Abstract
FANCD2, a key factor in the FANC-BRCA1 pathway is monoubiquitinated and targeted to discrete nuclear foci following DNA damage. Since monoubiquitination of FANCD2 is a crucial indicator for cellular response to DNA damage, we monitored the fate of FANCD2 and its monoubiquitination following DNA damage. Disappearance of FANCD2 protein was induced following DNA damage in a dose-dependent manner, which correlated with degradation of BRCA1 and poly-ADP ribose polymerase (PARP), known targets for caspase-mediated apoptosis. Disappearance of FANCD2 was not affected by a proteasome inhibitor but was blocked by a caspase inhibitor. DNA damage-induced disappearance of FANCD2 was also observed in cells lacking FANCA, suggesting that disappearance of FANCD2 does not depend on FANC-BRCA1 pathway and FANCD2 monoubiquitination. In keeping with this, cells treated with TNF-α, an apoptotic stimulus without causing any DNA damage, also induced disappearance of FANCD2 without monoubiquitination. Together, our data suggest that FANCD2 is a target for caspase-mediated apoptotic pathway, which may be an early indicator for apoptotic cell death.
Keywords: Fanconi Anemia, DNA Damage, Apoptosis, Caspase, Cisplatin, Mitomycin C, DNA Repair
Apoptosis, also known as programmed cell death, is a tightly regulated phenomenon ensuring that cells that accumulate irreversible DNA damage do not replicate [Das et al., 2008; Boulon et al., 2010]. Apoptosis is a distinct form of cell death that occurs not only in response to DNA damage but also to other stimuli [Wyllie, 1980; Song et al., 1996]. Although the mechanisms by which DNA damage-induced apoptotic responses are not well understood, evidence suggests that the transcription machinery might be used in DNA-damage surveillance and in triggering DNA-damage responses to suppress mutagenesis [Ljungman and Lane, 2004]. Apoptosis is mediated by a group of stimuli-activated proteases, called caspases [Yuan et al., 1993]. Caspases constitute a family of cysteine proteases that share a stringent specificity for cleaving their substrates after aspartic acid residues in target proteins. In mammals, seven caspases are involved in apoptosis [Fuentes-Prior and Salvesen, 2004], and their targets include BRCA1, poly-ADP-ribose polymerase (PARP), DNA-dependent protein kinase-catalytic subunit (DNA-PKcs), and replication factor C (RF-C) [Lazebnik et al., 1994; Casciola-Rosen et al., 1995; Song et al., 1996; Rheaume et al., 1997; Blagosklonny et al., 1999; Zhan et al., 2002]. PARP, DNAPKcs, and BRCA1 are all nuclear proteins implicated in DNA double-strand break (DSB) repair pathway [Jackson and Jeggo, 1995; Schultz et al., 2003; Susse et al., 2004].
Fanconi anemia (FANC) is a rare autosomal recessive genetic condition associated with a defect in damage-induced cell cycle arrest, a high level of chromosomal aberrations, and hypersensitivity to DNA crosslinking agents. Thirteen FANC complementation groups have been identified (A, B, C, D1, D2, E, F, G, I, J, L, M, and N) [Moldovan and D’Andrea, 2009], and recently, mutation in Rad51C gene also exhibited FANC-like disorder [Vaz et al., 2010]. Among them, 8 FANC proteins (A, B, C, D1, D2, E, F, G, L) cooperate in a common cellular pathway (also known as the FANC/BRCA pathway) leading to monoubiquitination of FANCD2 [Meetei et al., 2003; Vandenberg et al., 2003; Kennedy and D’Andrea, 2005; Moldovan and D’Andrea, 2009]. Upon DNA damage, FANCD2 cooperates with BRCA1 and forms nuclear foci at DNA damage sites [Garcia-Higuera et al., 2001]. In addition, monoubiquitinated form of FANCD2 appeared during S-phase or in response to DNA damage [Garcia-Higuera et al., 2001; Taniguchi et al., 2002]. Monoubiquitination of FANCD2 may be necessary for its function in DNA repair since a mutant protein lacking the monoubiquitination site cannot complement the MMC sensitivity of human FANCD2-deficient cells [Garcia-Higuera et al., 2001]. In this study, we found that DNA damage induced disappearance of FANCD2 in a caspase-mediated pathway. Disappearance of FANCD2 also occurred following treatment with non-DNA damaging agent, tumor necrosis factor-α (TNF-α), and was not dependent on its monoubiquitination status, suggesting that FANCD2 is a target for caspase-mediated apoptotic pathway.
MATERIALS AND METHODS
ANTIBODIES AND CHEMICALS
An anti-FANCD2 (monoclonal Ab; Novus Biologicals), anti-Ku70 and Ku80 polyclonal antibodies from goat (Santa Cruz Biotechnology), anti-BRCA1 and -PARP polyclonal antibodies from rabbits (Santa Cruz Biotechnology), and an anti-DNA-PKcs polyclonal antibody (BD Pharmingen) were commercially purchased. Mitomycin C (MMC) and cisplatin (CDDP) were obtained from Sigma Chem. Co. (St. Louis, MO), and the MTT cell proliferation kit I was from Roche Diagnostic Corp. (Germany). All caspase inhibitor peptides and caspase-family inhibitor were purchased from Gentaur (Belgium) and ALLN was from Sigma Chem. Co. (St. Louis, MO).
CELL CULTURES AND PREPARATION OF CELL EXTRACTS
HeLa cells were grown in Dulbecco’s modified Eagle’s media-F12 (DMEM/F12, GIBCO-BRL) supplemented with 10% fetal calf serum (GIBCO-BRL), penicillin (10 U/ml, Sigma), and streptomycin (0.1 mg/ml, Sigma). Wild-type fibroblast (GM637), human FANCA mutant (PD220), and human FANCG mutant cells (PD352) were grown in the same culture media except using DMEM. Cells in exponential growth phase were used for all the experiments described in this study. To prepare cell extracts, cells were washed with phosphate-buffered saline (PBS) and lysed directly on the 100 mm plates by adding 500 µl of cold lysis buffer (1% NP-40 in PBS in the presence of 1 mM Na3VO4, 1 mM NaF, and mammalian protease inhibitor cocktail) and scraped into microcentrifuge tubes. Cell lysates were centrifuged for 30 min at 20,000g at 4°C. The supernatants were collected as a soluble protein fraction and used for SDS–PAGE electrophoresis and immunoblot analysis.
SDS–PAGE AND WESTERN BLOT ANALYSIS
Cell lysates (25 µg per lane) or purified proteins were resolved on 6–8% SDS–PAGE under reducing conditions (10 mM DTT or 1% β-mercaptoethanol) or under non-reducing conditions. Proteins were then transferred to polyvinylidene difluoride membrane, probed with an anti-FANCD2 antibody (monoclonal mouse IgG, Novus) followed by horseradish peroxidase-conjugated secondary antibody [Park et al., 2004]. Proteins were visualized by using the ECL system (Amersham Biosciences).
PREPARATION OF CELL LYSATES AND WESTERN BLOT ANALYSIS
Cell lysates were prepared as described previously [Park et al., 2001]. Cells (1 × 105 cells/sample) were collected by centrifugation, washed in PBS, and lysed in JNK lysis buffer containing 25 mM HEPES (pH 7.5), 0.3M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5% Triton X-100, 20 mM β-glycerolphosphate, 1 mM sodium vanadate, 1 mM DTT, protease inhibitor cocktail. Cell lysates (50 µg) were loaded onto a 8% or 10% SDS–PAGE and following gel electrophoresis, proteins were transferred to a PVDF membrane (Millipore, Billerica, MA) and immunoblotted with primary antibody followed by peroxidase-coupled secondary antibody (Amersham, Piscataway, NJ) and an enhanced chemiluminescence (Amersham) reaction prior to visualization on Kodak-o-mat film.
CELL SURVIVAL ASSAY
Cells (1.0 × 104 cells/ well) were seeded in a 96-well plate and incubated for 24 h prior to the treatment of cells with DNA damaging agent. Following further incubation at 37°C 5% CO2 for 72 h, cell survival was measured using a colorimetric cell survival assay from Boehringer Mannheim (MTT Cell Proliferation Kit). Each point represents mean values ± SE, each conducted with triplicate plates. The P-values were obtained from two separate experiments using one-way ANOVA method (SigmaStat for Windows, version 2.03).
TUNNEL ASSAY
Cells (50,000–100,000 cells/well) were cultured on sterilized cover slide in six-well non-tissue culture plate containing 2 ml culture medium (α-MEM + 15% FBS) per condition. After overnight incubation for attachment of cells to cover slide, culture medium was removed, and each well was washed with PBS to get ride of dead cells. Following addition of new culture medium, cells were treated with various concentration of MMC (10, 50, 100, 500, and 1,000 nM) diluted in the same culture medium, and were incubated for 72 h at 37°C. After 72 h, all the culture medium in each well were collected, centrifuged, washed, and cytospined. Then, cells collected from each well and from cover slide were analyzed by terminal deoxynucleotidyltransferase-mediated nick-end labeling (TUNEL) assay according to the manufacturer’s recommendation (Roche, Indianapolis, IN). For each sample, at least 100 cells were evaluated (DAPI+) to determine the percentage of apoptotic cells (TUNEL+) using fluorescence microscope. The mean of three independent experiments was calculated and considered for the figure.
RESULTS
DISAPPEARANCE OF FANCD2 PROTEIN IN HeLa CELLS FOLLOWING TREATMENT WITH DNA DAMAGING AGENTS
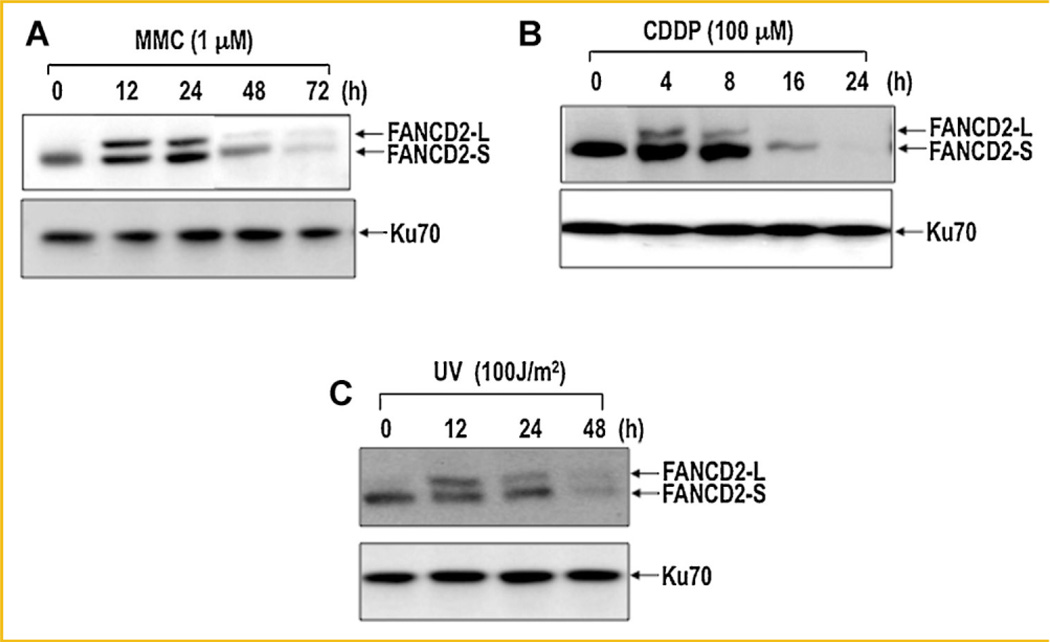
FANCD2 is a key member of FANC-BRCA1 pathway and is monoubiquitinated following DNA damage prior to colocalization with BRCA1 at the DNA damage sites [Garcia-Higuera et al., 2001; Moldovan and D’Andrea, 2009]. Monoubiquitination of FANCD2 usually found in chromatin fractions [Wang et al., 2004b; Bogliolo et al., 2007; Yuan et al., 2009; Chou et al., 2010; Liu et al., 2010] requires other FANC proteins [Moldovan and D’Andrea, 2009], suggesting that FANCD2 and its monoubiquitination is crucial for the FANC-BRCA1 pathway. Since monoubiquitination of FANCD2 is an indicator for cellular response to DNA damage, we prepared whole cell extracts and monitored FANCD2 and its monoubiquitination following DNA damage (Fig. 1). Treatment of HeLa cells with DNA damaging agents (MMC, CDDP, and UV) induced monoubiquitination of FANCD2, but caused disappearance of FANCD2 protein in later time points (Fig. 1). Disappearance of FANCD2 is likely due to a proteolytic degradation, although we were not able to detect proteolytic fragment(s) from our immunoblot analysis even in cells showing almost complete disappearance of full length of FANCD2. It is possible that the epitope(s) that the anti-FANCD2 antibody recognizes may exist within a small degradation fragment that is difficult to detect on 10% SDS–PAGE. In fact, FANCD2 has 9 DXXD motifs, a potential cleavage site for caspase-3 protease. Among them, 4 sites were located very close to the C-terminus (data not shown).
Fig. 1.
Disappearance of FANCD2 occurs following treatment of cells with DNA damaging agents. HeLa monolayer cells with 50% confluence were treated with 1 µM mitomycin C (MMC, panel A), 100 µM cisplatin (CDDP, panel B), or 100 J/m2 germicidal UV light (panel C). Cells were harvested at various time points and analyzed for FANCD2 by Western blot. FANCD2-L and FANCD2-S represent monoubiquitinated and non-ubiquitinated forms of FANCD2, respectively. Ku70 was used as an internal loading control for disappearance of FANCD2.
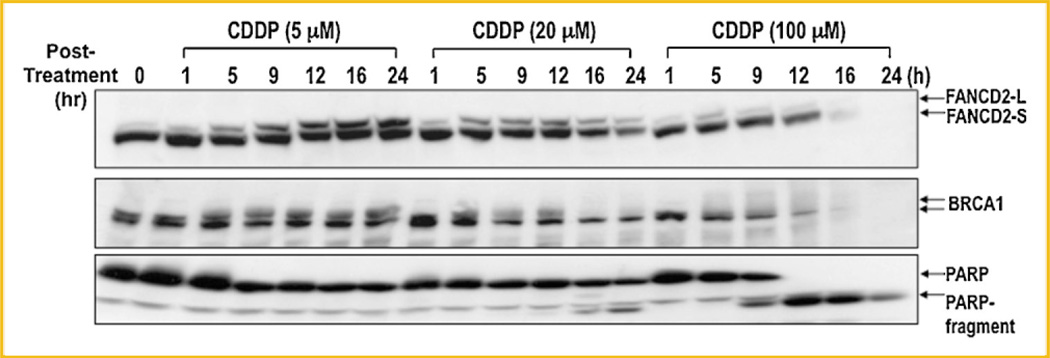
To further understand monoubiquitination and disappearance of FANCD2 following DNA damage, we carried out kinetic analysis of FANCD2 following treatment of cells with varying concentrations of CDDP. At a low concentration (5 µM), cells induced monoubiquitination of FANCD2 that was maintained up to 24 h without any change in FANCD2 protein level (Fig. 2). Cells treated with a high concentration of CDDP (100 µM) induced a massive disappearance of FANCD2 protein in 12–16 h, whereas only a fraction of FANCD2 was monoubiquitinated (Fig. 2). Kinetics of FANCD2 disappearance correlated with proteolytic degradation of BRCA1 and poly-ADP ribose polymerase (PARP; Fig. 2, middle & bottom panels), both of which not only play crucial role in DNA repair and protecting cells from DNA damage but also are the targets for caspase-mediated apoptotic cell death.
Fig. 2.
Kinetic analysis of disappearance of FANCD2 following CDDP treatment. HeLa monolayer cells were treated with indicated amount of cisplatin (CDDP), harvested at various time points, and analyzed for disappearance of FANCD2. Cell extracts were subjected to SDS–PAGE and analyzed by Western blot for FANCD2 (top panel), BRCA1 (middle panel), and PARP (bottom panel). FANCD2-L and FANCD2-S represent monoubiquitinated and non-ubiquitinated forms of FANCD2, respectively.
FANCD2 IS A TARGET FOR APOPTOSIS MEDIATED BY CASPASE(S)- NOT PROTEASOME-MEDIATED PATHWAY FOLLOWING DNA DAMAGE
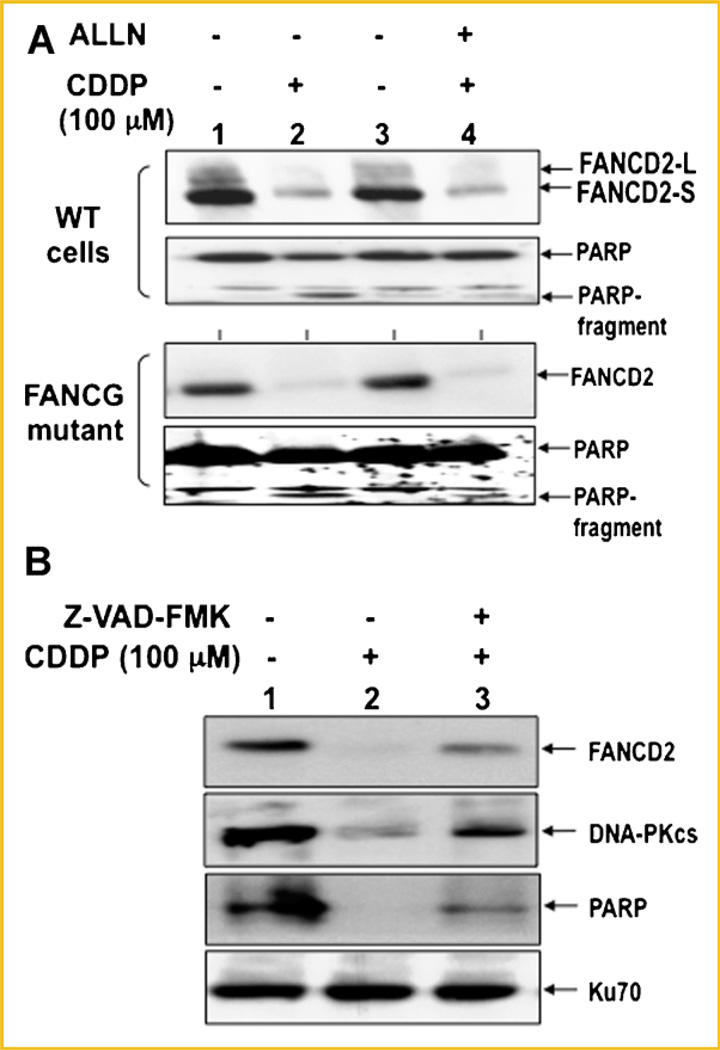
Similar to PARP and BRCA1 [Lazebnik et al., 1994; Zhan et al., 2002], disappearance of FANCD2 shown in Figures 1 and 2 may be due to the proteolytic degradation mediated by caspase(s). Alternatively, disappearance of FANCD2 may occur in a proteasome-dependent degradation pathway since FANCD2 is monoubiquitinated upon DNA damage [Haglund et al., 2003]. To test these two possibilities, HeLa fibroblast cells were pretreated with either a proteasome inhibitor (ALLN) or a caspase inhibitor (Z-VAD-FMK) and examined for disappearance of FANCD2 following CDDP treatment (Fig. 3). Disappearance of FANCD2 was not affected by pretreatment with a ALLN (Fig. 3A, top panel), but was significantly blocked by a caspase inhibitor (Z-VAD-FMK; Fig. 3B). More importantly, FANCG mutant cells defective in monoubiquitination of FANCD2 also showed disappearance of FANCD2 that was not affected by treatment with a ALLN (Fig. 3A, bottom panel). Together, our data suggest that FANCD2 is a target for apoptosis mediated by caspase(s)-dependent not proteasome-dependent pathway.
Fig. 3.
Disappearance of FANCD2 is mediated by the caspases and not proteasome-dependent pathway. A: WT fibroblast or FANCG-deficient cells were treated with 100 µM CDDP for 6 h and harvested for immunoblot analysis. Where indicated, 25 µM of a proteasome inhibitor (ALLN) was added at the time of CDDP treatment. B: WT fibroblast cells were treated with 0 or 100 µM CDDP for 16 h and harvested for protein analysis by immunoblot. Where indicated (lane 3), 10 µM of caspase inhibitor (Z-VAD-FMK) was treated at the time of CDDP treatment. DNA-PKcs and PARP were included as positive controls, while Ku70 was used as a loading control.
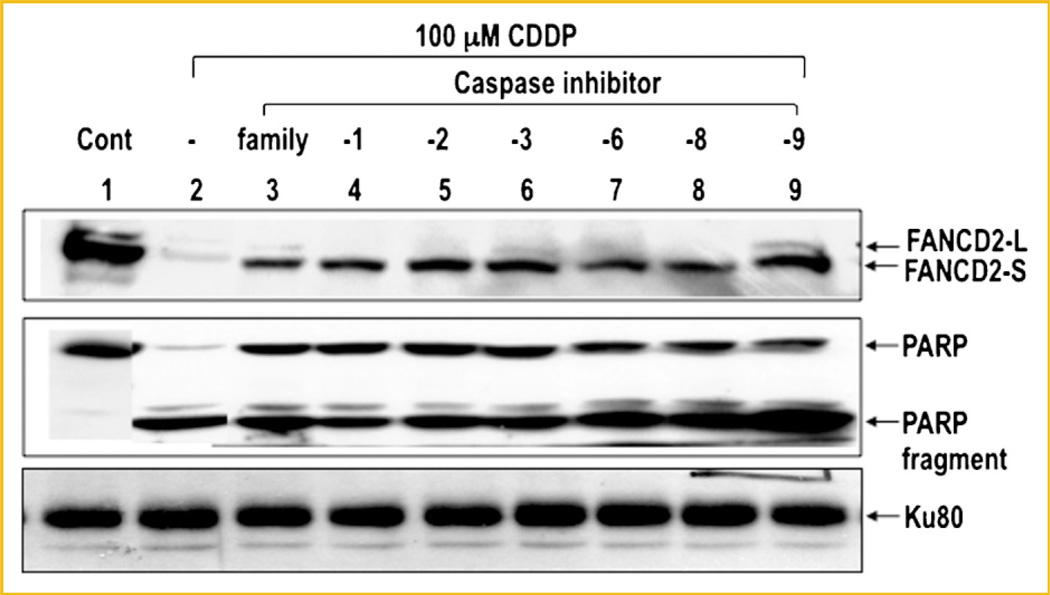
To see whether DNA damage-induced disappearance of FANCD2 is mediated by a specific caspase(s), we examined the effect of various caspase inhibitors on disappearance of FANCD2 (Fig. 4). FANCD2 was almost completely disappeared upon treatment of cells with CDDP (100 µM), but its disappearance was significantly blocked in the presence of caspase inhibitor or a caspase family inhibitor (Fig. 4). A similar inhibitory effect of these peptide inhibitors on degradation of PARP was also observed.
Fig. 4.
Effect of the caspase inhibitors on disappearance of FANCD2 following CDDP treatment. HeLa cells were treated with 100 µM CDDP for 24 h and harvested for immunoblot analysis. Cell extracts were subjected to SDS–PAGE and immunoblot using an antibody for FANCD2 (top panel), PARP (middle panel), and Ku80 (bottom panel). Where indicated, 10 µM of caspase inhibitor [lane 3, caspase family (Z-VAD-FMK); lane 4, caspase 1 inhibitor (Z-YVAD-FMK); lane 5, caspase 2 inhibitor (Z-VDVAD-FMK); lane 6, caspase 3 inhibitor (Z-DEVD-FMK); lane 7, caspase 6 inhibitor (Z-VEID-FMK); lane 8, caspase 8 inhibitor (Z-IETD-FMK); lane 9, caspase 9 inhibitor (Z-LEHD-FMK)] was added at the time of CDDP treatment. Ku80 was used as a loading control.
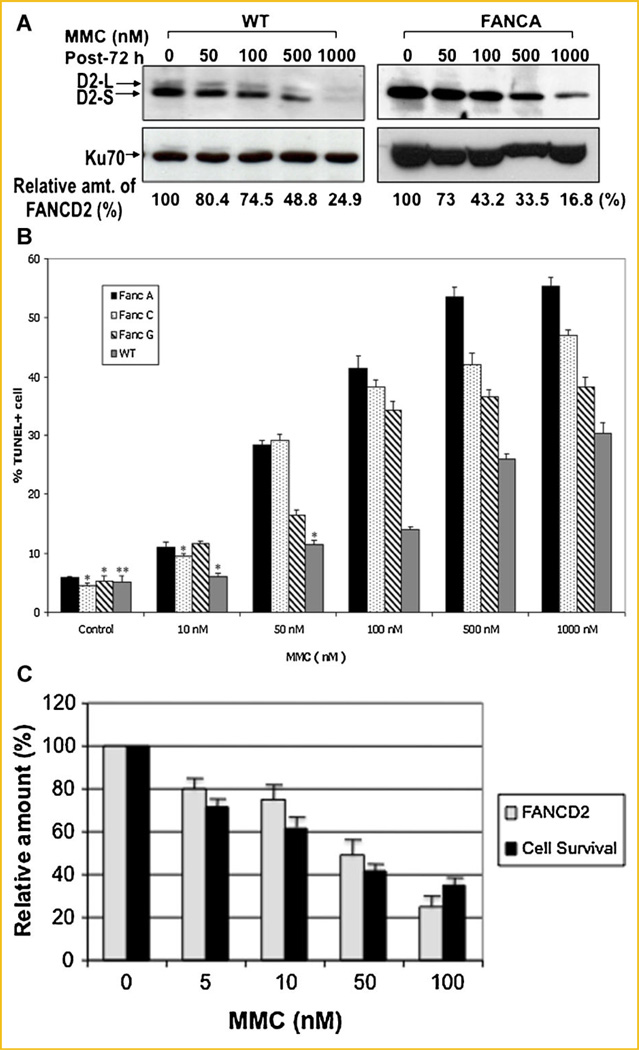
DISAPPEARANCE OF FANCD2 CORRELATES WITH CELL KILLING FOLLOWING MMC TREATMENT
Since FANCD2 is a likely target for caspase following DNA damage(s), we reasoned that disappearance of FANCD2 is an early indicator for apoptotic cell death. We therefore examined whether there is a correlation between disappearance of FANCD2 and apoptotic cell death. WT fibroblast cells and FANC mutant cells were treated with various concentrations of MMC and analyzed for disappearance of FANCD2 in parallel with apoptotic cell death (Fig. 5). Amount of FANCD2 disappearance following MMC treatment was more evident in FANCA-deficient cells (Fig. 5A), and correlated with MMC-induced apoptotic cell death (Fig. 5B), suggesting that disappearance of FANCD2 may be an early indicator for apoptotic cells death.
Fig. 5.
Disappearance of FANCD2 correlates with cell death following MMC treatment. A: Disappearance of FANCD2 in WT fibroblast cells following treatment with various concentrations of MMC for 72 h. Cell extracts were subjected to SDS–PAGE and immunoblot using an anti-FANCD2 antibody (top panel). Relative amount of FANCD2 protein (%) was obtained from the Western blot using the NIH image system (version 1.62). Ku70 (bottom panel) was used as an internal loading control. B: Apoptotic cell death (% TUNEL+ cells) of WT and FANC mutant cells following MMC treatment. Values are the average (±SEM) of three separate experiments. P <0.01; *P=0.05; **P=0.07.C: Disappearance of FANCD2 protein correlates with apoptotic cell death following MMC treatment.
MONOUBIQUITINATION OF FANCD2 IS NOT REQUIRED FOR DEGRADATION OF FANCD2
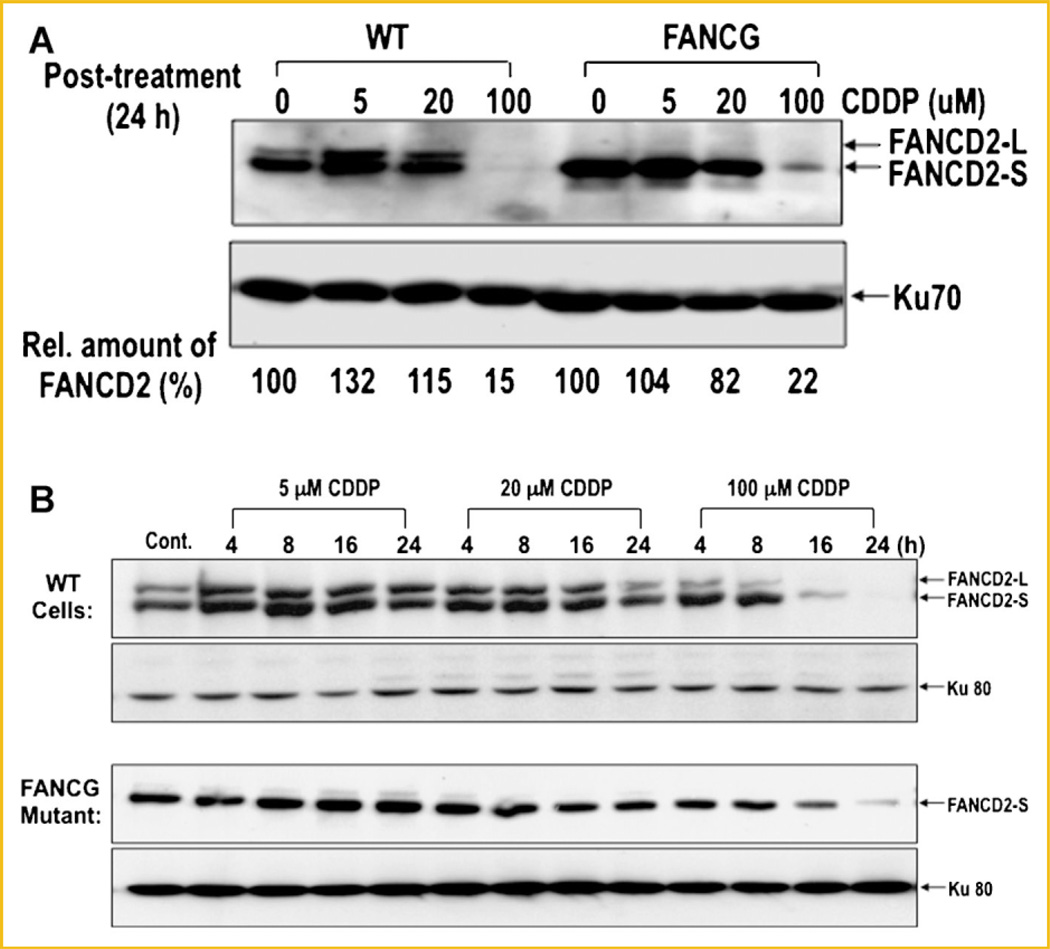
Both monoubiquitination and disappearance of FANCD2 were induced upon DNA damage. Monoubiquitination of FANCD2 occurred immediately after DNA damage and is required for colocalization of FANCD2 with BRCA1 at the damaged DNA sites during early stage of DNA repair, while FANCD2 disappearance occurs much later (Fig. 2). We therefore examined whether FANCD2 monoubiquitination affects its disappearance following DNA damage. For this, we compared WT cells and FANCA-deficient cells for disappearance of FANCD2 following CDDP treatment (Fig. 6A). FANCA-deficient cells showed no modification (monoubiquitination) of FANCD2, while the protein from WT cells were effectively monoubiquitinated. Nonetheless, no significant difference in FANCD2 degradation was observed between WT cells and FANCA mutant cells following CDDP treatment (Fig. 6A), suggesting that monoubiquitination of FANCD2 is not required for disappearance of FANCD2.
Fig. 6.
Disappearance of FANCD2 in WT cells and FANCG mutants following CDDP treatment. A: WT fibroblast and FANCG mutant cells were analyzed for disappearance of FANCD2 protein following CDDP treatment for 24 h. Cell extracts were subjected to 8% SDS–PAGE followed by Western blot using an anti-FANCD2 antibody. Ku70 was used as a loading control. Relative amount of FANCD2 protein (%) was quantified from theWestern blot (top panel) using the NIH image system. B: Kinetic analysis of disappearance of FANCD2 in WT cells and the FANCG mutant following CDDP treatment. Cells were treated with indicated amount of CDDP, harvested at various times, and analyzed for relative amounts of FancD2-L and FancD2-S by Western blot.
To further analyze a possible relationship between monoubiquitination and disappearance of FANCD2, we carried out a kinetic analysis with WT cells and FANCG-deficient cells following treatment with varying concentrations of CDDP (Fig. 6B). Both WT cells and the FANCG mutant showed disappearance of FANCD2 in a CDDP dose-dependent manner (Fig. 6B). Again, no significant difference was observed between WT cells and the FANCG mutant in disappearance of FANCD2 following CDDP treatment.
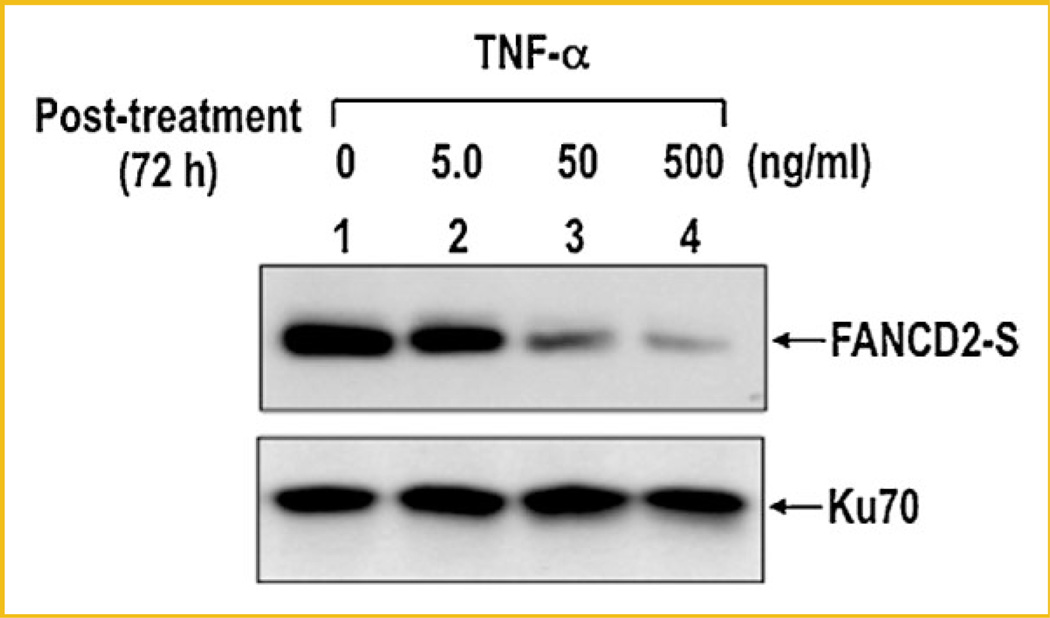
DISAPPEARANCE OF FANCD2 ALSO OCCURRED FOLLOWING TNF-α TREATMENT
In addition to MMC treatment, FANC-deficient cells are also sensitive to a non-DNA damaging agent, TNF-α and, at least, one of the FANC proteins, FANCC, modulates apoptotic responses to TNF-α [Freie et al., 2003]. We therefore examined whether disappearance of FANCD2 can also be observed in cells treated with TNF-α. Similar to MMC and CDDP, TNF-α also induced disappearance of FANCD2 in a dose-dependent manner (Fig. 7), while it had little or no effect on FANCD2 monoubiquitination. Our recent study showed that disappearance of FANCD2 following TNF-α treatment was also blocked in the presence of a caspase inhibitor (data not shown), suggesting that disappearance of FANCD2 following TNF-α treatment was also mediated by caspase(s). This finding suggests that disappearance of FANCD2 is likely a response to an apoptotic signal that is independent of monoubiquitination of FANCD2.
Fig. 7.
Treatment of TNF-α induces disappearance of FANCD2 protein. HeLa monolayer cells were treated with indicated amount of TNF-α for 72 h. Cells were harvested and analyzed for disappearance of FANCD2 by immunoblot. Ku70 was used as a loading control.
DISCUSSIONS
Upon DNA damage, monoubiquitination of FANCD2 is induced in the presence of other FANC proteins, which is likely targeted to the chromatin for the assembly of FANCD2 nuclear foci [Garcia-Higuera et al., 2001; Moldovan and D’Andrea, 2009]. In this study, we investigated the fate of FANCD2 following cellular exposure to DNA damaging agents. We found that treatment of cells with DNA damaging agent induced monoubiquitination of FANCD2 initially, but caused disappearance of FANCD2 protein later. Disappearance of FANCD2 correlated with proteolytic degradation of other caspase-mediated apoptotic targets such as BRCA1 and PARP, and was in keeping with damage-induced cell death, suggesting that FANCD2 is a key apoptotic target for caspases.
FANCD2 shares some of its biochemical properties with two known targets for apoptosis, PARP and BRCA1. Both PARP and BRCA1 are DNA repair factors that immediately respond to DNA breaks. In addition, both proteins become targets of caspase(s) for apoptotic cell death following severe DNA damages. FANCD2 is associated with chromatin following DNA damage and localized to the damaged DNA sites, suggesting a direct role for FANCD2 in DNA repair [Garcia-Higuera et al., 2001; Nakanishi et al., 2002]. In addition, DNA damage induces interaction of FANCD2 with BRCA1 at the damaged DNA site(s), although the exact role for BRCA1-FANCD2 interaction is not clear. FANCD2 also interacts with Nijmegen breakage syndrome 1 protein (NBS1) and forms subnuclear foci [Nakanishi et al., 2002]. Purpose of targeted proteolytic degradation of FANCD2 upon DNA damage or stress would be in line with degradation of PARP and BRCA1. Proteolytic degradation of key DNA repair factors is a part of the damage-signaling pathway towards apoptosis when cells encounter enormous amount of DNA damage. Considering both PARP and BRCA1 are the key repair factors essential for protecting cells from DNA damages, it is not surprising that FANCD2 is also a target for caspase(s) following DNA damages. A previous study indicated that FANCC is also proteolytically degraded in a process of caspase-mediated apoptotic response [Brodeur et al., 2004]. Lack of proteolytic modification at the putative cleavage site delays apoptosis but does not affect MMC complementation. It would be interesting to see whether proteolytic cleavage of FANCC is linked to disappearance of FANCD2 following DNA damage.
Both monoubiquitination and disappearance of FANCD2 were induced following DNA damage. Monoubiquitination is for redistribution of FANCD2 to nuclear foci containing BRCA1 following DNA damage [Meetei et al., 2003], which is a part of a protective response involved in the repair of damaged DNA. On the other hand, caspase-mediated disappearance of FANCD2 likely leads to a non-reversible cell death. It should be noted that both monoubiquitinated and non-ubiquitinated forms were disappeared in response to treatment with DNA damaging agent (Fig. 1). Disappearance of both mono- and non-ubiquitinated forms of FANCD2 was not only correlated with proteolytic degradation of PARP and BRCA1 (Fig. 2), but also blocked by caspase inhibitor(s) (Fig. 4), suggesting that both mono- and non-ubiquitinated forms are targeted by caspases following DNA damages. Monoubiquitination of FANCD2 is an early response to DNA damage and usually occurred within several hours following CDDP treatment (Figs. 1 and 2), while disappearance of FANCD2 is an apoptotic response that was observed in 16–24 h after DNA damage. FANC-deficient cells also induced disappearance of FANCD2 without post-translational modification (monoubiquitination) following CDDP treatment. Cells treated with non-DNA damaging agent, TGF-a, also induced degradation of FANCD2 without monoubiquitination, suggesting that disappearance of FANCD2 can occur in either DNA damage-dependent or -independent manner, although it was not dependent on FANCD2 monoubiquitination. It should be noted that disappearance of FANCD2 was also observed in mouse cells (data not shown). It would be interesting to see in the future whether disappearance of FANCD2 correlates with the p53-dependent apoptosis.
CDDP-induced disappearance of FANCD2 was blocked by treatment with either caspase inhibitors or a caspase family inhibitor, suggesting that its disappearance was due to proteolytic degradation mediated by caspase(s) (Fig. 4 and data not shown). We noticed, however, none of the peptide-based caspase inhibitor was able to completely block disappearance of FANCD2. The peptide-based inhibitors may have limited capacity in blocking caspase action in vivo. It is also possible that other protease such as granzymes may be involved in either caspase-dependent or-independent apoptotic mechanism [Fan et al., 2003]. Another noticeable finding was that several specific caspase inhibitors were able to block disappearance of FANCD2 as well as PARP degradation. It has been reported that CDDP differentially activated caspase cascades in various cells [Del Bello et al., 2003; Toyozumi et al., 2004; Wang et al., 2004a]. For example, CDDP activated the caspase-8 pathway through TNFR superfamily receptors such as Fas, but not caspase-9 in HeLa cells [Toyozumi et al., 2004]. On the other hand, the caspase-9 pathway was significantly activated in HEp-2 cells, while the activation of caspase-8 by CDDP was absent. Activation of both caspase-9 and caspase-3 was detected in other cell types after treatment with CDDP [Wang et al., 2004a].
ACKNOWLEDGMENTS
This research was supported by Grants from NIH (CA92111 to S.-H.L.), U.S. Army (DAMD17-00-1-0295 to S.-H.L.), and the Fanconi Anemia Research Foundation. S.-J.P. was supported by NIH Postdoctoral Fellowship (F32 GM20167-01).
Grant sponsor: NIH; Grant numbers: CA92111, F32 GM20167-01; Grant sponsor: U.S. Army; Grant number: DAMD17-00-1-0295.
REFERENCES
- Blagosklonny MV, An WG, Melillo G, Nguyen P, Trepel JB, Neckers LM. Regulation of BRCA1 by protein degradation. Oncogene. 1999;18:6460–6468. doi: 10.1038/sj.onc.1203068. [DOI] [PubMed] [Google Scholar]
- Bogliolo M, Lyakhovich A, Callen E, Castella M, Cappelli E, Ramirez MJ, Creus A, Marcos R, Kalb R, Neveling K, Schindler D, Surralles J. Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability. EMBO J. 2007;26:1340–1351. doi: 10.1038/sj.emboj.7601574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40:216–227. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur I, Goulet I, Tremblay CS, Charbonneau C, Delisle MC, Godin C, Huard C, Khandjian EW, Buchwald M, Levesque G, Carreau M. Regulation of the Fanconi anemia group C protein through proteolytic modification. J Biol Chem. 2004;279:4713–4720. doi: 10.1074/jbc.M301291200. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995;182:1625–1634. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DM, Adamson B, Dephoure NE, Tan X, Nottke AC, Hurov KE, Gygi SP, Colaiacovo MP, Elledge SJ. A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive Polycomb and NuRD complexes to sites of DNA damage. Proc Natl Acad Sci USA. 2010;107:18475–18480. doi: 10.1073/pnas.1012946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Boswell SA, Aaronson SA, Lee SW. P53 promoter selection: Choosing between life and death. Cell Cycle. 2008;7:154–157. doi: 10.4161/cc.7.2.5236. [DOI] [PubMed] [Google Scholar]
- Del Bello B, Valentini MA, Comporti M, Maellaro E. Cisplatin-induced apoptosis in melanoma cells: role of caspase-3 and caspase-7 in Apaf-1 proteolytic cleavage and in execution of the degradative phases. Ann N Y Acad Sci. 2003;1010:200–204. doi: 10.1196/annals.1299.034. [DOI] [PubMed] [Google Scholar]
- Fan Z, Beresford PJ, Zhang D, Xu Z, Novina CD, Yoshida A, Pommier Y, Lieberman J. Cleaving the oxidative repair protein Ape1 enhances cell death mediated by granzyme A. Nat. Immunol. 2003;4:145–153. doi: 10.1038/ni885. [DOI] [PubMed] [Google Scholar]
- Freie B, Li X, Ciccone SL, Nawa K, Cooper S, Vogelweid C, Schantz L, Haneline LS, Orazi A, Broxmeyer HE, Lee SH, Clapp DW. Fanconi anemia type C and p53 cooperate in apoptosis and tumorigenesis. Blood. 2003;102:4146–4152. doi: 10.1182/blood-2003-03-0971. [DOI] [PubMed] [Google Scholar]
- Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D’Andrea AD. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Jeggo PA. DNA double-strand break repair and V(D)J recombination: involvement of DNA-PK. Trends Biochem Sci. 1995;20:412–415. doi: 10.1016/s0968-0004(00)89090-8. [DOI] [PubMed] [Google Scholar]
- Kennedy RD, D’Andrea AD. The Fanconi anemia/BRCA pathway: New faces in the crowd. Genes Dev. 2005;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Liu T, Ghosal G, Yuan J, Chen J, Huang J. FAN1 acts with FANCIFANCD2 to promote DNA interstrand cross-link repair. Science. 2010;329:693–696. doi: 10.1126/science.1192656. [DOI] [PubMed] [Google Scholar]
- Ljungman M, Lane DP. Transcription–Guarding the genome by sensing DNA damage. Nat Rev Cancer. 2004;4:727–737. doi: 10.1038/nrc1435. [DOI] [PubMed] [Google Scholar]
- Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, Oostra AB, Yan Z, Ling C, Bishop CE, Hoatlin ME, Joenje H, Wang W. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35:165–170. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- Moldovan GL, D’Andrea AD. How the Fanconi anemia pathway guards the genome. Annu Rev Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Taniguchi T, Ranganathan V, New HV, Moreau LA, Stotsky M, Mathew CG, Kastan MB, Weaver DT, D’Andrea AD. Interaction of FANCD2 and NBS1 in the DNA damage response. Nat Cell Biol. 2002;4:913–920. doi: 10.1038/ncb879. [DOI] [PubMed] [Google Scholar]
- Park SJ, Oh EJ, Yoo MA, Lee SH. Involvement of DNA-dependent protein kinase in regulation of stress-induced JNK activation. DNA Cell Biol. 2001;20:637–645. doi: 10.1089/104454901753340622. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ciccone SL, Beck BD, Hwang B, Freie B, Clapp DW, Lee SH. Oxidative stress/damage induces multimerization and interaction of Fanconi anemia proteins. J Biol Chem. 2004;279:30053–30059. doi: 10.1074/jbc.M403527200. [DOI] [PubMed] [Google Scholar]
- Rheaume E, Cohen LY, Uhlmann F, Lazure C, Alam A, Hurwitz J, Sekaly RP, Denis F. The large subunit of replication factor C is a substrate for caspase-3 in vitro and is cleaved by a caspase-3-like protease during Fasmediated apoptosis. EMBO J. 1997;16:6346–6354. doi: 10.1093/emboj/16.21.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz N, Lopez E, Saleh-Gohari N, Helleday T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003;31:4959–4964. doi: 10.1093/nar/gkg703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Lees-Miller SP, Kumar S, Zhang Z, Chan DW, Smith GC, Jackson SP, Alnemri ES, Litwack G, Khanna KK, Lavin MF. DNA-dependent protein kinase catalytic subunit: A target for an ICE-like protease in apoptosis. EMBO J. 1996;15:3238–3246. [PMC free article] [PubMed] [Google Scholar]
- Susse S, Scholz CJ, Burkle A, Wiesmuller L. Poly(ADP-ribose) polymerase (PARP-1) and p53 independently function in regulating doublestrand break repair in primate cells. Nucleic Acids Res. 2004;32:669–680. doi: 10.1093/nar/gkh227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D’Andrea AD. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002;100:2414–2420. doi: 10.1182/blood-2002-01-0278. [DOI] [PubMed] [Google Scholar]
- Toyozumi Y, Arima N, Izumaru S, Kato S, Morimatsu M, Nakashima T. Loss of caspase-8 activation pathway is a possible mechanism for CDDP resistance in human laryngeal squamous cell carcinoma, HEp-2 cells. Int J Oncol. 2004;25:721–728. [PubMed] [Google Scholar]
- Vandenberg CJ, Gergely F, Ong CY, Pace P, Mallery DL, Hiom K, Patel KJ. BRCA1-independent ubiquitination of FANCD2. Mol Cell. 2003;12:247–254. doi: 10.1016/s1097-2765(03)00281-8. [DOI] [PubMed] [Google Scholar]
- Vaz F, Hanenberg H, Schuster B, Barker K, Wiek C, Erven V, Neveling K, Endt D, Kesterton I, Autore F, Fraternali F, Freund M, Hartmann L, Grimwade D, Roberts RG, Schaal H, Mohammed S, Rahman N, Schindler D, Mathew CG. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet. 2010;42:406–409. doi: 10.1038/ng.570. [DOI] [PubMed] [Google Scholar]
- Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004a;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- Wang X, Andreassen PR, D’Andrea AD. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol Cell Biol. 2004b;24:5850–5862. doi: 10.1128/MCB.24.13.5850-5862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Yuan F, El Hokayem J, Zhou W, Zhang Y. FANCI protein binds to DNA and interacts with FANCD2 to recognize branched structures. J Biol Chem. 2009;284:24443–24452. doi: 10.1074/jbc.M109.016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q, Jin S, Ng B, Plisket J, Shangary S, Rathi A, Brown KD, Baskaran R. Caspase-3 mediated cleavage of BRCA1 during UV-induced apoptosis. Oncogene. 2002;21:5335–5345. doi: 10.1038/sj.onc.1205665. [DOI] [PubMed] [Google Scholar]