Abstract
Previous work demonstrates that early life stress (ELS) and HPA-axis function predict later psychopathology. Animal work and cross-sectional human studies suggest that this process might operate through amygdala-ventromedial prefrontal cortical (vmPFC) circuitry implicated in emotion regulation. The current study prospectively investigated the roles of ELS and childhood basal cortisol in the development of adolescent resting-state functional connectivity (fcMRI) in the amygdala-PFC circuit. In females only, greater ELS predicted increased childhood cortisol levels, which, in turn, predicted decreased amygdala-vmPFC fcMRI 14 years later. Further, for females, amygdala-vmPFC fcMRI was inversely correlated with concurrent anxious symptoms, but positively associated with depressive symptoms, suggesting differing pathways from childhood cortisol function through adolescent amygdala-vmPFC functional connectivity to anxiety and depression. These data highlight that, for females, the effects of ELS and early HPA-axis function may be detected much later in the intrinsic processing of emotion-related brain circuits.
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is related to many adverse physiologic and psychological outcomes1. Cortisol, a principle hormone end product of HPA-axis activity, has a direct influence on brain circuits implicated in emotion regulation, and these circuits in turn regulate HPA-axis activity. Increased cortisol levels early in life are associated with poor emotion regulation, less social competence, and later symptoms of anxiety and depression2,3. These individual differences may be primed by exposure to early life stress (ELS), and the timing and magnitude of these effects can vary by gender4.
There are multiple distinct lines of research that investigate questions related to how ELS and the HPA-axis work together to influence emotion-related brain circuits and the development of psychopathology. For example, our group finds that children exposed to elevated levels of maternal stress in infancy are more likely to display higher afternoon basal cortisol levels later in childhood, which, in turn, is associated with increased mental health symptoms two years later4. Other work suggests that aspects of ELS may sensitize the neural circuitry that regulates HPA-axis feedback resulting in atypical basal cortisol levels (e.g., heightened or blunted cortisol function2,3), as well as structural and functional changes in brain regions involved in affective reactivity and regulation, such as the amygdala5 and ventromedial prefrontal cortex (vmPFC)6,7. The amygdala-vmPFC pathway may be of particular importance in these associations given non-human primate work that demonstrates that the prefrontal cortex has a dense collection of glucocorticoid receptors8. Elevated expression and concentrations of corticosteroid releasing hormone (CRH) receptors in the amygdala and other limbic regions are also linked to both ELS and anxiety-like behaviors in non-human primate and other animal (e.g., rat) studies, particularly in females9,10,11,12. In fact, task-based fMRI studies of emotion regulation suggest that the vmPFC is involved in top-down regulation of the amygdala, and functional coupling of the amygdala and vmPFC is related to trait anxiety in both task-related and resting-state studies13,14,15.
Recent work implicates ELS in morphometric differences in amygdala and PFC5,16. Strength of white matter tracts connecting these regions may also be inversely associated with anxious symptoms in adults17, suggesting evidence of disruptions in structural, as well as functional, connectivity. Dysregulated diurnal cortisol patterns are associated with the functional interplay among these brain regions18. Thus, while interactions between ELS and HPA-axis function are known to affect brain structure and behavior, the roles of ELS and neuroendocrine processes in the development of the functional interplay, or connectivity, of the amygdala-vmPFC circuit, and its associations with internalizing problems later in life are not yet clear19.
Resting-state functional connectivity MRI (fcMRI) is an excellent metric for probing the functional integrity of the amygdala-vmPFC circuit20. This metric is postulated to reflect critical individual differences in spontaneous brain activity that can be captured independent of task demands and has evidenced patterns of functional connectivity consistent with the white matter structure of the brain21,22. Highly anxious individuals exhibit reduced amygdala-vmPFC resting connectivity14, while higher levels of current basal cortisol are associated with less amygdala-vmPFC connectivity in adults15. These differences in the amygdala-vmPFC circuit may be particularly relevant to anxiety given the role this pathway is postulated to have on the down-regulation of threat- or fear-related activity in the amygdala6,13,18. In addition, the vmPFC may also mediate perceptions of stress-controllability in behavioral inhibition, particularly in females23,24.
Resting-state data from samples with major depressive disorders demonstrate hyperactivity in the vmPFC and limbic structures (e.g., amygdala, bed nucleus of the stria terminalis [BNST])25, as well as increased connectivity between medial prefrontal regions (including the vmPFC) and other networks including an affective network to which the amygdala is interconnected26. Such data are consistent with prolonged and persistent experience of negative emotion and affect dysregulation, which may be associated with treatment-resistant symptomatology and disruptions in the stress-response circuitry of the brain27. However, given the high comorbidity of anxiety and depression, as well as the consistent involvement of amygdala-vmPFC circuitry in studies of anxiety and depression28, systematic disentangling of the role of vmPFC-amygdala circuitry in anxious and depressive symptoms is required. There is very limited evidence to address this issue, and in a rare example of rigorous parsing of anxious and depressive symptoms in adolescence, researchers observe reliable differences in amygdala activation as a function of specific task requirements29. However, to the best of our knowledge, no research to date has examined the differential relations between amygdala-vmPFC resting state connectivity and anxious and depressive symptoms and explored how these associations are impacted by ELS and childhood HPA function.
Results
The current study investigated 1) the associations between ELS, early neuroendocrine function, and adolescent resting-state fcMRI; and 2) the differential association of adolescent resting-state fcMRI, as well as ELS and early neuroendocrine function, with concurrent symptoms of anxiety and depression. To affirm that any associations observed were due to early experience, adolescent life stress and afternoon basal cortisol from this period were also examined. Our central prediction was that ELS would be associated with the development of heightened late afternoon childhood basal cortisol levels and that both ELS and childhood cortisol would predict variations in adolescent fcMRI. Specifically, we predicted that ELS and cortisol would be positively correlated, and that both would be inversely related to connectivity estimates between the amygdala and vmPFC, which would reflect basal abnormalities in this key regulatory circuit. Following from this, we also predicted that resting-state amygdala-vmPFC fcMRI would be associated with concurrent adolescent internalizing symptoms, especially anxiety. Additional analyses explored whether resting-state amygdala-vmPFC fcMRI mediated the association of childhood cortisol and internalizing symptoms. Finally, based on gender differences found in numerous studies summarized above, gender differences were investigated in all analyses.
In the present study, we acquired structural MRI and resting-state fMRI data, along with self-reported psychiatric symptoms, from 57 (28 female, Mage: 18.44, SD = 0.19 years) participants selected from the Wisconsin Study of Families and Work (WSFW)4, a prospective longitudinal study of a community sample beginning during the prenatal period. FcMRI of the amygdalae at age 18 years was computed by first defining masks of the left and right amygdala using previously-developed30 probabilistic maps in Talairach space31. The averaged preprocessed signal time-courses from each amygdala were extracted and regressed with resting-state time-courses from each voxel in the rest of the brain. Connectivity values were then regressed across participants against measures of mean salivary basal cortisol obtained in childhood at age 4.5 years, which had been collected on 3 consecutive days at a pre-selected target time between 3 and 7pm (actual collection time M = 5:56pm, SD = 88 min). We chose this time as most likely to reflect children’s experiences of stress during the day32. Cortisol was then assayed using the Pantex RIA (Santa Monica, CA), modified for salivary data. Mean late afternoon basal cortisol values were log10 transformed and residualized for time of collection and concomitant over-the-counter medication usage4. ELS exposure was measured using a composite measure of early maternal stress that was computed using maternal reports of depressive symptoms, parenting stress, marital conflict, role overload, and financial stress, averaged over assessments conducted when children were 1, 4, and 12 months old4. Current adolescent psychiatric symptoms were indexed via self-report on the MacArthur Health and Behavior Questionnaire33 (HBQ). The general anxiety and depressive subscales were computed; the externalizing subscale was also used as a control variable in all analyses of psychiatric symptoms. Adolescent recent life stress was measured with a self-report measure derived from the Adolescent Perceived Events Scale34 and the Life Events Survey35. Finally, all data were winsorized to within three standard deviations of the mean to normalize the distribution in the subsample.
Initial analyses examined voxel-wise functional connectivity estimates using the left amygdala seed (associations using the right amygdala were similar). Results indicated significant positive fcMRI with the contralateral amygdala, dorsomedial and ventrolateral PFC, ventral striatum, superior temporal gyrus, and vmPFC at rest, consistent with earlier studies10.
ELS, Childhood Cortisol, and Adolescent fcMRI
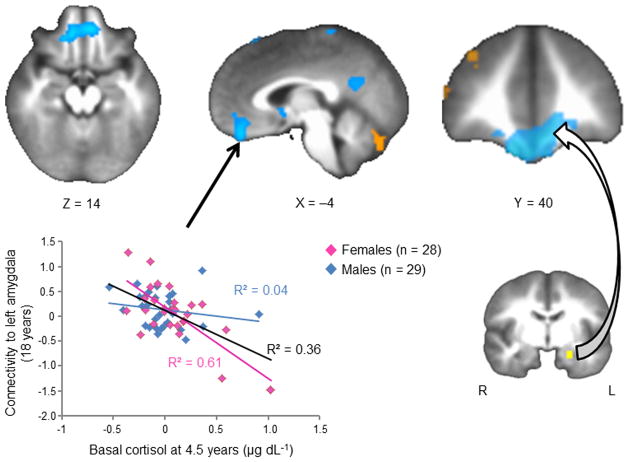
Voxel-wise analyses with the left amygdala seed revealed no statistically significant associations between ELS and resting-state fcMRI between the amygdala and any other brain regions. However, as predicted, childhood cortisol was negatively correlated with functional connectivity estimates between the left amygdala and a voxel cluster in the vmPFC (peak t-statistic: t(56) = −5.97, R2 = 0.36, FDR-corrected p = 0.01). Higher late afternoon cortisol levels during childhood were associated with decreased amygdala-vmPFC fcMRI fourteen years later (see Figure 1 and Table 1). No voxel-wise relations were detected between amygdala-vmPFC fcMRI estimates and either adolescent basal cortisol or current life stress, suggesting that the observed effects were specific to childhood late afternoon basal cortisol function, not current stress or cortisol levels.
Figure 1.
Correlation between late afternoon cortisol at child age 4.5 yrs and resting-state fcMRI to the left amygdala at 18 yrs. Connectivity between the left amygdala and vmPFC is significantly negatively associated with childhood cortisol (R2 = 0.36, FDR-corrected p = 0.01). This effect is driven entirely by females, represented by the magenta data points (R2 = 0.61, FDR-corrected p = 0.01).
Table 1.
Summary of Resting State fcMRI Estimates with Left Amygdala ROI as Predicted by Childhood Late Afternoon Basal Cortisol Levels.
| Identified Clusters | Talairach coordinates of peak voxel
|
peak t-stat | volume (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| vmPFC | 10 | 38 | −24 | −5.97 | 978 |
| Posterior cingulate | −2 | −54 | 22 | −4.74 | 321 |
| Cerebellum | |||||
| L posterior | −16 | −78 | −42 | 4.74 | 357 |
| R posterior | 16 | −76 | −42 | 5.06 | 318 |
| R anterior | 36 | −44 | −54 | −5.95 | 266 |
| L anterior | −34 | −56 | −56 | −5.37 | 210 |
Note: n = 57. FDR corrected with q < 0.05 (p < 7e–4)
Bivariate Pearson-r (two-tailed) correlations for the total sample revealed a significant positive association between ELS and childhood late afternoon cortisol, and a significant inverse association between childhood cortisol and adolescent amygdala-vmPFC fcMRI (Table 2). When considered separately for males and females, results suggested that these effects were largely specific to females. Moreover, only females demonstrated a detectable, but marginally-significant (p = 0.07), inverse relationship between ELS and amygdala-vmPFC fcMRI, with higher ELS exposure linked to lower functional connectivity.
Table 2.
Summary of Pearson-r Bivariate Correlation Statistics for ELS, Childhood Cortisol, and Adolescent Amygdala-vmPFC Functional Connectivity, Life Stress and Cortisol
| Average Values of Predictors | Childhood Cortisol
|
Adolescent Amygdala-vmPFC fcMRI
|
||||
|---|---|---|---|---|---|---|
| Females r-value | Males r-value | Total r-value | Females r-value | Males r-value | Total r-value | |
| ELS | 0.45* | 0.08 | 0.32* | −0.36t | 0.07 | −0.22 |
| Childhood Cortisol | --- | --- | --- | −0.78** | −0.19 | −0.60** |
| Adolescent Life Stress | −0.13 | −0.19 | −0.13 | 0.16 | −0.10 | 0.05 |
| Adolescent Cortisol | 0.31 | −0.02 | 0.22 | −0.21 | −0.13 | −0.21 |
Note: n = 57 (28 female).
p < 0.10,
p < 0.05,
p < 0.001.
There were no significant zero-order two-tailed correlations between ELS and adolescent life stress (females r = −0.13, NS; males: r = −0.06, NS; total: r = −0.08, NS).
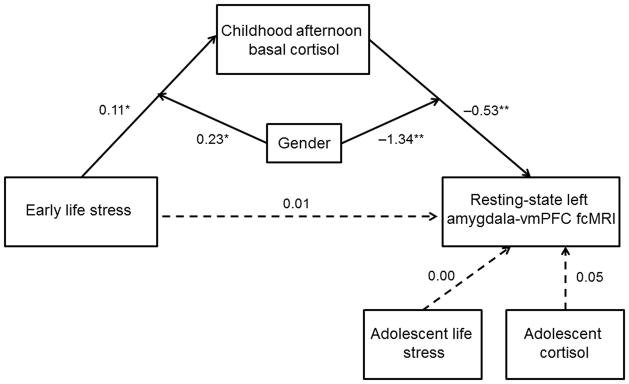
These results, and the temporal ordering of the variables, suggested that the associations of ELS and childhood cortisol with adolescent amygdala-vmPFC fcMRI might reflect a moderated mediational process. Thus, we constructed a structural equation model using Mplus36 (version 5.2) to determine if childhood cortisol mediated the association between ELS and amygdala-vmPFC fcMRI, and whether gender moderated this association. In addition, adolescent recent life stress and current afternoon basal cortisol were included as controls to confirm that the observed effects were indeed specific to early stress and childhood basal cortisol function. This model (Figure 2) showed excellent fit and accounted for 39.1% of the variance in fcMRI. There was no direct effect of ELS on adolescent amygdala-vmPFC fcMRI, and the mediating effect of childhood cortisol was marginally significant (Indirect Est = −0.06, p = 0.09). Further, gender significantly moderated both pathways. For females only, higher levels of ELS predicted increased levels of childhood afternoon basal cortisol, which, in turn, predicted decreased amygdala-vmPFC functional connectivity.
Figure 2.
Structural equation model examining the moderating effect of gender on the mediation through childhood late afternoon basal cortisol of the association between early life stress and amygdala-vmPFC fcMRI. The SEM model demonstrated good fit: Chi2 = 1.89, p > 0.05; RMSEA = 0.00; SRMR = 0.03; CFI = 1.00. Paths are marked with unstandardized coefficients. Gender significantly moderated associations between ELS and childhood cortisol and childhood cortisol and amygdala-vmPFC fcMRI estimates and the mediating effect of childhood cortisol was marginally significant (Indirect Est. = −0.06, p = 0.09). In females only, higher levels of ELS predicted increased levels of childhood afternoon basal cortisol, which, in turn, predicted decreased amygdala-vmPFC functional connectivity. * p < 0.05, ** p < 0.01
Adolescent Resting-State fcMRI and Concurrent Internalizing Symptoms
Bivariate Pearson-r (two-tailed) correlations revealed no significant associations between adolescent amygdala-vmPFC fcMRI and concurrent anxiety or depressive symptoms prior to controlling for each other and for externalizing symptoms (Table 3). However, when their covariation was considered, Pearson-r partial correlations revealed that, for the total sample, adolescent amygdala-vmPFC fcMRI was significantly inversely correlated with adolescent anxiety and significantly positively correlated with adolescent depression. When considered separately for males and females, results suggested, again, that these effects were largely specific to females. Further, significant associations between childhood cortisol and adolescent internalizing symptoms suggested that amygdala-vmPFC fcMRI might play a mediating role, especially for females.
Table 3.
Summary of Pearson-r Bivariate and Partial Correlation Statistics for Adolescent Anxiety and Depression Symptoms with ELS, Childhood Cortisol, and Adolescent Amygdala-vmPFC Resting-state Functional Connectivity
| Average Values of Predictors | Anxiety
|
Depression
|
||||
|---|---|---|---|---|---|---|
| Females r-value | Males r-value | Total r-value | Females r-value | Males r-value | Total r-value | |
| Correlations | ||||||
| ELS | 0.21 | 0.33t | 0.30* | 0.13 | 0.20 | 0.21 |
| Childhood Cortisol | 0.35t | 0.03 | 0.30* | 0.07 | −0.13 | 0.07 |
| Adolescent Amygdala-vmPFC fcMRI | −0.32t | 0.18 | −0.20 | −0.001 | 0.14 | 0.001 |
| Partial Correlations | ||||||
| ELS | 0.20 | 0.22 | 0.22 | 0.02 | −0.05 | −0.03 |
| Childhood Cortisol | 0.47* | 0.13 | 0.40* | −0.35t | −0.21 | −0.27* |
| Adolescent Amygdala-vmPFC fcMRI | −0.56* | 0.15 | −0.35* | 0.45* | 0.05 | 0.27* |
Note: n = 57 (28 female).
p < 0.10,
p < 0.05.
Partial correlations: To remove shared variance among the types of symptoms, depression and externalizing were partialled out of correlations with anxiety, and anxiety and externalizing were partialled out of the correlations with depression.
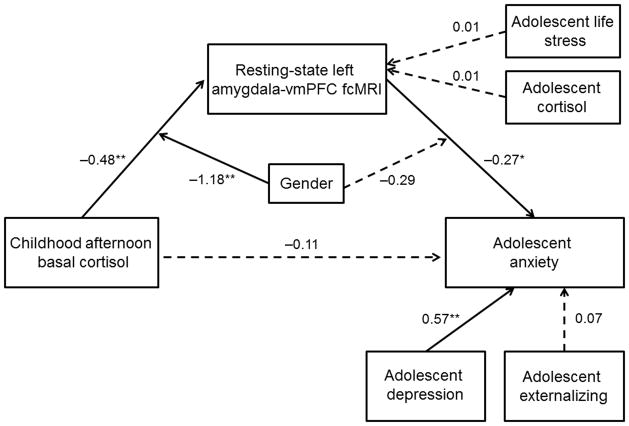
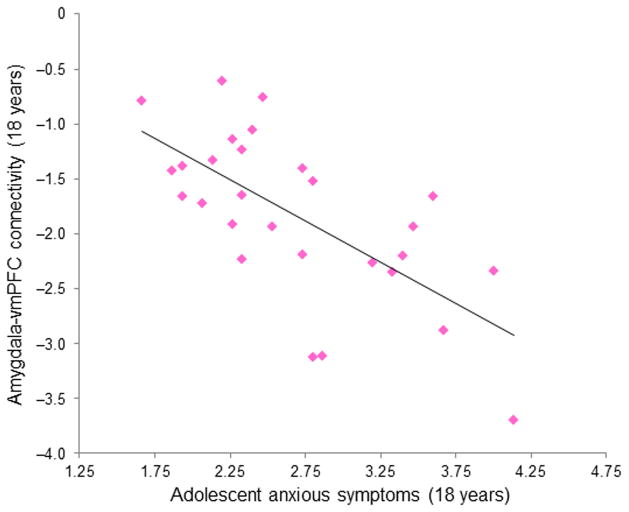
We constructed a second moderated mediation SEM to examine whether adolescent amygdala-vmPFC fcMRI was a mediator in the association of childhood cortisol and adolescent anxiety symptoms, and whether this process was moderated by gender. This model (Figure 3) demonstrated good fit and accounted for 64.6% of the variance in adolescent anxiety symptoms. There was no direct effect of childhood cortisol on adolescent anxiety symptoms, and the mediating effect of adolescent amygdala-vmPFC fcMRI was marginally significant (Indirect Est = 0.13, p = 0.08). Further, gender significantly moderated the association of childhood cortisol with fcMRI. However, because this model revealed a non-significant moderating effect of gender on the association of fcMRI with anxiety (p = 0.23), an alternative model was constructed without this pathway. In addition, because fcMRI estimates were obtained concurrently with anxiety symptoms, and there was no temporal precedence by which to define mediation, we built a second alternative model with the positions of anxiety symptoms and fcMRI reversed. For both of these alternative models, the fit statistics were decreased and the model fit was poor (see Online Methods). Together, these results show that, especially for females, amygdala-vmPFC fcMRI is negatively correlated with concurrent symptoms of anxiety (see Figure 4). Further, for females only, the developmental pathway between childhood cortisol and adolescent anxiety symptoms operates through adolescent amygdala-vmPFC fcMRI.
Figure 3.
Structural equation model examining the moderating effect of gender on the mediation through amygdala-vmPFC fcMRI of the relation between childhood late afternoon basal cortisol and adolescent anxiety. The SEM model demonstrated good fit: Chi2 = 9.95, p > 0.05; RMSEA = 0.11; SRMR = 0.04; CFI = 0.94. Paths are marked with unstandardized coefficients. Gender only significantly moderated the association between childhood cortisol and amygdala-vmPFC fcMRI and the mediating effect of adolescent amygdala-vmPFC fcMRI was marginally significant (Indirect Est. = 0.13, p = 0.08). For females only, the developmental pathway between childhood cortisol and adolescent anxiety symptoms operates through inverse associations with adolescent amygdala-vmPFC fcMRI. * p < 0.05, ** p < 0.01
Figure 4.
Partial correlation between resting-state left amygdala-vmPFC fcMRI and concurrent self-reported anxious symptoms in adolescent females, controlling for concurrent symptoms of depression and externalizing behaviors (R2 = 0.31, p = 0.004).
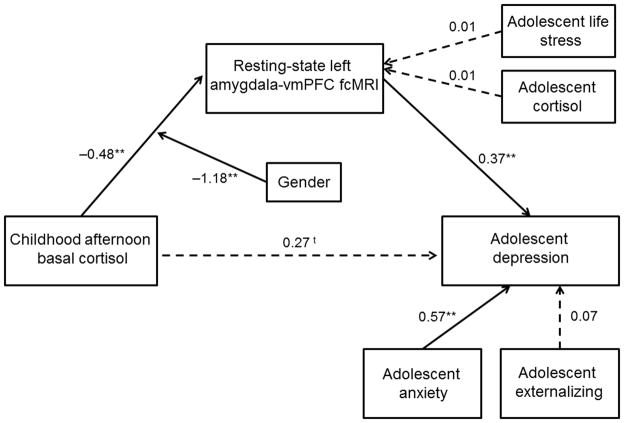
To examine depression as an outcome, we built a parallel moderated mediational model. Although this model demonstrated good fit, gender did not moderate the path from fcMRI to depression and thus, that pathway was omitted (see Online Methods). This modified model demonstrated improved fit and accounted for 56.2% of the variance in depression (Figure 5). There was a marginally-significant direct effect of childhood cortisol on adolescent depressive symptoms, and the mediating effect of adolescent amygdala-vmPFC fcMRI was significant (Indirect Est = −0.18, p = 0.02). Further, as with anxiety, when depressive symptoms and amygdala-vmPFC were transposed in an alternative model, the fit statistics were decreased and the model fit was poor (see Online Methods). Together, these results show that, for all adolescents, increased amygdala-vmPFC functional connectivity is associated with the development of depressive symptoms. In addition, for females only, the developmental pathway between childhood cortisol and adolescent depressive symptoms operates, in part, through adolescent amygdala-vmPFC fcMRI.
Figure 5.
Structural equation model examining the moderating effect of gender on the mediation through amygdala-vmPFC fcMRI of the relation between childhood afternoon cortisol and adolescent depression. The SEM model demonstrated good fit: Chi2 = 7.56, p > 0.05; RMSEA = 0.07; SRMR = 0.03; CFI = 0.97. Paths are marked with unstandardized coefficients. There was a marginally-significant direct effect of childhood cortisol on adolescent depressive symptoms, and the mediating effect of adolescent amygdala-vmPFC fcMRI was significant (Indirect Est. = −0.18, p = 0.02). In both male sand females, increased amygdala-vmPFC functional connectivity predicted increased depressive symptoms. For females, a developmental pathway between childhood cortisol and adolescent depressive symptoms operates partially through an inverse association with adolescent amygdala-vmPFC fcMRI. t p = 0.07, * p < 0.05, ** p < 0.01
Discussion
These findings establish a compelling developmental sequence whereby, in females, exposure to ELS during infancy was associated with higher levels of cortisol in childhood, and higher levels of cortisol at this early age predicted more negative amygdala-vmPFC fcMRI at age 18. Moreover, females with greater inverse adolescent amygdala-vmPFC fcMRI had higher levels of anxiety at 18 years, and subsequent analyses suggested that the developmental pathway from cortisol to anxiety worked through amygdala-vmPFC resting-state connectivity. Despite their temporal proximity, concurrent measures of adolescent life stress and cortisol were not significantly associated with amygdala-vmPFC resting-state fcMRI, implying that patterns of resting-state connectivity in adolescence likely reflect the cumulative impact of influences that begin early in life. Importantly, models examining anxiety controlled for depressive and externalizing symptoms suggesting that this pathway is specific to anxious symptoms, adding to a growing literature that underscores the role of the amygdala-vmPFC circuit in anxiety14,15,16.
Our findings on relations between amygdala-vmPFC resting-state fcMRI and depression stand in marked contrast to those for anxiety and they are consistent with recent findings that show increased connectivity between medial prefrontal and emotion circuits26. Such increased connectivity has been interpreted as reflecting emotionally-charged self-focused rumination in depression. Whether there exist partially separable zones within vmPFC that may inhibit versus activate the amygdala should be the focus of explicit investigation to better understand how the vmPFC may be playing these different roles in the etiology of anxious and depressive symptoms.
Our findings are the first to suggest a neurodevelopmental mechanism by which ELS in infancy increases vulnerability for internalizing disorders in adolescence. Our model indicates that early life stress increases basal cortisol, which in turn modulates emotion regulation circuitry in adolescence. The functional status of this circuitry at rest predicts both anxious and depressive symptoms and displays strong gender differences with the effects stronger in females. The fact that only females evinced these longitudinal associations between ELS during infancy, childhood late afternoon basal cortisol, and neural connectivity in adolescence extends previous work suggesting that females may be more sensitive to the effects of ELS on neuroendocrine function11 and work showing that females may also show more robust epigenetic changes in response to early social environmental factors37.
These data provide new insight regarding the interplay among gender, ELS, HPA-axis function, resting-state functional brain activity, and behavior. However, the causal relation among these constructs is not known. It is critical that future studies obtain measures of early childhood brain structure and function to determine if connectivity differences are present early in life or, rather, are the product of developmental influences of ELS and early variation in HPA-axis function. Our understanding of these associations would also benefit from studies designed to examine relations among resting-state fcMRI, task-induced connectivity assessed with fMRI, and white matter microstructure with diffusion tensor imaging to probe for associations among these different indices of connectivity.
In conclusion, our findings imply that, in females, ELS and early cortisol function may leave an imprint on the brain that can be detected in adolescent resting-state connectivity within a circuit important for emotion regulation, and variation in the function of this circuit plays an important role in adolescent anxiety and depressive symptoms. The difference in direction of association between connectivity and anxious versus depressive symptoms is consistent with prior data. However, the factors that are responsible for these diverging patterns of association require future study. It may be that among individuals predisposed to rumination, high levels of childhood cortisol sensitize the emotion regulation circuitry in a way that results in greater emotional arousal to self-relevant rumination, resulting in a positive coupling between vmPFC and amygdala. Future research with systematic observations of stress, neuroendocrine function, and brain activity throughout childhood and adolescence is needed to determine the causal role ELS may play in influencing HPA-axis function and resting-state connectivity in emotion regulation circuits and to identify the factors that account for the pronounced gender difference we uncovered.
Online Methods
Participants
Participants were 66 adolescents (32 female; Mage = 18.44, SD = 0.19 years) from the larger Wisconsin Study of Families and Work (WSFW)4, 57 (28 female) of whom had childhood cortisol data. Recruitment for the WSFW began in 1990 and was designed to gather information on parental leave and health outcomes from a subsample of the general population in southern Wisconsin. A total of 570 women and their partners were initially recruited from clinics and hospitals while attending routine prenatal visits. Mothers had to be over 18-years-old, in their second trimester of pregnancy, and living with their husband/partner. Selection for the present study was based on proximity to the laboratory and MRI exclusionary criteria. Participants’ racial background was 53 Caucasian, 2 Native American/Alaskan, and 2 African American. Data were collected during a 4-hour lab visit. Informed consent (and parental permission in childhood) was obtained for all assessments, and participants received monetary compensation. University of Wisconsin-Madison Institutional Review Boards approved all procedures.
Measures of Early Life Stress and Childhood Basal Cortisol
Early life stress (ELS) was indexed via a composite of maternal stress comprised of maternal reports of post-natal depression symptoms, marital conflict, parenting stress, financial stress, and role overload as detailed elsewhere4. Scores from 1, 4, and 12 months following birth were averaged to yield an infant maternal stress exposure measure.
Childhood basal cortisol (Mage = 4.58 years, SD = 0.05 years) was collected over three days. Parents were instructed to collect salivary samples at a target time between 3:00 and 7:00pm. In the current subsample, 84% complied with the prescribed time frame; 9 had average collection times >8pm. No differences in cortisol levels were detected as a function of time of collection (r = 0.03, p = 0.90), consistent with previous work demonstrating that evening cortisol is less likely to be impacted by small differences in collection time19,38. Children were allowed to be taking over-the-counter (OTC) medications including NSAIDs (e.g., ibuprofen) and/or allergy/cold medicine. The use of these medications was not associated with observed cortisol levels (r = −0.11, p = 0.40). Samples were assayed for cortisol in duplicate using a radioimmunoassay modified for saliva (Pantex, Santa Monica, CA). The detection limit of the assay (ED80) was 0.03 μg/dL, with mean inter- and intra-assay variations of 7.4% and 3.8% respectively. Participants were included if they provided sufficient, uncontaminated samples on at least 2 of 3 days. Mean levels were normalized via log10 transformation and residualized for time of collection and OTC medication usage4.
Current Cortisol, Anxiety, Depression, and Stress Measures
Adolescent basal cortisol collection followed similar procedures as in childhood, with participants collecting 3 days of samples at home near the date of the imaging visit. Average collection time in the current subsample was 5:21pm (SD = 72 min). Samples were assayed in duplicate using a high-sensitivity salivary enzyme immunoassay kit (Salimetrics, State College, PA) with calibrator range of 0.012 – 3.000 μg/dL and mean intra- and inter-assay coefficients of variation of 3.5% and 5.1% respectively. All other procedures mirrored those for the childhood cortisol.
Adolescent psychiatric symptoms were assessed via self-report with the adolescent version of the MacArthur Health and Behavior Questionnaire33 (HBQ), a well-validated measure of mental health, physical health, and social and academic functioning. HBQ subscales measuring symptoms of anxiety, depression, and externalizing behaviors (e.g., aggression, oppositional defiant disorder, conduct problems) were used in current analyses.
Lastly, current life stress was indexed using a 61-item life-events inventory modeled on the Adolescent Perceived Events Scale34 and the Life Events Survey35. Events covered age-appropriate life domains (e.g., relationships with friends/family, changes in parental marital status or finances, serious illnesses and deaths). The current analyses include the summed impact of negative events in the past 6 months.
Imaging Data Acquisition and Processing
Structural and functional images were collected on a 3T MRI scanner (Discovery MR750, General Electric Medical Systems, Milwaukee, WI, USA) with an 8-channel RF head coil array. T1-weighted structural images (1mm3 voxels) were acquired axially with an isotropic MPRAGE sequence (TE = 3.18 ms, TR = 8.13 ms, TI = 450, flip angle = 12 degrees). Participants were instructed to rest silently with their eyes closed while remaining “clear, calm, and awake” during the collection of a T2*-weighted gradient-echo echo-planar pulse sequence lasting 420 seconds (210 volumes) with a TE, TR, and flip angle of 25 ms, 2000 ms, and 60 degrees, respectively. Image volumes had a resolution of 3.5 × 3.5 × 5 mm3 (matrix size = 64 × 64, 30 sagittal slices).
Most data reduction steps were performed using AFNI software package39. Images were corrected for slice-dependent time shifts and motion and field-map corrected using FSL’s PRELUDE40 and in-house software. Anatomical images were aligned to the fifth volume of EPI time-series using a Local Pearson Correlation cost function41. The first four volumes of the time-series were removed due to T1-equillibrium effects. Images were then transformed to Talairach Atlas space31 using a 9-parameter affine transformation and resampled to 2 mm cubic voxels.
Resting-state fMRI time-courses were temporally filtered (band-pass: 0.001 Hz < 33< 0.01 Hz). To further reduce the influence of motion, time-points were censored if the motion of a point 87 mm from the center of rotation was >2 mm/degrees. Variance from sources of non-interest was then regressed (AFNI’s 3dDeconvolve function). Six rigid-body motion parameters were included as nuisance regressors, along with the voxel-wise average signal and derivatives from both eroded cerebral spinal fluid (CSF) and 2x eroded white matter (WM) masks. Masks were generated with an automated segmentation of the T1-weighted structural scan using FSL’s FAST routine40,42 and transformed to Talairach Atlas space31. EPI time-series were spatially-smoothed with a 6 mm full-width half-maximum (FWHM) Gaussian kernel (post-nuisance regression to avoid partial volume averaging within CSF and WM masks).
Statistical Analyses
FcMRI estimates were computed using a seed-region-based approach43. Binary masks of the left and right amygdala were defined in AFNI using previously-developed boundaries29. The average pre-processed fMRI signal intensity time-course over each amygdala ROI was then regressed against the signal intensity time-courses of all other voxels in the brain. Time points were motion censored as outlined above. The correlation coefficient of each voxel in the resultant statistical parametric maps was converted into a Z-score using the Fisher Z-transformation. Participant connectivity maps were then entered into two-tailed regressions (AFNI’s 3dttest++) while co-varying behavioral variables of interest. Cluster sizes were selected based on α significance values less than or equal to 0.05 and were estimated with AFNI’s 3dClustStim and 3dFWHMx.
In addition to the vmPFC, voxel-wise analyses with childhood evening cortisol revealed significant negative correlations with functional connectivity estimates between the left amygdala and clusters in the posterior cingulate and cerebellum (see Table 1 for complete list). Only the vmPFC-amygdala fcMRI estimates remained significant when the two individuals with the highest cortisol values were completely excluded from analyses (prior to winsorization), t(54) = −5.69, β = −0.62, R2 = 0.37, p < 0.001. To rule out the effects of subject motion, basal cortisol in adolescence, and current life stress, each of these variables were entered into the previous models as covariates. None contributed significant variance to the observed relations between childhood cortisol and amygdala-vmPFC connectivity, nor did they appreciably change these findings (individual β’s ranged from −0.014 to −0.066, NS).
Next, to determine whether the maternal stress composite was the best predictor of the observed fcMRI connectivity, as mediated by childhood cortisol, each maternal stress variable’s association with childhood cortisol and fcMRI was examined independently. No significant associations were detected among the variables of interest in males. In females, only the financial stress subscale was marginally correlated with both childhood cortisol, and significantly correlated with amygdala-vmPFC fcMRI, (see Supplementary Table 1).
Bivariate Pearson-r (two-tailed) correlations revealed significant comorbidity of anxiety and depression (females: r = 0.74, p < 0.001; males: r = 0.68, p < 0.001; total: r = 0.76, p < 0.001), as well as anxiety and externalizing (females: r = 0.57, p = 0.001; males: r = 0.56, p = 0.002; total: r = 0.49, p < 0.001), and depression and externalizing symptoms (females: r = 0.51, p = 0.005; males: r = 0.47, p = 0.01; total: r = 0.45, p = 0.001) in the current sample. To ensure specificity of symptom type, partial correlations were used which controlled for depression and externalizing problems when associations with anxiety were being examined, and anxiety and externalizing symptoms when associations with depression were being examined (see Table 3).
Three primary structural equation models (SEM) were constructed to test anticipated mediating and moderating associations using the software package Mplus36 (v. 5.2). SEM provides estimates, or path coefficients, which indicate the direction and significance of the association between constructs, as well as several fit indices which evaluate the fit of the proposed model. A Chi-squared significance test, considered good when non-significant, suggests the specified model is congruent with the observed data and is a reasonable measure of fit, particularly for models with small sample sizes44. The Root Mean Square Error of Approximation (RMSEA) is considered adequate below 0.10, but may be used with caution as a fit index in small sample sizes. The Standardized Root Mean Square Residual (SRMR) is the standardized difference between the observed correlation and the predicted correlation, and considered acceptable with values at 0.08 or less45. The Comparative Fit Index (CFI) considers the number of parameters, or paths, in the model and is considered good at 0.93 or above. While ideally models would have a sample size to number of free parameters ratio of 20 to 1, a more realistic ratio of 5 to 1 is acceptable46. To test for mediation in all three models, both direct and indirect effects were examined. To test for moderation, main and appropriate interactive effects of sex were included on all indirect pathways47.
The first model examined whether childhood cortisol mediated the association between ELS exposure and amygdala-vmPFC fcMRI and tested to see if both portions of the indirect pathway were moderated by gender. Additionally, adolescent current life stress and basal afternoon cortisol were included as predictors of fcMRI to ascertain whether the observed effects were indeed specific to ELS and/or childhood basal cortisol function. The second model tested whether adolescent amygdala-vmPFC fcMRI was a mediator in the association of childhood cortisol and adolescent anxiety symptoms. Again adolescent current life stress and basal cortisol were included as predictors as well as depression and externalizing symptoms. This model (Figure 3) accounted for 64.6% of the variance in adolescent anxiety symptoms, and demonstrated a marginally-significant mediating effect of adolescent amygdala-vmPFC fcMRI (Indirect Est = 0.13, p = 0.08). While this model revealed a non-significant moderating effect of gender on the association of fcMRI with anxiety (p = 0.23), removing this pathway resulted in poor model fit (Chi2 = 17.15, p < 0.05, RMSEA = 0.19, CFI = 0.84). Finally, because fcMRI estimates were obtained concurrently with anxious symptoms an additional SEM was constructed with the positions of anxiety symptoms and fcMRI reversed. The fit statistics of this model were poor (Chi2 = 17.09, p < 0.05, RMSEA = 0.19, CFI = 0.84), and there was no mediational effect (Indirect Est = −0.01, p = 0.56).
A third model, parallel to the model for adolescent anxiety, tested the same associations in relation to adolescent symptoms of depression. This model demonstrated good fit (Chi2 = 9.67, p > 0.05, RMSEA = 0.11, CFI = 0.94), however, gender did not moderate the path from fcMRI to depression (p = 0.34). When that pathway was omitted, the final model demonstrated improved fit (Chi2 = 7.56, p > 0.05, RMSEA = 0.07, CFI = 0.97) and accounted for 56.2% of the variance in depression (Figure 5). Further, as with anxiety, when depressive symptoms and amygdala-vmPFC were transposed in a subsequent model, the fit statistics were reduced to unacceptable levels (Chi2 = 25.44, p < 0.05, RMSEA = 0.25, CFI = 0.66) and there was no mediational effect (Indirect Est = 0.02, p = 0.61).
Supplementary Material
Acknowledgments
This work was supported by the NIH grants P50 MH084051, R01-MH044340, P50-MH052354, and P30-HD003352-46; the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development; and the HealthEmotions Research Institute, Department of Psychiatry, University of Wisconsin School of Medicine and Public Health. Support for PLR was provided by the Canadian Institutes for Health Research Post-doctoral Fellowship. We would like to thank Michael Anderle, Ron Fisher, Lisa Angelos, Courtney Hermes, Adam Koppenhaver, and Corinne Boldt for assistance with data collection and recruitment. We would also like to thank Daren Jackson, John Ollinger, Gregory Kirk, Nate Vack, John Koger, and Isa Dolski for general, technical, and administrative assistance.
Footnotes
Conflict of Interest?
None of the authors of this manuscript have any biomedical financial interests or potential conflicts of interest.
Author Contributions
CAB, DES, PLR, JMA, MJE, RJD, and RMB wrote and revised the manuscript. CAB, DES, PJR, EKM, NHK, MJE, and RMB performed data processing, statistical, and/or image analyses. MEF, ASH collected data, created Figures, and assisted with editing the manuscript. CAB, DES, PLR, JAO, MJE, RJD, RMB contributed to the interpretation of the data. MJE, RJD, and RMB supervised the project.
References
- 1.McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 2.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 3.Essex M, et al. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23:1039. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiat. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 5.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. PNAS. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cog Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharm. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neuro. 2000;20(12):4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Nat Reviews. 2009;10:434–445. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 11.Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Research. 2007;32(10):1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- 12.Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Hor & Beh. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MJ, et al. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen P. J Cer Cortex. 2011;21:1667. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veer IM, et al. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. PNEC. 2012;37:1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuro. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MJ, Whalen PJ. The structural integrity of an amygdala–prefrontal pathway predicts trait anxiety. J Neuro. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neuro. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruessner JC, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations-2008 Curt Richter Award Winner. PNEC. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Thomason ME, Hamilton JP, Gotlib IH. Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents. J Child Psychol Psychiat. 2011;52(10):1026–1034. doi: 10.1111/j.1469-7610.2011.02422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damosiseaux JS, Greicus MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 22.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12(5):445–450. doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakamata Y, et al. Gender difference in relationship between anxiety-related personality traits and cerebral brain glucose metabolism. Psychiat Res Neuro. 2009;172:206–211. doi: 10.1016/j.pscychresns.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Northoff G, Wiebking C, Feinberg T, Panksepp J. The ‘Resting-state Hypothesis’ of Major Depressive Disorder-A Translational Subcortical-Cortical Framework for a System Disorder. Neurosci Biobehav R. 2010;35(9):1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Sheline YI, et al. The default mode network and self-referential processes in depression. PNAS. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lui S, et al. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiat. 2011:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- 28.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Molec Bio. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beesdo K, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiat. 2009;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amunts K, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryo. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 31.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme; 1988. [Google Scholar]
- 32.Schreiber JE, et al. Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. PNEC. 2006;31:1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Essex MJ, et al. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. JAACAP. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Compas BE. Coping with stress during childhood and adolescence. Psychol Bull. 1987;101:393. [PubMed] [Google Scholar]
- 35.Sarason I, et al. Assessing the Impact of Life Changes: Development of the Life Experiences Survey. JCCP. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 36.Muthén LK, Muthén BO. Mplus User’s Guide. 5. Los Angeles, CA: 2008. [Google Scholar]
- 37.Murgatroyd C, Spengler D. Epigenetic programming of the HPA axis: early life decides. Stress: Intl J Bio Str. 2011;14(6):581–589. doi: 10.3109/10253890.2011.602146. [DOI] [PubMed] [Google Scholar]
- 38.Michels N, et al. Negative life events, emotions and psychological difficulties as determinants of salivary cortisol in Belgian primary school children. PNEC. 2012 doi: 10.1016/j.psyneuen.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 41.Saad ZS, et al. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. NeuroImage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. Med Imag, IEEE Trans. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 43.Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Mag Res Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 44.Barrett P. Structural equation modeling: adjudging model fit. Pers Indiv Dif. 2007;42:815–824. [Google Scholar]
- 45.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Str Eq Modeling. 1999;6(1):1–55. [Google Scholar]
- 46.Bentler PM, Chou CP. Practical issues in structural modeling. Soc Meth & Res. 1987;16:78–117. [Google Scholar]
- 47.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Beh Res Meth. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.