow-derived lymphocyte memory phenomenon modulated by thymus-derived lymphocytes. J Exp Med. 1974 Dec 1;140(6):1571–1578. doi: 10.1084/jem.140.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Zissis G., Brandt C. D., Rodriguez W. J., Kim H. W., Parrott R. H., Urrutia J. J., Mata L., Greenberg H. B. Epidemiology of human rotavirus Types 1 and 2 as studied by enzyme-linked immunosorbent assay. N Engl J Med. 1978 Nov 23;299(21):1156–1161. doi: 10.1056/NEJM197811232992103. [DOI] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P. Different serotypes of human rotaviruses. Lancet. 1978 Jan 7;1(8054):38–39. doi: 10.1016/s0140-6736(78)90380-x. [DOI] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P. Enzyme-linked immunosorbent assays adapted for serotyping of human rotavirus strains. J Clin Microbiol. 1980 Jan;11(1):1–5. doi: 10.1128/jcm.11.1.1-5.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]