Abstract
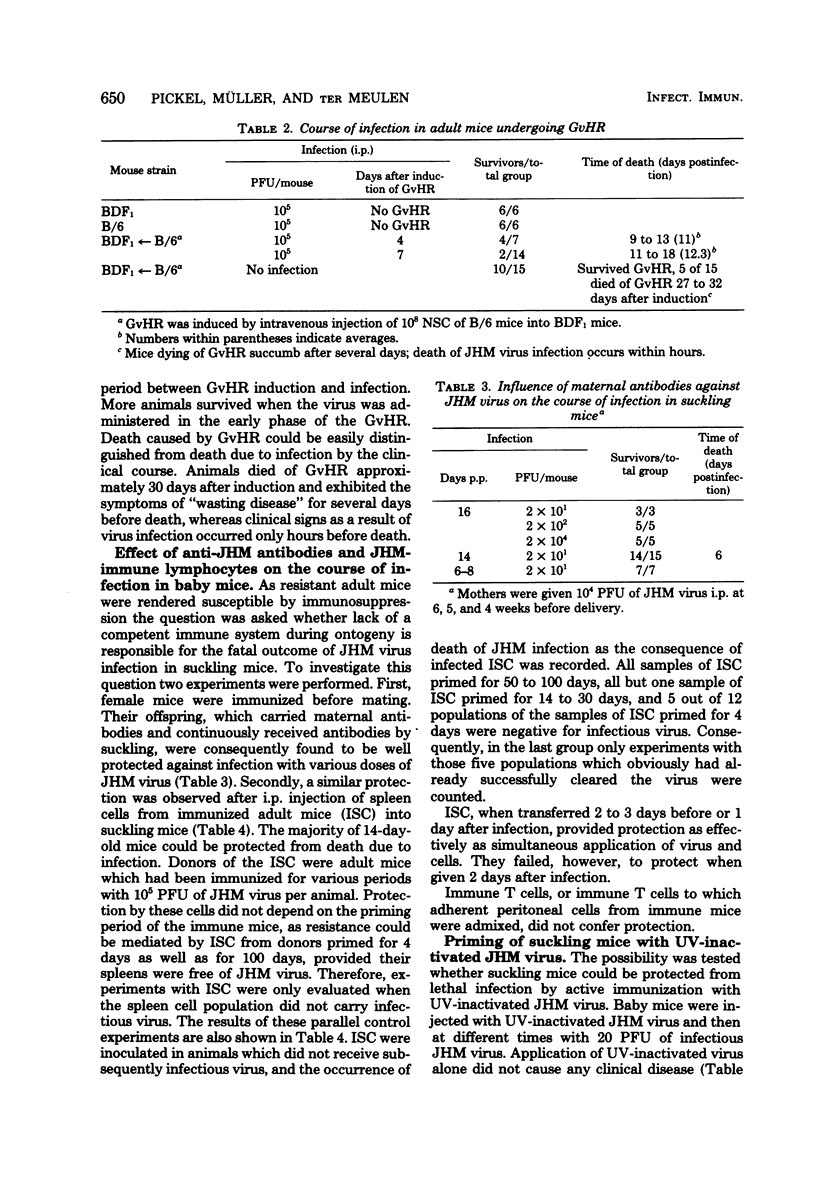
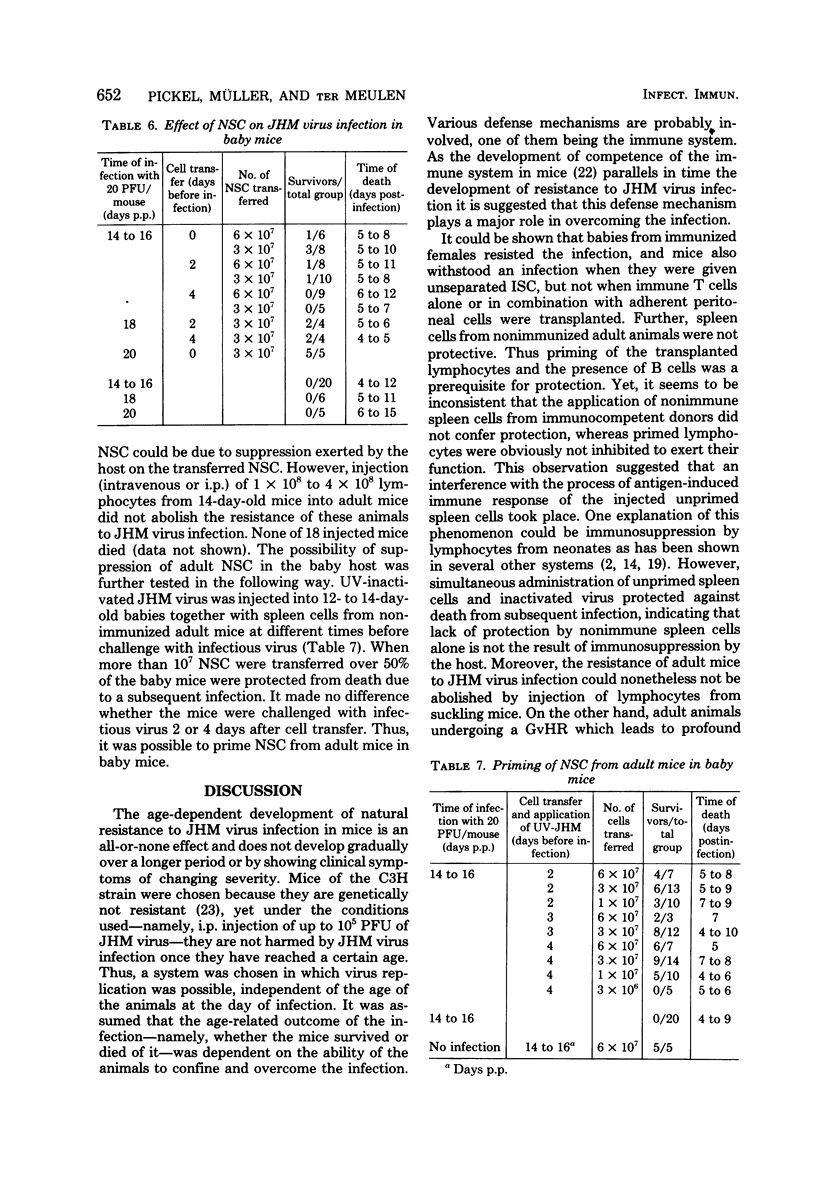
Resistance to intraperitoneal murine coronavirus JHM infection in mice develops with age. C3H mice were found to be fully susceptible up to the age of 20 days and resistant after 23 days of age. Protection of susceptible animals from death due to infection could be achieved by maternal antibodies or by transfer of spleen cells from immunized, but not from nonimmunized, donor mice. Lack of protection by transfer of unprimed adult spleen cells was not related to immunosuppression by the host. Moreover, resistance of adult mice could not be abrogated by application of lymphocytes from suckling mice, although immune suppression by other means did affect the resistance of adult animals. On the other hand, spleen cells from nonimmunized mice could be primed with inactivated JHM virus in suckling mice and protected these mice from death due to a subsequent virus infection. Thus, the outcome of infection with JHM virus in suckling and adult mice can be influenced by immunological events, but is not exclusively due to the different stages of immune competence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dupuy J. M., Levey-Leblond E., Le Prevost C. Immunopathology of mouse hepatitis virus type 3mii. effect of immunosuppression in resistant mice. J Immunol. 1975 Jan;114(1 Pt 1):226–230. [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Active suppression of B lymphocyte maturation by two different newborn T lymphocyte subsets. J Immunol. 1979 Dec;123(6):2644–2650. [PubMed] [Google Scholar]
- GALLILY R., WARWICK A., BANG F. B. EFFECT OF CORTISONE OF GENETIC RESISTANCE TO MOUSE HEPATITIS VIRUS IN VIVO AND IN VITRO. Proc Natl Acad Sci U S A. 1964 Jun;51:1158–1164. doi: 10.1073/pnas.51.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallily R., Warwick A., Bang F. B. Ontogeny of macrophage resistance to mouse hepatitis in vivo and in vitro. J Exp Med. 1967 Apr 1;125(4):537–548. doi: 10.1084/jem.125.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N., Takenaka S., Fujiwara K. Pathogenicity of mouse hepatitis virus for mice depending upon host age and route of infection. Jpn J Exp Med. 1975 Aug;45(4):285–292. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., McFarland H. F., Levy S. E. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972 Mar;125(3):257–262. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Levy-Leblond E., Dupuy J. M. Neonatal susceptibility to MHV3 infection in mice. I. Transfer of resistance. J Immunol. 1977 Apr;118(4):1219–1222. [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Johnson B. M. Ontogeny of mouse lymphocyte function. II. Development of the ability to produce antibody is modulated by T lymphocytes. J Exp Med. 1975 Jan 1;141(1):216–226. doi: 10.1084/jem.141.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978 Oct 13;44(1):63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Demyelinating encephalomyelitis induced by a long-term corona virus infection in rats. A preliminary report. Acta Neuropathol. 1979 Mar 15;45(3):205–213. doi: 10.1007/BF00702672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel K., Hoffmann M. K. Suppressor T cells arising in mice undergoing a graft-vs-host response. J Immunol. 1977 Feb;118(2):653–656. [PubMed] [Google Scholar]
- Pickel K., Hoffmann M. K. The Ly phenotype of suppressor T cells arising in mice subjected to a graft-versus-host reaction. J Exp Med. 1977 May 1;145(5):1169–1175. doi: 10.1084/jem.145.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez G., Andersson G., Wigzell H., Peck A. B. Non-T cell nature of the naturally occurring, spleen-associated suppressor cells present in the newborn mouse. Eur J Immunol. 1979 Sep;9(9):737–746. doi: 10.1002/eji.1830090913. [DOI] [PubMed] [Google Scholar]
- SIGEL M. M. Influence of age on susceptibility to virus infections with particular reference to laboratory animals. Annu Rev Microbiol. 1952;6:247–280. doi: 10.1146/annurev.mi.06.100152.001335. [DOI] [PubMed] [Google Scholar]
- Sheets P., Shah K. V., Bang F. B. Mouse hepatitis virus (MHV) infection in thymectomized C3H mice. Proc Soc Exp Biol Med. 1978 Oct;159(1):34–38. doi: 10.3181/00379727-159-40278. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Frelinger J. A., Weiner L. P. Resistance to fatal central nervous system disease by mouse hepatitis virus, strain JHM. II. Adherent cell-mediated protection. J Immunol. 1980 Apr;124(4):1733–1739. [PubMed] [Google Scholar]
- Taguchi F., Yamada A., Fujiwara K. Factors involved in the age-dependent resistance of mice infected with low-virulence mouse hepatitis virus. Arch Virol. 1979;62(4):333–340. doi: 10.1007/BF01318107. [DOI] [PubMed] [Google Scholar]
- Tardieu M., Héry C., Dupuy J. M. Neonatal susceptibility to MHV3 infection in mice. II. Role of natural effector marrow cells in transfer of resistance. J Immunol. 1980 Jan;124(1):418–423. [PubMed] [Google Scholar]
- Willenborg D. O., Shah K. V., Bang F. B. Effect of cyclophosphamide on the genetic resistance of C 3 H mice to mouse hepatitis virus. Proc Soc Exp Biol Med. 1973 Mar;142(3):762–766. doi: 10.3181/00379727-142-37111. [DOI] [PubMed] [Google Scholar]


