Summary
Understanding the nature of the biofilm component in the pathogenesis of otitis media [OM] will likely have a meaningful influence on the development of novel strategies to prevent and/or treat this highly prevalent pediatric disease. The design of vaccine candidates for OM that currently focus on preventing colonization are predicated on the assumption that by reducing the burden of bacteria present in the pediatric nasopharynx, one could reduce or eliminate the likelihood of retrograde ascension of the Eustachian tube by bacteria from the nasopharynx to the middle ear. If effective, this strategy could prevent biofilms from ever forming in the middle ear. Additionally, gaining an improved understanding of the unique properties of bacteria resident within a biofilm and the proteins they express while growing as part of this organized community has the potential to identify novel and perhaps biofilm-specific molecular targets for the design of either therapeutic agents or vaccine candidates for the resolution of existing OM.
Keywords: Otitis media, biofilms, nontypeable Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae
OM is a highly prevalent paediatric disease.1–4 In 1990, 24.5 million physician’s office visits were made for OM, representing a greater than 200% increase over that reported in the 1980's.5 As such, OM is the most frequently diagnosed illness in children under 15 yrs of age and is the primary cause for ER visits.2 Hearing loss is the most common complication of OM6,7 with behavioral, educational and language development delays being additional consequences of early onset OM with effusion.3,8 The socioeconomic impact of OM is also great, with total costs of diagnosing and managing OM exceeding $5 billion annually in the U.S. alone.4,9–11 In addition to the high prevalence of OM in developed countries, worldwide it is reported that between 65 and 330 million children suffer from chronic secretory OM (CSOM), 60% of which have an associated hearing loss from this disease state which is characterized by chronically draining ears.12–14 Shockingly, in 1990, ~28,000 childhood deaths were attributed to OM.13 The most cost-effective way to manage OM and have a transformational effect on the health of children worldwide, would be through the development of innovative vaccines to prevent OM. Nonetheless, due to the high prevalence of existing disease in older children, development of novel methods to treat OM is a much needed and highly significant goal as well.
Historically, widespread use, and many would say overuse, of both narrow- and broad-spectrum antibiotics has been heavily relied upon for medical management of OM.4 This practice has resulted in the emergence of multiple-antibiotic resistant microorganisms.4,15,16 This alarming increase in bacterial resistance to antimicrobials, including members of all three genera commonly associated with OM,17–20 is not surprising when one considers that antibiotic use in children is more than three times greater than that in any other age group, and in fact, 40% of all outpatient antibiotic use in children is for treatment of OM.10,21 Surgical management of chronic OM involves the insertion of tympanostomy tubes while a child is under general anesthesia. While common-place,22 and effective in terms of relieving painful symptoms by draining the middle ear of accumulated fluids, due to its invasive nature and the incumbent risks of general anesthesia, tube insertion has met with criticism.4,5,15,22,23 Moreover, tube insertion does not prevent OM, it simply ameliorates the symptoms. As will be discussed later, the need for extended and/or repeated use of broad spectrum antibiotics, the failure of tympanostomy tubes in some children, and the chronic drainage through perforated ear drums in CSOM is both predicated, as well as complicated, by the fact that OM is a ‘biofilm disease’.24–28
Role of bacterial biofilms in OM
By definition, a biofilm is a highly-organized, multicellular community encased in an extracellular polymeric matrix or substance (often referred to as the EPS) that is affixed to a surface. Biofilms are the preferred state of all bacterial lifestyles in nature. Bacterial populations within a biofilm, as opposed to their planktonic or free-living counterparts, have a reduced growth rate (due to a nutrient limited environment), and a distinct transcriptome.29,30 Moreover, they exchange genetic material at an increased frequency thereby augmenting their ability to acquire traits favorable to their persistence. Bacteria in a biofilm also have substantially increased resistance not only to effectors of innate and acquired immunity, but to the action of antibiotics as well.31 For example, antibiotics that target the cell wall are not effective against bacteria such as those within a biofilm that have slowed their metabolism and are no longer dividing. Moreover, the EPS presents a formidable physical barrier to cellular effectors of immunity and is highly recalcitrant to removal.32 Biofilm diseases, such as OM, thus require novel methods for diagnosis, treatment and prevention.
As stated above, the biofilm paradigm was originally put forth because OM is a spectrum of diseases that are very difficult to treat with antibiotics and are often chronic and recurrent in nature. Moreover, effusions recovered from middle ears are often bacteriologically sterile, yet although bacteria cannot be cultured from these effusions, they are nonetheless typically PCR-positive for bacterial DNA.27 Moreover, Rayner et al27 demonstrated that, in addition to bacterial DNA, there was also bacterial messenger RNA present in middle ear fluids. The presence of this very short-lived messenger RNA suggested the existence of metabolically active bacteria within those fluids, despite an inability to culture them. Importantly, direct detection of bacterial biofilms in association with mucosa samples recovered from the middle ears of children with chronic and recurrent OM was recently shown.26
Biochemistry of bacterial biofilms created by otopathogens
Once the biofilm hypothesis was put forth for OM, early descriptive data of middle ear histopathology in support of the presence of biofilms appeared. Several laboratories began to investigate the capability of bacteria commonly associated with OM to develop biofilms in greater detail; they also better characterized the biochemical composition of the matrix formed by these microorganisms. Despite being a relatively young concept, substantial data have already been collected, illustrating that the 3 primary causative agents of OM, Streptococcus pneumoniae, nontypeable Haemophilus influenzae (NTHI), and Moraxella catarrhalis, readily form biofilms in vitro33–38 and in vivo.26,39–44 To date, data for the biofilming phenotype of H. influenzae is more extensive, while those for S. pneumoniae and M. catarrhalis are yet evolving. Briefly, in 2002, Ehrlich et al demonstrated that a clinical isolate of H. influenzae was able to form a well-developed biofilm in the middle ear of the chinchilla host within 5 days after direct challenge of the middle ear cavity.41 Work from several other groups subsequently indicated that proteins (or epitopes of them) expressed by H. influenzae when growing in a biofilm are unique from those expressed when grown planktonically.34,35 Webster et al, using electron microscopy, showed that certain proteins, adhesins, bacterial enzymes and lipo-oligosaccharide(s) (LOS) occupied distinct compartments within the H. influenzae-formed biofilm.45
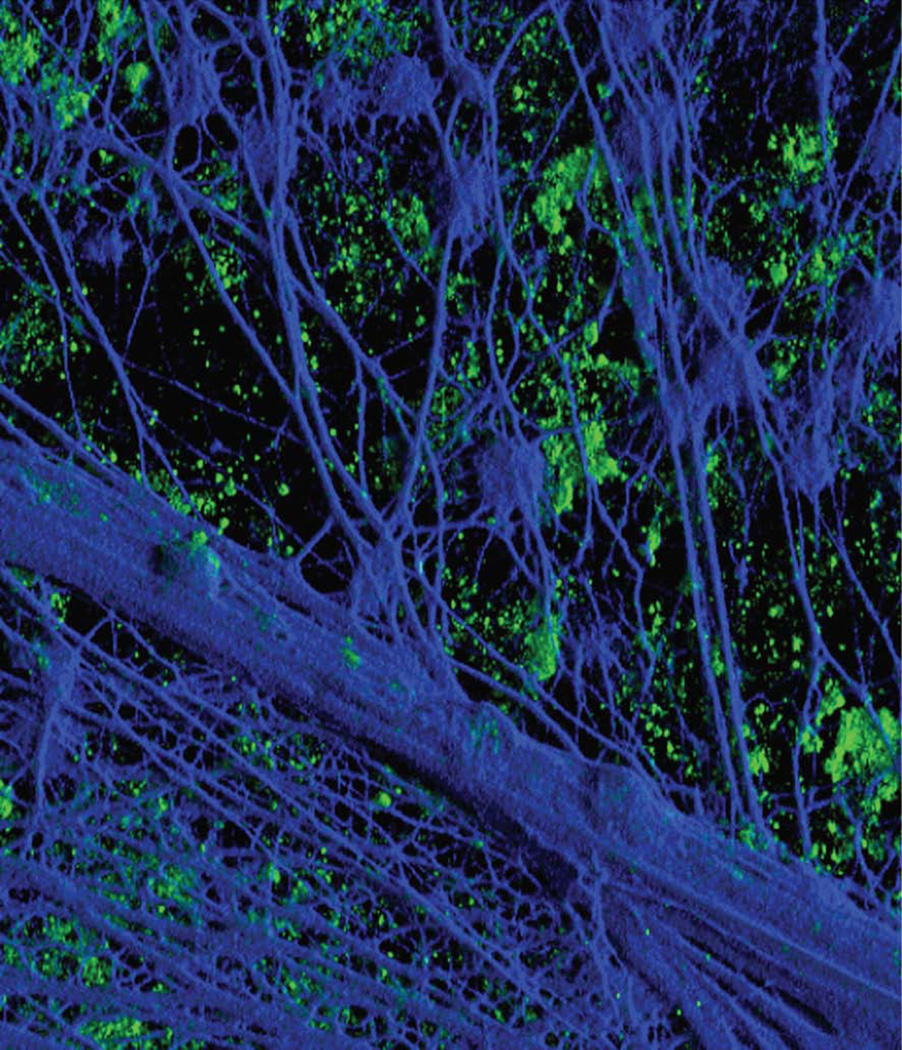
Since 2002, there has been a tremendous increase in our understanding of biofilms formed by NTHI in both the lower airway37 and middle ear.26,29,46–48 Bouchet et al39 demonstrated in an experimental chinchilla model of OM that sialylation of H. influenzae LOS is a major virulence factor; and Greiner et al,49 and later, Jurcisek et al,43 demonstrated that sialylated LOS, or endotoxin is a key component of biofilms formed in vitro and in vivo. Swords et al elucidated that sialylation of the LOS expressed by H. influenzae promoted formation of a biofilm;44 they also demonstrated that when the phosphorylcholine content of H. influenzae LOS increases within a biofilm, stimulation of the host inflammatory response decreases. H. influenzae may also be promoting persistence and residence in the human airway through enhanced resistance to host bacterial clearance.38,39,42,50–53 Further, whereas NTHI biofilms do not include a unique polysaccharide,46 the matrix contains several outer membrane proteins (OMPs), LOS, type IV pilin protein and extracellular or eDNA, among others. As shown in Figure 1, the biofilm formed by NTHI in the middle ear of the chinchilla includes an abundant amount of eDNA (blue).54 The source of this eDNA was determined to be both of eukaryotic origin (likely PMN released) as well as of prokaryotic origin, with bacterial dsDNA predominating in the inner most reaches of the biofilm EPS matrix (author’s unpublished data).
Figure 1.
Three-dimensional reconstruction of z-stack images from a frozen section of a 21-day biofilm formed in the chinchilla middle ear by nontypeable Haemophilus influenzae and labeled for type IV pilin protein (green fluorescence) and with DAPI for detection of double-stranded DNA (dsDNA) (blue fluorescence). DAPI labeling of dsDNA that has formed a dense interwoven meshwork within the biofilm is evident. Pilin protein, or possibly bacteria expressing type IV pili, is seen as small aggregates in a plane that sits slightly below the meshwork of dsDNA strands. Reprinted with permission from the cover of J. Bacteriol 189(10), © American Society for Microbiology (2007).
Characteristics of biofilms formed in the middle ear
Images from the middle ear have revealed interesting aspects of OM-related biofilms including the fact that these communities of microbes are viable, they are well organized, and highly structured. In the middle ear of the chinchilla host, gross microscopic images reveal the presence of a creamy colored biomass of semisolid and uniform consistency anchored to the mucosal epithelium of the inferior bulla, the thin bone that surrounds the middle ear space of the chinchilla host (Fig. 2A).43 When this biomass is snap frozen over liquid nitrogen and subjected to a vital fluorescent dye (in which live bacteria fluoresce green; dead bacteria fluoresce red), one can readily see the fingerlike projections of viable bacteria that extend from the mucosal surface into the middle ear space (Fig. 2B). These fingerlike projections are separated by dark areas that represent regularly spaced, but bacteria-free, water channels whose function is to bathe, feed, and detoxify the biofilm community.
Figure 2.
A - Gross whole mount image of bulla recovered 5 days after direct challenge of the middle ear with H. influenzae. Bracketed area indicates presence of biofilm present in the middle ear space. B - Confocal microscopy image of a whole mount section of the biofilm shown in Panel A stained with a vital fluorescent dye. Long finger-like projections of viable bacteria (fluoresce green) extend from the mucosa (M) into the middle ear cavity. Reprinted with permission from Infection and Immunity 2005;73:3210–3218. © American Society for Microbiology (2005).
Using middle ear mucosal biopsy specimens recovered from the children with chronic or recurrent OM, combined with fluorescent in situ hybridization (FISH) and confocal scanning laser microscopy (CSLM), Hall-Stoodley et al demonstrated that H. influenzae, as well as S. pneumoniae and M. catarrhalis had formed biofilms on recovered middle ear mucosal samples.26 CSLM images of mucosa recovered from a 2-year-old with OME after staining with a live-dead fluorescent stain, demonstrate that despite the culture-negative status of the effusion, the specimen was heavily populated with viable bacteria.26 Given that the effusion recovered from this child was PCR-positive for H. influenzae, at least a portion of the bacteria shown in the CSLM image contained within that report are likely to be H. influenzae. Similarly, additional CSLM FISH images of middle ear mucosa recovered from children with culture-negative OME or recurrent OM clearly showed the presence of both S. pneumoniae and M. catarrhalis within the biofilms present on these tissue specimens. The mixed microbial etiology of these biofilms is an observation with broad clinical implications in terms of the standard medical practice of empirically prescribing antimicrobials for OM as well as for vaccine development.
Whereas current data support the role of biofilms in recurrent and chronic OM, it would be counterintuitive to not consider the possibility that biofilms also contribute to acute otitis media (AOM) as well, since bacteria typically require only minutes to begin building a biofilm in a favourable environment. This concept is highly relevant to the therapeutic management of OM because the antibiotic susceptibility profiles of bacteria resident within a biofilm are distinctly different than those for broth-grown microorganisms. Bacteria in a biofilm are up to 1000 times more resistant to the action of antibiotics than their planktonic counterparts.31,55,56 Due to their notable resistance to antibiotic treatment, methods to mechanically or enzymatically disperse biofilms from the middle ear are being developed despite challenges as to how to best apply/deliver these methods in order to achieve efficacy. As an additional complication, it has been suggested that the practice of “watchful waiting” with regard to medical management of OM and of ventilation tube insertion itself (for surgical management of chronic and recurrent OM) may both actually promote biofilm formation in the middle ear. Tube insertion may also actually favor the development of tube-associated otorrhea wherein the tympanostomy tubes themselves provide a substratum for biofilm growth. Others however have suggested that tube-associated aeration of the middle ear favors disruption of the bacterial biofilms.57–59
Targeting the biofilm for preventative vaccine development efforts
In our attempts over the years to design vaccine candidates for the prevention of NTHI-induced diseases of the respiratory tract, we have primarily focused our efforts on two of several adhesins expressed by this group of microorganisms - the OMP P5 homologous adhesin and type IV pili, or Tfp, both of which have been shown to be expressed during biofilm growth by NTHI.34,35,54,60–64 Here, we show data from a pre-clinical trial wherein we tested vaccine candidates derived from these two adhesins for their relative ability to prevent experimental OM due to NTHI. The candidates tested included: rsPilA, LB1 and ChimV4 as described in greater detail below.65
The vaccine candidate ‘rsPilA’ is a recombinant protein derived from the type IV pilus of NTHI, ‘LB1’ is a synthetic peptide derived from the OMP P5 homologous adhesin, and ‘chimV4’ is a chimeric recombinant vaccine candidate that combines parts of both of these bacterial adhesin proteins. As can be seen in Figure 3, all three candidates performed well in a chinchilla model of experimental OM following passive immunization, wherein an ongoing viral upper respiratory tract infection precedes bacterial superinfection of the middle ear. In animals within the cohort that received antiserum directed against adjuvant only (negative control, see gray bars), OM developed in 100% of ears by day 21. In animals that received antiserum directed against LB1 + adjuvant (see yellow bars), 78% of ears did not develop ascending OM due to NTHI (52% overall protective efficacy). Animals that received antiserum directed against rsPilA (see red bars), 65% of ears did not develop OM (42% overall protective efficacy), whereas those chinchillas that received antiserum directed against the chimeric vaccine candidate (see green bars) 60% of ears did not develop OM (43% overall protective efficacy). This level of protection was statistically significant (p < 0.001) for all three immunized cohorts.
Figure 3.
Relative percentage of middle ears with OM. The greatest incidence of OM was observed in the negative control cohort, which received anti-adjuvant serum (gray bars). In contrast, the greatest protection against the development of experimental NTHI-induced OM was achieved in the cohort administered antiserum against LB1 + AS04 (p < 0.001; yellow bars), as expected since this cohort served as the positive control. Further, significant protection against ascending NTHI-induced OM was conferred by receipt of either anti-rsPilA (p < 0.001; red bars) or anti-chimV4 serum pools (p < 0.001; blue-green bars).
Reprinted from Vaccine, 28(1), Novotny LA, Adams LD, Kang DR, Wiet GJ, Cai X, Sethi S, Murphy TF, Bakaletz LO “Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates”, pp. 279–289. Copyright 2009, with permission from Elsevier.
Whereas these vaccine candidates are all being developed further, chimV4 is of great potential interest to us as it was designed to have the added benefit of including epitopes derived from two NTHI adhesins (OMP P5 and Tfp), each of which have been shown to confer key biological functions to NTHI and each further shown to be required for pathogenesis,60–64 in a single immunogen.
Potential for therapeutic vaccine and/or approaches for OM that target the biofilm
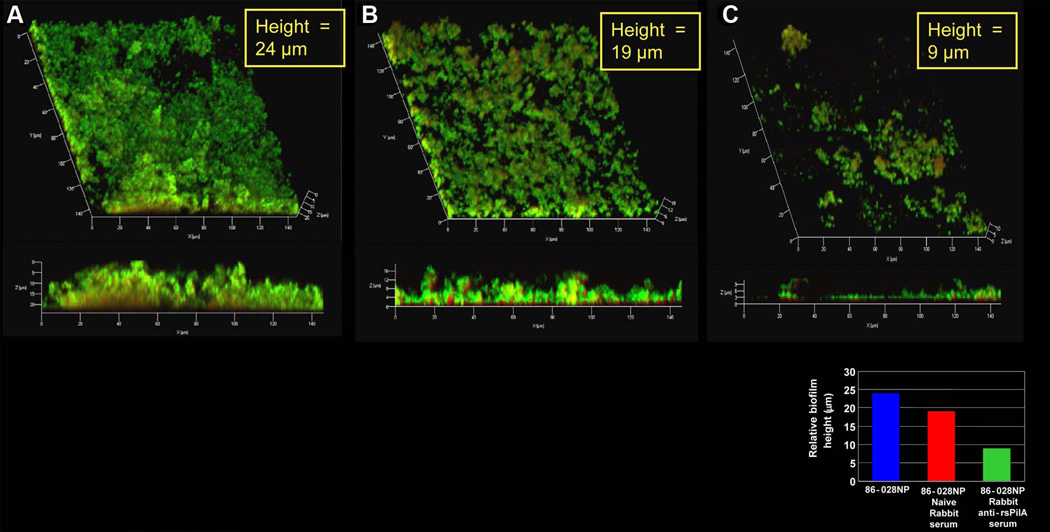
As detailed above, the chronic and recurrent nature of OM is attributed, at least in part, to the formation of biofilms which are recalcitrant to traditional antibiotic therapy as well as eradication by antibodies. Given our encouraging results when targeting adhesins expressed within biofilms for our preventative vaccine development, it was of interest to us to now see if we could potentially harness the immune system to not only prevent OM but also perhaps to treat existing OM. We were encouraged in this regard by the fact that we were able to demonstrate that antibody directed against PilA, the majority subunit of Tfp, eradicated an existing biofilm when using an in vitro assay system. Treatment of an already established biofilm with antibody directed against a recombinant soluble form of PilA (rsPilA) resulted in a 37% reduction in height, compared to naive serum (Fig. 4). We are currently attempting to demonstrate the feasibility of using this approach in vivo, wherein chinchillas with experimentally induced biofilms already present within their middle ears will be immunized against NTHI proteins, including PilA and the OMP P5 homologous adhesin to determine whether we can mediate a therapeutic cure via active immunization.
Figure 4.
Rabbit anti-rsPilA ‘reversed’ an biofilm formed by NTHI. Whereas incubation of an established biofilm, (which had been allowed to form in a chamber slide in vitro for 24 hours prior to treatment) with either sterile medium (Panel A) or naïve rabbit serum (Panel B) had minimal effect on relative biofilm height, incubation with antiserum directed against rsPilA resulted in a marked diminution in biofilm height compared to controls.
In addition to immunization strategies, there is a great deal of interest in developing biochemical methods to disrupt an existing biofilm, including those present in the middle ear cleft. Developing these methods will benefit from efforts to detail both the mechanisms of biofilm development, including perhaps quorem sensing (used by bacteria to determine both their own relative density and the presence of other bacterial species), and the biochemical composition of the biofilm matrix.
Clinical relevance of biofilms
Having an understanding that one of the most common paediatric diseases includes a biofilm component has important clinical relevance. The susceptibility profiles of sessile versus planktonically growing bacteria are very different.31 To date, selection of antimicrobials for treating OM has been based on testing of planktonically grown bacteria. Further evaluation of the difference(s) in antibiotic susceptibility between planktonic and sessile bacterial populations will be needed to more fully understand the implications of biofilm formation on therapeutic options. Knowledge that biofilms formed in the middle ears are possibly of mixed microbial etiology might help explain the high frequency of treatment failures among children where antibiotic choice has been largely empiric, and are likely to influence future guidelines for medical management of treatment-failures.
Concluding Comments
Understanding the nature of the biofilm component in the pathogenesis of OM is likely to have a meaningful influence on the development of novel strategies for preventing and/or treating this prevalent pediatric disease. The design and derivation of vaccine candidates for OM that currently focus on preventing colonization are predicated on the hypothesis that by reducing the burden of bacteria present in the pediatric nasopharynx, one could reduce or eliminate the likelihood of retrograde ascension of the Eustachian tube by bacteria from the nasopharynx to the middle ear. If effective, this strategy could prevent biofilms from ever forming in the middle ear. Additionally, gaining an improved understanding of the unique properties of bacteria resident within a biofilm, and the proteins they express while growing as part of this organized community, has the potential to identify novel and perhaps biofilm-specific molecular targets for the design of either therapeutic agents or vaccine candidates for the resolution of existing OM. Moreover, whereas this report has focused on OM, the same three predominant pathogens of otitis media also cause multiple additional diseases of both the upper and lower respiratory tract. Thereby lessons learned in pursuit of a better understanding of the role of bacterial biofilms in the pathogenesis of OM are very likely to be translatable to that for other diseases of the human airway as well.
Educational Aims.
To review the role of bacterial biofilms in the pathogenesis of otitis media
To outline the challenges presented to medical and surgical management of OM due to the presence of bacterial biofilms
To discuss the current state of understanding of the biochemical characteristics of biofilms caused by common otopathogens
To present the reader with potential options for improved treatment and/or prevention modalities for OM that can be gained by having a better understanding of biofilms
Practice Points
Current treatment of OM is largely empirical
Widespread use of both narrow and broad-spectrum antibiotics for OM has resulted in the sobering emergence of multiple-antibiotic resistant bacteria in all major genera of otopathogens
Diseases wherein biofilms contribute to pathogenesis require unique methods for diagnosis, treatment and prevention
Development of a vaccine to prevent OM would be the most cost-effective way to manage OM
Research Directions
Whereas biofilms are now acknowledged to contribute to chronic and recurrent OM, the role of biofilms in acute OM is yet understudied
Having an increased understanding of the interrelationship between otopathogens present within a biofilm of mixed bacterial etiology is highly desirable
Gaining an improved understanding of the biology and biochemistry of bacteria resident within a biofilm community will likely greatly enhance our ability to develop improved methods for treatment and prevention
Acknowledgements
I thank Jennifer Neelans for preparation of this manuscript. This work was funded in part by NIH/NIDCD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akira S. Toll-like receptors: lessons from knockout mice. Biochem Soc Trans. 2000;28:551–556. doi: 10.1042/bst0280551. [DOI] [PubMed] [Google Scholar]
- 2.Cassell GH, Archer GL, Beam TR, et al. Report of the ASM Task Force on Antibiotic Resistance. Washington D.C: 1994. [Google Scholar]
- 3.Infante-Rivard C, Fernandez A. Otitis media in children: frequency, risk factors, and research avenues. Epidemiol Rev. 1993;15:444–465. doi: 10.1093/oxfordjournals.epirev.a036129. [DOI] [PubMed] [Google Scholar]
- 4.Stool SE, Berg AO, Berman S, et al. AHCPR Publication No. 94-0622. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; 1994. Otitis Media with Effusion in Young Children. Clinical Practice Guideline. [Google Scholar]
- 5.Paap CM. Management of otitis media with effusion in young children. Ann Pharmacother. 1996;30:1291–1297. doi: 10.1177/106002809603001114. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin RL. Effects of otitis media on child development. Am J Otol. 1993;14:601–604. [PubMed] [Google Scholar]
- 7.Hunter LL, Margolis RH, Giebink GS. Identification of hearing loss in children with otitis media. Ann Otol Rhinol Laryngol Suppl. 1994;163:59–61. doi: 10.1177/00034894941030s516. [DOI] [PubMed] [Google Scholar]
- 8.Teele DW, Klein JO, Chase C, Menyuk P, Rosner BA. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J Infect Dis. 1990;162:685–694. doi: 10.1093/infdis/162.3.685. [DOI] [PubMed] [Google Scholar]
- 9.Alsarraf R, Jung CJ, Perkins J, Crowley C, Alsarraf NW, Gates GA. Measuring the indirect and direct costs of acute otitis media. Arch Otolaryngol Head Neck Surg. 1999;125:12–18. doi: 10.1001/archotol.125.1.12. [DOI] [PubMed] [Google Scholar]
- 10.Cassell GH. New and Reemerging Infectious Diseases: A Global Crisis and Immediate Threat to the Nation's Health: The Role of Research. Washington, D.C: American Society for Microbiology; 1997. pp. 1–11. [Google Scholar]
- 11.Kaplan B, Wandstrat TL, Cunningham JR. Overall cost in the treatment of otitis media. Pediatr Infect Dis J. 1997;16:S9–S11. doi: 10.1097/00006454-199702001-00003. [DOI] [PubMed] [Google Scholar]
- 12.Woodfield G, Dugdale A. Evidence behind the WHO guidelines: hospital care for children: what is the most effective antibiotic regime for chronic suppurative otitis media in children? J Trop Pediatr. 2008;54:151–156. doi: 10.1093/tropej/fmn042. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Chronic suppurative otitis media: burden of illness and management options. 2004
- 14.Leach AJ, Morris PS, Mathews JD. Compared to placebo, long-term antibiotics resolve otitis media with effusion (OME) and prevent acute otitis media with perforation (AOMwiP) in a high-risk population: a randomized controlled trial. BMC Pediatr. 2008;8:23. doi: 10.1186/1471-2431-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cimons M. Watchful Waiting Advised When Treating Otitis Media. ASM News. 1994;60:527–528. [Google Scholar]
- 16.Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha [published erratum appears in JAMA 1994 Feb 9;271(6):430] [see comments] JAMA. 1993;270:1344–1351. [PubMed] [Google Scholar]
- 17.Cohen R, Bingen E, Varon E, et al. Change in nasopharyngeal carriage of Streptococcus pneumoniae resulting from antibiotic therapy for acute otitis media in children. Pediatr Infect Dis J. 1997;16:555–560. doi: 10.1097/00006454-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Green M, Wald ER. Emerging resistance to antibiotics: impact on respiratory infections in the outpatient setting. Ann Allergy Asthma Immunol. 1996;77:167–173. doi: 10.1016/S1081-1206(10)63250-4. [DOI] [PubMed] [Google Scholar]
- 19.McLinn S, Williams D. Incidence of antibiotic-resistant Streptococcus pneumoniae and beta- lactamase-positive Haemophilus influenzae in clinical isolates from patients with otitis media. Pediatr Infect Dis J. 1996;15:S3–S9. doi: 10.1097/00006454-199609009-00001. [DOI] [PubMed] [Google Scholar]
- 20.Nelson CT, Mason EO, Jr, Kaplan SL. Activity of oral antibiotics in middle ear and sinus infections caused by penicillin-resistant Streptococcus pneumoniae: implications for treatment. Pediatr Infect Dis J. 1994;13:585–589. doi: 10.1097/00006454-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Wang EE, Einarson TR, Kellner JD, Conly JM. Antibiotic prescribing for Canadian preschool children: evidence of overprescribing for viral respiratory infections. Clin Infect Dis. 1999;29:155–160. doi: 10.1086/520145. [DOI] [PubMed] [Google Scholar]
- 22.Bright RA, Moore RM, Jr, Jeng LL, Sharkness CM, Hamburger SE, Hamilton PM. The prevalence of tympanostomy tubes in children in the United States, 1988. Am J Public Health. 1993;83:1026–1028. doi: 10.2105/ajph.83.7.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman S, Roard R, PA-C M, Luckey C. Theoretical cost effectiveness of management options for children with persisting middle ear effusions. Pediatrics. 1984;83:353–363. [PubMed] [Google Scholar]
- 24.Bakaletz LO. Bacterial biofilms in otitis media: evidence and relevance. Pediatr Infect Dis J. 2007;26:S17–S19. doi: 10.1097/INF.0b013e318154b273. [DOI] [PubMed] [Google Scholar]
- 25.Armbruster CE, Hong W, Pang B, et al. Indirect Pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in Polymicrobial Otitis Media Occurs via Interspecies Quorum Signaling. mBio. 2010;1 doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. Jama. 1998;279:296–299. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 28.Homoe P, Bjarnsholt T, Wessman M, Sorensen HC, Johansen HK. Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur Arch Otorhinolaryngol. 2009;266:1533–1538. doi: 10.1007/s00405-009-0940-9. [DOI] [PubMed] [Google Scholar]
- 29.Post JC, Hiller NL, Nistico L, Stoodley P, Ehrlich GD. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg. 2007;15:347–351. doi: 10.1097/MOO.0b013e3282b97327. [DOI] [PubMed] [Google Scholar]
- 30.Post JC, Stoodley P, Hall-Stoodley L, Ehrlich GD. The role of biofilms in otolaryngologic infections. Curr Opin Otolaryngol Head Neck Surg. 2004;12:185–190. doi: 10.1097/01.moo.0000124936.46948.6a. [DOI] [PubMed] [Google Scholar]
- 31.Slinger R, Chan F, Ferris W, et al. Multiple combination antibiotic susceptibility testing of nontypeable Haemophilus influenzae biofilms. Diagn Microbiol Infect Dis. 2006;56:247–253. doi: 10.1016/j.diagmicrobio.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 33.Allegrucci M, Hu FZ, Shen K, et al. Phenotypic characterization of Streptococcus pneumoniae biofilm development. J Bacteriol. 2006;188:2325–2335. doi: 10.1128/JB.188.7.2325-2335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy TF, Kirkham C. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2002;2:7. doi: 10.1186/1471-2180-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallaher TK, Wu S, Webster P, Aguilera R. Identification of biofilm proteins in non-typeable Haemophilus Influenzae. BMC Microbiol. 2006;6:65. doi: 10.1186/1471-2180-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson MM, Laurence CA, Guinn SE, Hansen EJ. Biofilm formation by Moraxella catarrhalis in vitro: roles of the UspA1 adhesin and the Hag hemagglutinin. Infect Immun. 2006;74:1588–1596. doi: 10.1128/IAI.74.3.1588-1596.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starner TD, Zhang N, Kim G, Apicella MA, McCray PB., Jr Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Respir Crit Care Med. 2006;174:213–220. doi: 10.1164/rccm.200509-1459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West-Barnette S, Rockel A, Swords WE. Biofilm growth increases phosphorylcholine content and decreases potency of nontypeable Haemophilus influenzae endotoxins. Infect Immun. 2006;74:1828–1836. doi: 10.1128/IAI.74.3.1828-1836.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouchet V, Hood DW, Li J, et al. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci U S A. 2003;100:8898–8903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daines DA, Bothwell M, Furrer J, et al. Haemophilus influenzae luxS mutants form a biofilm and have increased virulence. Microb Pathog. 2005;39:87–96. doi: 10.1016/j.micpath.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Ehrlich GD, Veeh R, Wang X, et al. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA. 2002;287:1710–1715. doi: 10.1001/jama.287.13.1710. [DOI] [PubMed] [Google Scholar]
- 42.Hong W, Mason K, Jurcisek J, Novotny L, Bakaletz LO, Swords WE. Phosphorylcholine decreases early inflammation and promotes the establishment of stable biofilm communities of nontypeable Haemophilus influenzae strain 86-028NP in a chinchilla model of otitis media. Infect Immun. 2007;75:958–965. doi: 10.1128/IAI.01691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jurcisek J, Greiner L, Watanabe H, Zaleski A, Apicella MA, Bakaletz LO. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect Immun. 2005;73:3210–3218. doi: 10.1128/IAI.73.6.3210-3218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swords WE, Moore ML, Godzicki L, Bukofzer G, Mitten MJ, VonCannon J. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect Immun. 2004;72:106–113. doi: 10.1128/IAI.72.1.106-113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webster P, Wu S, Gomez G, Apicella M, Plaut AG, St Geme JW., 3rd Distribution of bacterial proteins in biofilms formed by non-typeable Haemophilus influenzae. J Histochem Cytochem. 2006;54:829–842. doi: 10.1369/jhc.6A6922.2006. [DOI] [PubMed] [Google Scholar]
- 46.Erwin AL, Smith AL. Nontypeable Haemophilus influenzae: understanding virulence and commensal behavior. Trends Microbiol. 2007;15:355–362. doi: 10.1016/j.tim.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Leroy M, Cabral H, Figueira M, et al. Multiple consecutive lavage samplings reveal greater burden of disease and provide direct access to the nontypeable Haemophilus influenzae biofilm in experimental otitis media. Infect Immun. 2007;75:4158–4172. doi: 10.1128/IAI.00318-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris DP. Bacterial biofilm in upper respiratory tract infections. Curr Infect Dis Rep. 2007;9:186–192. doi: 10.1007/s11908-007-0030-3. [DOI] [PubMed] [Google Scholar]
- 49.Greiner LL, Watanabe H, Phillips NJ, et al. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect Immun. 2004;72:4249–4260. doi: 10.1128/IAI.72.7.4249-4260.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin R, Cox AD, Makepeace K, Richards JC, Moxon ER, Hood DW. Elucidation of the monoclonal antibody 5G8-reactive, virulence-associated lipopolysaccharide epitope of Haemophilus influenzae and its role in bacterial resistance to complement-mediated killing. Infect Immun. 2005;73:2213–2221. doi: 10.1128/IAI.73.4.2213-2221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutsmann T, Hagge SO, David A, et al. Lipid-mediated resistance of Gram-negative bacteria against various pore-forming antimicrobial peptides. J Endotoxin Res. 2005;11:167–173. doi: 10.1179/096805105X37330. [DOI] [PubMed] [Google Scholar]
- 52.Hong W, Pang B, West-Barnette S, Swords WE. Phosphorylcholine expression by nontypeable Haemophilus influenzae correlates with maturation of biofilm communities in vitro and in vivo. J Bacteriol. 2007;189:8300–8307. doi: 10.1128/JB.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Severi E, Randle G, Kivlin P, et al. Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol Microbiol. 2005;58:1173–1185. doi: 10.1111/j.1365-2958.2005.04901.x. [DOI] [PubMed] [Google Scholar]
- 54.Jurcisek JA, Bakaletz LO. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol. 2007;189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Starner TD, Shrout JD, Parsek MR, Appelbaum PC, Kim G. Subinhibitory concentrations of azithromycin decrease nontypeable Haemophilus influenzae biofilm formation and diminish established biofilms. Antimicrob Agents Chemother. 2008;52:137–145. doi: 10.1128/AAC.00607-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaji C, Watanabe K, Apicella MA, Watanabe H. Antimicrobial effect of fluoroquinolones for the eradication of nontypeable Haemophilus influenzae isolates within biofilms. Tohoku J Exp Med. 2008;214:121–128. doi: 10.1620/tjem.214.121. [DOI] [PubMed] [Google Scholar]
- 57.Barakate M, Beckenham E, Curotta J, da Cruz M. Bacterial biofilm adherence to middle-ear ventilation tubes: scanning electron micrograph images and literature review. J Laryngol Otol. 2007;121:993–997. doi: 10.1017/S0022215107008870. [DOI] [PubMed] [Google Scholar]
- 58.Jang CH, Cho YB, Choi CH. Structural features of tympanostomy tube biofilm formation in ciprofloxacin-resistant Pseudomonas otorrhea. Int J Pediatr Otorhinolaryngol. 2007;71:591–595. doi: 10.1016/j.ijporl.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Vlastarakos PV, Nikolopoulos TP, Korres S, Tavoulari E, Tzagaroulakis A, Ferekidis E. Grommets in otitis media with effusion: the most frequent operation in children. But is it associated with significant complications? Eur J Pediatr. 2007;166:385–391. doi: 10.1007/s00431-006-0367-x. [DOI] [PubMed] [Google Scholar]
- 60.Bakaletz LO, Baker BD, Jurcisek JA, et al. Demonstration of type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect Immun. 2005;73:1635–1643. doi: 10.1128/IAI.73.3.1635-1643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jurcisek JA, Bookwalter JE, Baker BD, et al. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol. 2007;65:1288–1299. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 62.Novotny LA, Bakaletz LO. A novel transcutaneous immunization regimen with OMP P5 and type IV pilus-derived immunogens confers protection against nontypeable Haemophilus influenzae-induced otitis media. 6th Extraordinary International Symposium on Recent Advances in Otitis Media; Seoul, Korea. 2009. [Google Scholar]
- 63.Sirakova T, Kolattukudy PE, Murwin D, et al. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang Z, Nagata N, Molina E, Bakaletz LO, Hawkins H, Patel JA. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect Immun. 1999;67:187–192. doi: 10.1128/iai.67.1.187-192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Novotny LA, Adams LD, Kang DR, et al. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine. 2009;28:279–289. doi: 10.1016/j.vaccine.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]