Abstract
Whole-cell patch-clamp recordings and high resolution morphometry were used to assess functional and structural properties of layer 3 pyramidal neurons in early (< 4 months) and advanced (> 8 months) stages of tauopathy in frontal cortical slices prepared from rTg4510 tau mutant (P301L) mice. In early tauopathy, dendritic architecture is preserved. In advanced tauopathy, neurons can be categorized as either “atrophic” (58%)- exhibiting marked atrophy of the apical tuft, or “intact” (42%)- with normal apical tufts and, in some instances, proliferative sprouting of oblique branches of the apical trunk. Approximately equal numbers of atrophic and intact neurons contain neurofibrillary tangles (NFTs) or are tangle-free, lending further support to the idea that NFTs per se are not toxic. Spine density is decreased due to a specific reduction in mushroom spines, but filopodia are increased in both atrophic and intact neurons. By contrast to these morphological changes, which are robust only in the advanced stage, significant electrophysiological changes are present in the early stage and persist in the advanced stage in both atrophic and intact neurons. The most marked of these changes are: a depolarized resting membrane potential, an increased depolarizing sag potential and increased action potential firing rates- all indicative of hyperexcitability. Spontaneous excitatory postsynaptic currents are not reduced in frequency or amplitude in either stage. The difference in the time course of functionally important electrophysiological changes versus regressive morphological changes implies differences in pathogenic mechanisms underlying functional and structural changes to neurons during progressive tauopathy.
Keywords: in vitro slice, whole-cell patch-clamp, dendrite, dendritic spine, excitability
INTRODUCTION
During the pathogenesis of neurodegenerative tauopathies, the microtubule-associated protein tau becomes hyperphosphorylated, disengages from microtubules and missorts from the axon to the somatodendritic compartment where it ultimately aggregates into a mature neurofibrillary tangle (NFT). NFTs were long believed to contribute to neuronal dysfunction and ultimately lead to neuron death in tauopathies such as Alzheimer’s disease (AD), in which the number of NFTs correlates with the degree of neuron loss and the severity of cognitive impairment [16,4,17]. However, there is now strong evidence that NFTs per se are not toxic, but rather that soluble hyperphosphorylated tau species, present before and during NFT aggregation, are the key pathogenic entities (review [50]). Evidence that soluble hyperphosphorylated tau species drive neuronal dysfunction and degeneration in tauopathy has been generated primarily from studies of the regulatable rTg4510 mouse model, which expresses mutant (P301L) human tau and closely recapitulates key characteristics of human tauopathy [15,47,49,19,25,5]. Early in the progression of tauopathy in these mice (< 4 months of age) many different soluble hyperphosphorylated tau species are present in the neocortex, but NFTs and neurodegeneration are largely absent. Soluble hyperphosphorylated tau species persist in advanced tauopathy (> 8 months of age), when NFTs are also prevalent and neuron loss in the frontal cortex is extensive [41,47,49]. We have recently shown that individual layer 3 frontal cortical pyramidal neurons from 9-month-old rTg4510 mice exhibit major structural and functional changes independent of the presence or absence of a mature NFT [9,43]. These changes include: loss of the apical dendritic tuft; ~30% reduction in dendritic spine density; increased density of axonal boutons; depolarized resting membrane potential; markedly increased depolarizing sag potential; increased action potential firing rates, and; increased frequency of low amplitude excitatory synaptic currents [43,9].
In the present study, we extended our analyses of layer 3 frontal cortical pyramidal neurons in rTg4510 mice to a younger “early stage tauopathy” cohort (1 to 3 months of age). In addition, by expanding both the number of neurons and the age of mice assessed in the “advanced stage tauopathy” cohort (9 to 13 months of age), we were able to differentiate morphologically distinct types of neurons -atrophic and intact- and to demonstrate that this distinction occurs independent of the presence or absence of a mature NFT. Neurons from rTg4510 mice treated with lifelong doxycycline to suppress expression of the mutant tau transgene exhibited functional and structural properties that did not differ from those of neurons from non-transgenic littermates. These studies revealed that significant electrophysiological changes occur in early tauopathy and persist in the advanced stage, while morphological changes are prominent only in the advanced stage. Thus, we provide evidence for a dissociation between functional and structural changes to individual neurons during progressive neurodegenerative tauopathy in this widely used mouse model.
MATERIALS AND METHODS
Animals
A total of 19 rTg4510 (TG) and 17 age-matched non-transgenic (NT) littermate controls (strain FVB/129) were used in this study. Two age cohorts were used: an “early” 1- to 3-month-old cohort (n: TG- 8, NT- 8 mice) and an “advanced” 9- to 13-month-old cohort (TG: 11 mice, NT: 9 mice). Mice treated to suppress the tau transgene were aged to 9 months (n: TG- 5, NT- 3 mice) and received doxycycline (dox) from conception and then first in drinking water and subsequently in chow containing 200mg/kg dox (Harlan Teklad, South Easton, MA) to the time of sacrifice. Mice were group housed in a pathogen-free barrier facility on a 12/12-hour light/dark cycle with unrestricted access to food and water. DNA extracted from tail samples was screened for both activator and responder transgenes as described using standard PCR methodology [41]. All experiments were approved by the Boston University Institutional Animal Care and Use Committee (IACUC) and were conducted in strict accordance with the animal care guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Preparation of slices
Mice were sacrificed by decapitation and their brains were extracted and placed in oxygenated (95% O2 and 5% CO2) ice-cold Ringer’s solution, concentrations in mM: 25 NaHCO3, 124 NaCl, 1 KCl, 2 KH2PO4, 10 Glucose, 2.5 CaCl2, and 1.3 MgCl2, (pH 7.4; Sigma-Aldrich, St. Louis, MO). After blocking, frontal cortical hemispheres were sectioned into 8 to 10 coronal sections (300 µm thick) with a vibrating microtome. Slices were maintained in oxygenated room temperature (RT) Ringer’s solution for equilibration for at least 1 hour. Following equilibration, individual slices were situated in submersion-type recording chambers (Harvard Apparatus, Holliston, MA) affixed to the stages of Nikon E600 infrared-differential interference contrast (IR-DIC) microscopes (Micro Video Instruments, Avon, MA) for whole-cell patch-clamp recordings. During recordings (~15 min in duration), slices were superfused with oxygenated RT Ringer’s solution at a rate of 2.5 ml/min.
Whole-cell patch-clamp recordings
Whole-cell patch-clamp recordings were made using patch electrode pipettes fabricated from non-heparinized microhematocrit capillary tubes (Thermo Fisher Scientific, Pittsburg, PA) on a Flaming and Brown horizontal pipette puller (Model P-87, Sutter Instrument, Novato, CA). All neurons from which recordings were obtained were layer 3 frontal (dorsal premotor) cortical pyramidal cells identified under IR-DIC optics as previously described [43,9]. Electrodes were filled with potassium methanesulfonate internal solution, concentrations in mM: 122 KCH3SO3, 2 MgCl2, 5 EGTA, and 10 NaHEPES containing 1% biocytin (pH 7.4; Sigma-Aldrich, St. Louis, MO) and had resistances from 4 to 6 MΩ when immersed in Ringer’s solution. EPC-9 and EPC-10 patch-clamp amplifiers and PatchMaster software were used for data acquisition (HEKA Elektronik, Lambrecht, Germany). Access resistance was monitored throughout the experiment and all recordings were low-pass filtered at 10 kHz.
Intrinsic membrane properties
Passive membrane and action potential (AP) firing properties were assessed in current-clamp mode as previously described [43]. Passive membrane properties were determined using voltage responses to a series of 200 ms hyperpolarizing and depolarizing current steps (10 pA steps, −40 to +40 pA; 12.5 kHz sampling frequency). Resting membrane potential (Vr) was measured as the membrane voltage in the absence of current injection. Responses to current steps were measured at steady state and plotted on a voltage-current plot, and input resistance (Rn) was calculated as the slope of the line of best fit through the linear portion of the graph. Membrane time constant was determined by fitting a single-exponential function to the membrane voltage response to the 10 pA hyperpolarizing current step. Rheobase (amount of current required to evoke the first AP) was assessed with a 10 s depolarizing current ramp (0 to 200 pA; 3.03 kHz sampling frequency). Single AP properties were measured on the first AP elicited in a 200 ms current-clamp series using an expanded timescale and the linear measurement tool in FitMaster analysis software (HEKA Elektronik). Single AP properties assessed included threshold and amplitude (voltage difference from threshold to peak). The event detection tool in FitMaster analysis software was used to evaluate the number of APs generated during a series of 2 s hyperpolarizing and depolarizing current steps (50 pA steps, −170 to +380 pA; 12.5 kHz sampling frequency) and AP firing rates were subsequently calculated. In most neurons, a slow depolarizing voltage sag potential (an H-current-mediated response) was evoked by strong hyperpolarizing current steps. This sag potential was eliminated by the H-current (HCN channel) blocker ZD-7288 (10 µM; Tocris Bioscience, Ellisville, MO) [60]. Depolarizing sag potential amplitudes were measured using the 2 s, −170 pA hyperpolarizing current step with the FitMaster linear measurement tool.
Spontaneous excitatory postsynaptic currents
AMPA receptor-mediated spontaneous excitatory postsynaptic currents (sEPSCs) were recorded from a holding potential of −80 mV for a period of 2 min (6.67 kHz sampling frequency). sEPSCs were unaffected by the NMDA-receptor antagonist APV or by the GABAA receptor antagonist BMI, but were fully blocked by application of the non-NMDA receptor antagonist CNQX. MiniAnalysis software was used to quantify synaptic current properties including frequency, amplitude, and kinetics (rise time constant and decay time constant). For assessment of kinetics, the rise and decay of averaged traces were each fit to a single exponential function. The MiniAnalysis detection threshold was set at the maximum of the RMS noise level (5 pA) for all synaptic current analyses.
Tissue processing
All neurons from which recordings were obtained were simultaneously filled with 1% biocytin in the internal solution (pH 7.4; Sigma-Aldrich, St. Louis, MO). Following recording, slices containing biocytin-filled neurons were fixed in 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS) solution (pH 7.4) for 4 days at 4°C. After three rinses in PBS (10 min each), slices were bathed in 0.1% Triton X-100/PBS for a period of 2 h at RT. For visualization and subsequent confocal laser scanning of neurons, slices were incubated in Streptavidin-Alexa 546 (1:500; Vector Labs, Burlingame, CA) for 2 days at 4°C. For detection of mature neurofibrillary tangles (NFTs), slices from both TG and NT mice were incubated in filtered 0.1% Thioflavin-S dissolved in PBS/10% ethanol solution (30 min). Prolong Gold mounting medium (InVitrogen, Eugene, OR) was used to mount stained slices on slides, which were then cover-slipped.
Confocal laser-scanning microscopy and image processing
A Zeiss LSM-510 confocal laser-scanning microscope outfitted with a 40× Plan Apochromatic 1.3 NA oil-immersion objective lens (210 µm working distance) was used to obtain confocal images for subsequent 3D neuron reconstructions and for assessment of Thioflavin-S staining. Completely filled neurons were imaged in their entirety at a resolution of 0.4 × 0.4 × 0.41 µm per voxel (0.7× digital zoom) and required acquisition of 2 to 4 z-stacks per neuron. To determine whether neurons contained a Thioflavin-S positive NFT, one z-stack was captured through each soma at a resolution of 0.05 × 0.05 × 0.5 µm per voxel (3.0× digital zoom). For spine density and subtype assessments, 100× (2.0× digital zoom) image stacks were acquired using a UPlan-FL 100×/1.3 NA oil-immersion objective lens (100 µm working distance) from one distal apical dendritic branch and from one basal dendritic branch at a resolution of 0.022 × 0.022 × 0.1 µm per voxel; 2 to 4 z-stacks were acquired per dendritic branch. Emissions from Streptavidin-Alexa 546 (Helium/Neon laser excitation) and Thioflavin-S (Argon laser excitation) were detected using two separate channels with filters set at 560 nm and 480 to 520 nm, respectively. To reduce z-plane signal blurring, all confocal z-stacks were subject to deconvolution using AutoQuant software (Media Cybernetics, Bethesda, MD). Following deconvolution, z-stacks obtained for each individual neuron were aligned in 3D and integrated into a single volumetric dataset using Volume Integration and Alignment System (VIAS) software [44]. The distance from the soma to the pial surface was measured for each neuron using the VIAS measure tool.
Morphometric analyses of dendrites and somata
For 3D reconstruction of individual neurons, integrated volumetric datasets obtained following VIAS z-stack stitching of 40× confocal images were imported into Neurolucida neuron reconstruction software (MBF Bioscience, Williston, VT). First, Neurolucida automatically traced the entire dendritic structure and soma of each neuron and then reconstructions were manually corrected. Reconstruction data were subsequently imported into NeuroExplorer software (MBF Bioscience). Total dendritic length and complexity (number of bifurcation nodes) were assessed for the apical and basal dendritic arbor of each neuron. To determine regional measures of dendritic length and complexity, a Sholl analysis [48] was also used to divide apical and basal dendritic arbors separately into proximal, middle and distal thirds (based on the total 3D extent of the arbor) using concentric rings originating from the center of each soma. The enclosed volume of each soma was also determined using NeuroExplorer software.
Morphometric analyses of dendritic spines
Integrated volumetric datasets of 100× confocal images were imported into the 64-bit version of NeuronStudio [57,46; available at: http://www.mssm.edu/cnic]. First, using a Rayburst-based algorithm analysis method, NeuronStudio automatically traced each dendritic segment to generate a dendritic .swc file. Then, spines were automatically detected and then manually corrected on dendritic .swc files [45]. To calculate spine density, the dendritic length and the number of spines for a given dendritic segment were extracted from .swc and .txt files respectively. Spine densities were determined for one complete distal apical dendritic branch and one complete basal dendritic branch per neuron. For spine subtyping analyses, spines on distal apical segments were classified as mushroom-, stubby-, thin- or filopodia-type spines as previously described [9].
Cell inclusion criteria
Neurons included in this study were required to meet both electrophysiological and morphological inclusion criteria. Electrophysiological criteria were: a resting membrane potential of ≤ −55 mV, stable access resistance (< 10% change over the course of the recording), and the ability to fire a train of APs during sustained depolarizing current injection. Morphological criteria were: a well-filled dendritic arbor with no cut dendrites in the distal apical dendritic arbor, an intact soma, a soma-to-pial surface distance between 220 and 550 µm, and, for TG neurons from 9- to 13-month-old mice, unequivocal Thioflavin-S labeling for classification of neurons as NFT+ or NFT−.
Additional considerations
It is important to note that we cannot rule out that brains of transgenic mice are inherently more susceptible to damage during slice preparation. However, TG neurons from the early tauopathy cohort were assessed at an age prior to neurodegeneration and widespread tau pathology in the frontal cortices of TG mice. Thus, we believe that it is unlikely that neurons from TG mice are less resistant to insult than neurons from NT mice at this age. While it is possible that TG neurons may become increasingly vulnerable with increasing age, the membrane potential is depolarized to the same level in TG neurons from younger relative to older mice, suggesting that this finding is indeed a real phenomenon that occurs independent of tissue damage. In addition, basic electrophysiological responses of TG neurons examined in the present study met criteria for “healthy neurons” and in most respects did not differ from NT neurons, exhibiting a regular spiking pattern, high amplitude APs, and an AP threshold close to −30 mV. Lastly, under IR-DIC visualization, somata of all neurons from which recordings were obtained appeared healthy, lacking signs of neuron death such as pyknotic nuclei.
Statistical analyses
Statistical analyses were performed using SPSS Statistics software version 19 (SPSS Inc., Chicago, IL). A k-means cluster analysis of distal apical dendritic length (followed by a one-way ANOVA) was used to verify morphologically distinct populations of neurons (atrophic vs. intact) from advanced-stage TG mice. A Multivariate ANOVA with group as the fixed factor was used to compare all morphological and electrophysiological parameters. Fisher’s Least Significant Difference (LSD) post hoc tests were performed to assess between-group differences. Student’s t-tests (two-tailed, equal variance not assumed) were used to compare means between groups where indicated. A Pearson Product-Moment correlation was used to determine relationships between variables. Cumulative histograms were compared using a Kolmogorov-Smirnov (K-S) test. The significance level was p ≤ 0.05 and all data are presented as the mean ± the standard error of the mean.
RESULTS
Classification of neurons from the two stages of tauopathy
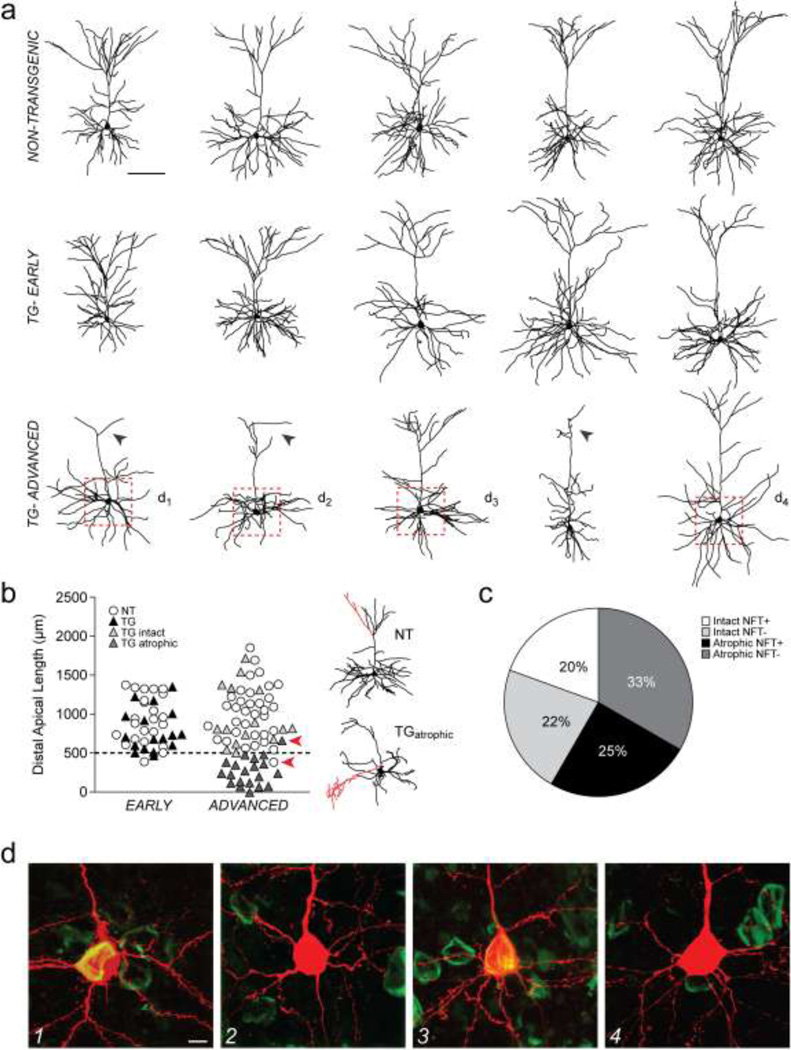
A total of 18 transgenic (TG) and 23 non-transgenic (NT) neurons in early tauopathy (1- to 3-month-old mice) and 36 TG and 34 NT neurons in advanced tauopathy (9- to 13-month-old mice) met both electrophysiological and morphological criteria for inclusion in the present study. Qualitative and quantitative approaches were combined to classify neurons from early and advanced-stage tauopathy. First, qualitative visual assessment of neuron reconstruction data revealed that while dendritic architecture was no different in TG compared to NT neurons in early tauopathy (Fig. 1a, top and middle rows), many TG (58%) neurons in advanced tauopathy exhibited marked regression of the apical dendritic tuft (Fig. 1a, bottom row, arrowheads). Then, to quantitatively distinguish distinct populations of TG neurons in advanced tauopathy, a cluster analysis was performed on total dendritic length within the distal third of the apical arbor (ANOVA, F(2,32) = 160.9, p < 0.001). Using this analysis, a 500 µm cutoff value was determined and hence the two populations of TG neurons in advanced tauopathy were: 1) neurons with intact apical dendritic tufts and distal apical lengths of > 500 µm, and; 2) neurons with dramatic regression of apical dendritic tufts and distal apical lengths of < 500 µm. These two populations are hereafter referred to as TGintact and TGatrophic respectively. A scatter plot of the distal apical dendritic lengths of neurons is shown in Figure 1b. The 500 µm cutoff value for distal apical length revealed discrete subpopulations of neurons within the advanced TG neuron group only (intact and atrophic); such subpopulations were not evident in the early TG, early NT, or advanced NT groups. When advanced NT and TG neurons were compared without clustering, significant differences between groups were found for each of the same parameters revealed by the cluster analysis (not shown).
Fig. 1. Classification of neurons using 3D reconstructions and Thioflavin-S staining.
a) Representative 3D reconstructions of layer 3 frontal cortical pyramidal neurons from NT (top row) and TG mice in early (middle row) and advanced (bottom row) stages of tauopathy. Arrowheads indicate atrophy of the apical tuft. Dashed boxes indicate regions from which confocal images in panel d were obtained. b) Left: scatter plot of the total lengths of the distal apical dendritic arbors of NT and TG neurons in early and advanced tauopathy. Arrowheads indicate distal apical values corresponding to neuron reconstructions shown on the right. Dashed line: 500 µm. Right: reconstructions of the NT and the TGatrophic neuron with distal apical values indicated by arrowheads in the scatter plot on the left. Dendrites highlighted in red indicate a small number of branches that extend several microns beyond the majority of branches in the distal apical tuft of the NT neuron, and an exuberant side branch of the apical dendritic trunk of the TGatrophic neuron; these branches contribute to the < 500 µm and the > 500 µm distal apical length values of these neurons respectively. Based on qualitative assessment of the apical tuft alone, the TGatrophic neuron was re-classified from the intact to the atrophic category. c) Pie chart showing relative proportions of TGintact and TGatrophic neurons that were either NFT+ or NFT−. d) Confocal images of the somata of TGintact and TGatrophic neurons indicated with dashed boxes in panel a. TG neurons either contained a Thioflavin-S positive NFT (d1,3) or were tangle-free (d2,4).
Scale bars: a, 100 µm; d, 5 µm; n: early- 23 NT, 18 TG neurons; advanced- 34 NT, 15 TGintact, 21 TGatrophic neurons.
Of TGintact neurons, 47% were Thioflavin-S positive (NFT+) and 53% were Thioflavin-S negative (NFT−), and of TGatrophic neurons, 43% were NFT+ and 57% were NFT−. Thus, approximately equal proportions of TGintact and TGatrophic neurons were NFT+ or NFT− indicating that atrophy of the apical tuft occurs in TG neurons regardless of the presence or absence of a mature NFT. A pie chart showing relative proportions of TG neurons in the advanced group that were NFT+ or NFT− and had either an intact or an atrophic apical dendritic tuft is shown in Figure 1c. Confocal images of the somata of representative neurons with and without NFTs are shown in Figure 1d.
Dendritic lengths and complexities are preserved in early tauopathy but are significantly altered in advanced tauopathy
Total and regional lengths and complexities of dendritic arbors were determined for each electrophysiologically characterized neuron. A multivariate ANOVA revealed significant differences between groups for all but four measures of dendritic morphology (the complexity of the middle third of the apical arbor; the length of the proximal third of the basal arbor, and; the length and complexity of distal third of the basal arbor). Significant differences in the apical dendritic arbor were: total and regional lengths (total: F(4,105) = 14.8, p < 0.001; proximal: F(4,105) = 7.0, p < 0.001; middle: F(4,105) = 3.2, p = 0.016; distal: F(4,105) = 18.4, p < 0.001) and, total complexity and complexity of the proximal and distal divisions (total: F(4,105) = 8.8, p < 0.001; proximal: F(4,105) = 4.7, p = 0.002; distal: F(4,105) = 9.9, p < 0.001). Significant differences in the basal dendritic arbor were: total length and length of the middle division (total: F(4,106) = 3.4, p = 0.012; middle: F(4,106) = 5.3, p = 0.001) and, total complexity and complexity of the proximal and middle divisions (total: F(4,106) = 8.3, p < 0.001; proximal: F(4,106) = 4.9, p = 0.001; middle: F(4,106) = 5.0, p = 0.001). Lastly, the volume of the soma did not differ significantly between groups (Table 1).
Table 1.
Morphological properties of neurons in early and advanced tauopathy
| EARLY | ADVANCED | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NT | TG | p value* | NT | TG intact | p value* | TG atrophic | p value* | ||
| Apical | |||||||||
| Total Length (µm) | 2757 ± 163 | 2716 ± 117 | ns | 2688 ± 122 | 3104 ± 301 | ns | 1425 ± 132 | < 0.001 | |
| Total Nodes (#) | 24.2 ± 1.5 | 21.5 ± 1.2 | ns | 23.6 ± 1.2 | 26.6 ± 3.1 | ns | 13.8 ± 1.4 | < 0.001 | |
| Basal | |||||||||
| Total Length (µm) | 2675 ± 108 | 2486 ± 240 | ns | 2781 ± 106 | 2764 ± 187 | ns | 2103 ± 185 | 0.001 | |
| Total Nodes (#) | 23.4 ± 1.1 | 17.8 ± 1.7 | 0.002 | 22.5 ± 0.8 | 17.1 ± 1.2 | 0.002 | 16.2 ± 1.5 | < 0.001 | |
| Soma | |||||||||
| Volume (µm3) | 1362 ± 122 | 1545 ± 230 | ns | 1595 ± 105 | 2055 ± 166 | ns | 1723 ± 175 | ns | |
Fisher’s LSD test; NT vs. TG
ns, not significant
n: early- 23 NT, 18 TG neurons; advanced- 34 NT, 15 TGintact, 21 TGatrophic neurons
Early-stage: TG versus NT neurons
Post hoc comparisons (Fisher’s LSD test) showed that in early tauopathy, the total lengths and complexities of the apical dendritic arbor were not significantly different between TG and NT neurons (Table 1). Similarly regional lengths (Fig. 2a, left) and complexities (not shown) did not differ. The total and regional lengths of the basal arbor were not significantly different between the two groups (Table 1; Fig. 2b, left). However, the total complexity of the basal arbor was significantly reduced (p = 0.002; Table 1) due to a decreased number of nodes in the proximal (p = 0.049) and middle (p = 0.006) divisions, but not the distal division in TG neurons (not shown). Thus, changes to dendritic architecture in early tauopathy were restricted to the basal dendritic arbor, which was less complex in TG than in NT neurons.
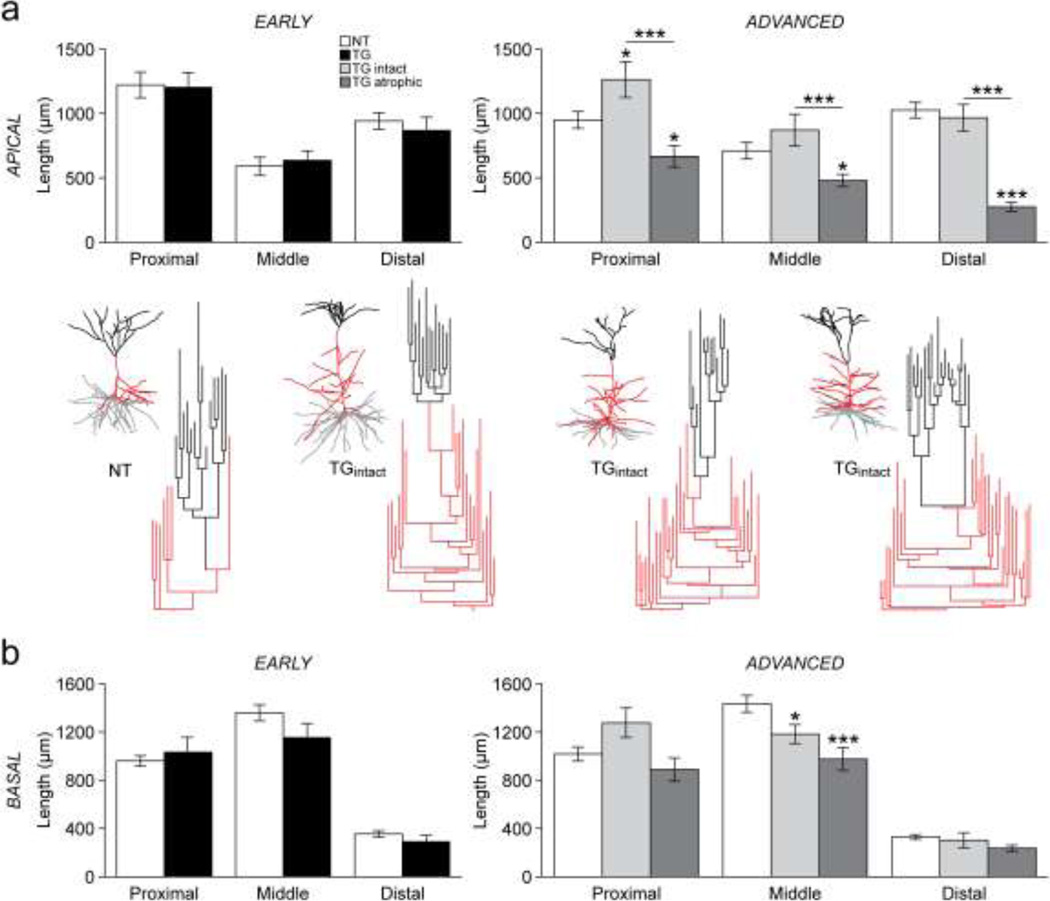
Fig. 2. Lengths of apical and basal dendritic arbors.
a) Top row: bar graphs of total dendritic length within proximal, middle and distal divisions of the apical arbors of NT and TG neurons in early (left) and advanced (right) tauopathy. Bottom row: reconstructions of entire dendritic arbors and dendrograms of apical dendritic arbors of a representative NT neuron and three TGintact neurons in advanced tauopathy. Basal dendritic arbors are indicated in grey, the apical trunks and oblique dendritic branches are indicated in red and apical dendritic tufts are indicated in black. b) Bar graphs of total dendritic length within proximal, middle and distal divisions of the basal dendritic arbors of TG and NT neurons in early (left) and advanced (right) tauopathy.
*p < 0.05; ***p < 0.001; Fisher’s LSD test. n: early- 23 NT, 18 TG neurons; advanced- 34 NT, 15 TGintact, 21 TGatrophic neurons.
Advanced-stage: TGintact versus NT neurons
In advanced tauopathy, the total length of the apical dendritic arbor was not significantly different between TGintact and NT neurons (Table 1). TGintact neurons showed an increase in length in proximal (p = 0.019) but not middle or distal divisions of the apical dendritic arbor compared to NT neurons (Fig. 2a, right). An unusually high number of oblique dendritic branches extending from the main apical trunk were apparent in a number of TGintact neurons (7/15 neurons). These branches are clearly demonstrated by the dendrograms shown in Figure 2a (bottom). Consistent with this observation, there was a significantly greater number of bifurcation nodes in the proximal division of the apical dendritic arbor (p = 0.039; not shown). However, the total complexity (Table 1) and the complexity of the middle and distal divisions of the apical dendritic arbor (not shown) were the same in TGintact and NT neurons. Basal dendritic length of TGintact neurons was significantly decreased in the middle division (p = 0.040; Fig. 2b, right); there were no additional differences in basal length between TGintact and NT neurons. However, the total complexity of the basal dendritic arbors of TGintact neurons was significantly reduced (p = 0.002; Table 1) due to fewer bifurcation nodes in the proximal (p = 0.044) and middle divisions (p = 0.007), but not the distal division (not shown). In summary, changes to dendritic arbors of TGintact neurons in advanced tauopathy occurred within relatively close proximity to the soma, with the most notable changes being increased length and complexity of the apical arbor and decreased complexity of the basal arbor.
Advanced-stage: TGatrophic versus NT neurons
Both apical and basal dendritic arbors showed marked regression in TGatrophic neurons. TGatrophic neurons exhibited a significant reduction in total apical length (p < 0.001; Table 1) as well as in all three divisions of the apical dendritic arbor (proximal: p = 0.020; middle: p = 0.019; distal: p < 0.001; Fig. 2a, right). Moreover, the overall complexity of the apical dendritic arbor was reduced in TGatrophic neurons (p < 0.001; Table 1) due to a decreased number of bifurcation nodes in the distal division (p < 0.001), but not the proximal or the middle division (not shown). Total basal dendritic length was significantly reduced in TGatrophic compared to NT neurons (p = 0.001; Table 1) due to a specific reduction in length in the middle division of the arbor (p < 0.001; Fig. 2b, right). TGatrophic neurons also had fewer bifurcation nodes in the proximal division of the basal dendritic arbor (p < 0.001; not shown) that contributed to a significant reduction in total basal dendritic complexity (p < 0.001; Table 1).
Advanced-stage: TGintact versus TGatrophic neurons
The total and regional lengths (total: p < 0.001; proximal: p < 0.001; middle: p = 0.001; distal: p < 0.001) and complexities (total: p < 0.001; proximal: p = 0.001; distal: p < 0.001) of apical dendritic arbors were significantly greater in TGintact versus TGatrophic neurons. Moreover, the total length of the basal arbor was greater in TGintact versus TGatrophic neurons (p = 0.008). Both total and regional complexity of basal dendritic arbors was the same in TGintact and TGatrophic neurons. Thus, while the length of the basal arbor was relatively more preserved in TGintact neurons, both groups of TG neurons in advanced tauopathy exhibit similar reductions in basal complexity.
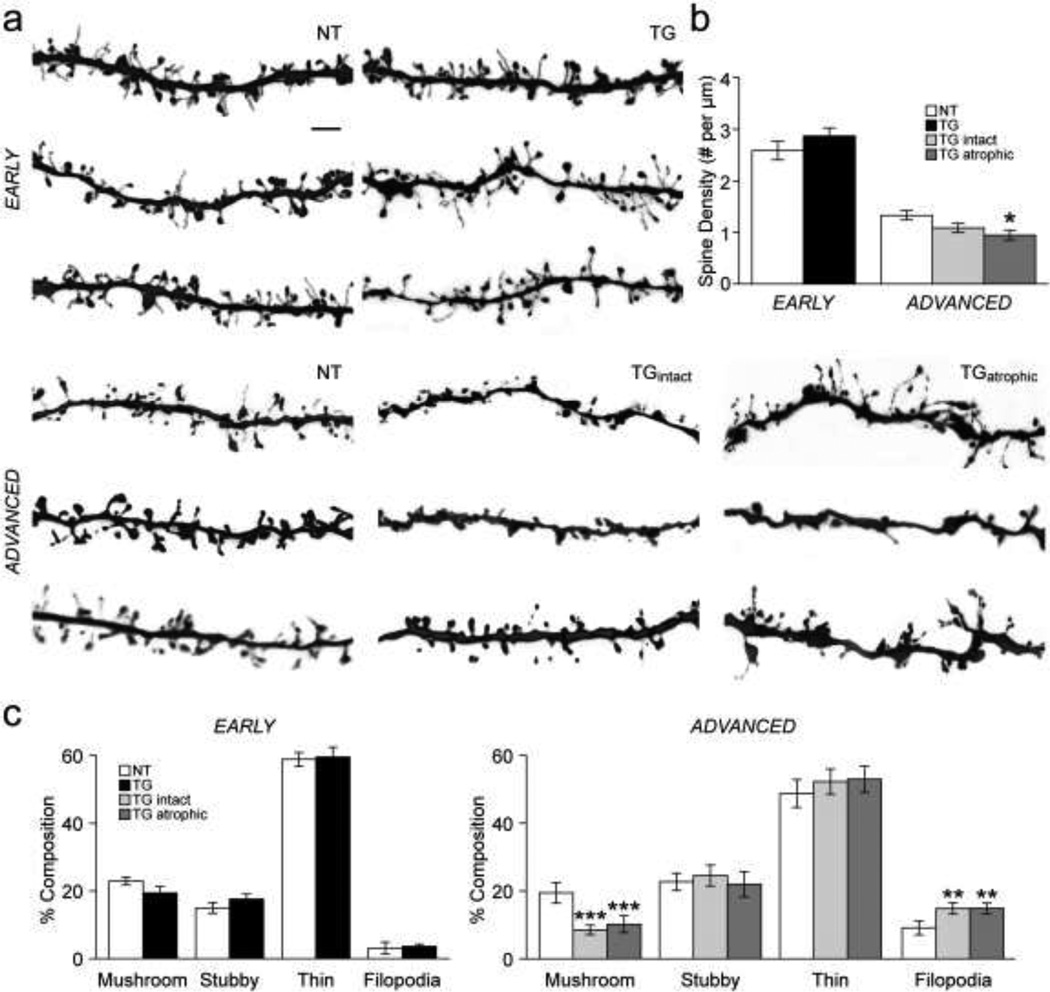
Spine density is unchanged in early tauopathy but is reduced in advanced tauopathy
Representative examples of apical dendritic segments imaged at 100× are shown in Figure 3a. Spine density was the same in apical and basal branches (data not shown) and thus these data were combined for each neuron. There was an overall difference in spine density between groups (F(4,77) = 57.7, p < 0.001). In early tauopathy, spine density was not significantly different in TG compared to NT neurons (Fig. 3a,b). In advanced tauopathy, spine density was significantly reduced in TGatrophic relative to NT neurons (p = 0.016; Fig. 3a,b). While spine density was lower in TGintact compared to NT neurons, this did not reach the significance level (TGintact vs. NT, p = 0.119); TGintact versus TGatrophic did not differ with regard to spine density (Fig. 3a,b). There was an overall difference in the relative proportion (percentage of total spines) of mushroom- (F(4,27) = 9.2, p < 0.001) and filopodia- (F(4,27) = 13.7, p < 0.001) type spines, but not of stubby- or thin-type spines along distal apical dendrites between groups. The relative proportion of mushroom- and filopodia-type spines was no different in TG versus NT neurons in early-stage tauopathy (Fig. 3c, left). However, in the advanced stage, the relative proportion of mushroom-type spines was significantly reduced (TGintact vs. NT, p = 0.001; TGatrophic vs. NT, p = 0.003), while the relative proportion of filopodia was significantly increased in TGintact and TGatrophic neurons relative to NT neurons (TGintact vs. NT, p = 0.011; TGatrophic vs. NT, p = 0.010); TGintact and TGatrophic neurons did not significantly differ with regard to relative proportions of spine subtypes (Fig. 3c, right).
Fig. 3. Dendritic spine density.
a) 100× images of apical dendritic segments typical of those used for assessment of spines. b) Bar graph of mean spine density in NT and TG neurons in early and advanced tauopathy. c) Bar graphs of mean percent composition of spine subtypes in apical dendritic branches of NT and TG neurons in early and advanced tauopathy.
*p < 0.05; **p < 0.01; ***p < 0.001; Fisher’s LSD test; scale bar: 3 µm; spine density, n: early- 12 NT, 16 TG branches; advanced- 18 NT, 18 TGintact, 18 TGatrophic branches; percent composition, n: early- 5 NT, 6 TG branches; advanced- 7 NT, 7 TGintact, 7 TGatrophic branches.
Passive properties are altered in both early and advanced tauopathy
Mean electrophysiological data are presented in Table 2. A multivariate ANOVA demonstrated a significant difference in resting membrane potential (Vr; F(4,97) = 14.0, p < 0.001) and input resistance (Rn; F(4,97) = 5.9, p < 0.001), but not in membrane time constant between groups. Importantly, TG neurons in both early and advanced tauopathy had a significantly depolarized mean resting membrane potential compared to NT neurons (early: TG vs. NT, p = 0.003; advanced: TGintact vs. NT, p < 0.001; TGatrophic vs. NT: p < 0.001). In early tauopathy, TG neurons had significantly higher Rn than did NT neurons (p = 0.018). Similarly in advanced tauopathy, TGatrophic neurons had a significantly higher Rn compared to both NT (p < 0.001) and TGintact neurons (p = 0.002); Rn was not different between TGintact and NT neurons in this stage.
Table 2.
Electrophysiological properties of neurons in early and advanced tauopathy
| EARLY | ADVANCED | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NT | TG | p value * | NT | TG intact | p value* | TG atrophic | p value* | ||
| Passive | |||||||||
| Resting Potential (mV) | −75.5 ± 1.8 | −68.1 ± 1.2 | 0.003 | −75.7 ± 1.0 | −64.7 ± 2.0 | < 0.001 | −66.0 ± 1.4 | < 0.001 | |
| Input Resistance (MΩ) | 192 ± 18 | 273 ± 25.0 | 0.018 | 177 ± 18 | 171 ± 30 | ns | 298 ± 34 | < 0.001 | |
| Time Constant (ms) | 37.5 ± 3.6 | 41.5 ± 4.2 | ns | 30.4 ± 3.2 | 35.6 ± 4.1 | ns | 36.3 ± 3.8 | ns | |
| Action Potential | |||||||||
| Rheobase (pA) | 143 ± 16 | 94.8 ± 14.6 | 0.010 | 114 ± 14 | 79.7 ± 10.4 | ns | 59.8 ± 7.8 | 0.002 | |
| Threshold (mV) | −34.1 ± 1.5 | −32.2 ± 1.2 | ns | −35.4 ± 0.7 | −35.2 ± 3.1 | ns | −33.8 ± 0.8 | ns | |
| Amplitude (mV) | 84.7 ± 1.5 | 80.4 ± 1.5 | ns | 83.4 ± 1.2 | 85.0 ± 1.7 | ns | 75.2 ± 5.0 | 0.020 | |
| Sag Amplitude (mV) | 2.2 ± 0.7 | 4.8 ± 0.7 | 0.005 | 1.8 ± 0.4 | 6.2 ± 0.8 | < 0.001 | 6.1 ± 0.8 | < 0.001 | |
| sEPSC | |||||||||
| Frequency (Hz) | 5.1 ± 0.5 | 6.1 ± 1.2 | ns | 6.3 ± 0.5 | 7.6 ± 1.3 | ns | 6.7 ± 0.8 | ns | |
| Amplitude (pA) | 11.8 ± 0.8 | 10.5 ± 1.0 | ns | 12.9 ± 0.6 | 15.3 ± 1.6 | 0.043 | 11.4 ± 0.4 | ns | |
| Rise Time (ms) | 1.7 ± 0.1 | 1.4 ± 0.1 | ns | 1.3 ± 0.1 | 1.3 ± 0.1 | ns | 1.4 ± 0.1 | ns | |
| Decay Time (ms) | 7.8 ± 0.6 | 7.7 ± 0.5 | ns | 6.7 ± 0.4 | 7.5 ± 1.2 | ns | 6.6 ± 0.4 | ns | |
Fisher’s LSD test; NT vs. TG
ns, not significant
n: early- 23 NT, 18 TG neurons; advanced- 34 NT, 15 TGintact, 21 TGatrophic neurons
Increased excitability in early and advanced tauopathy
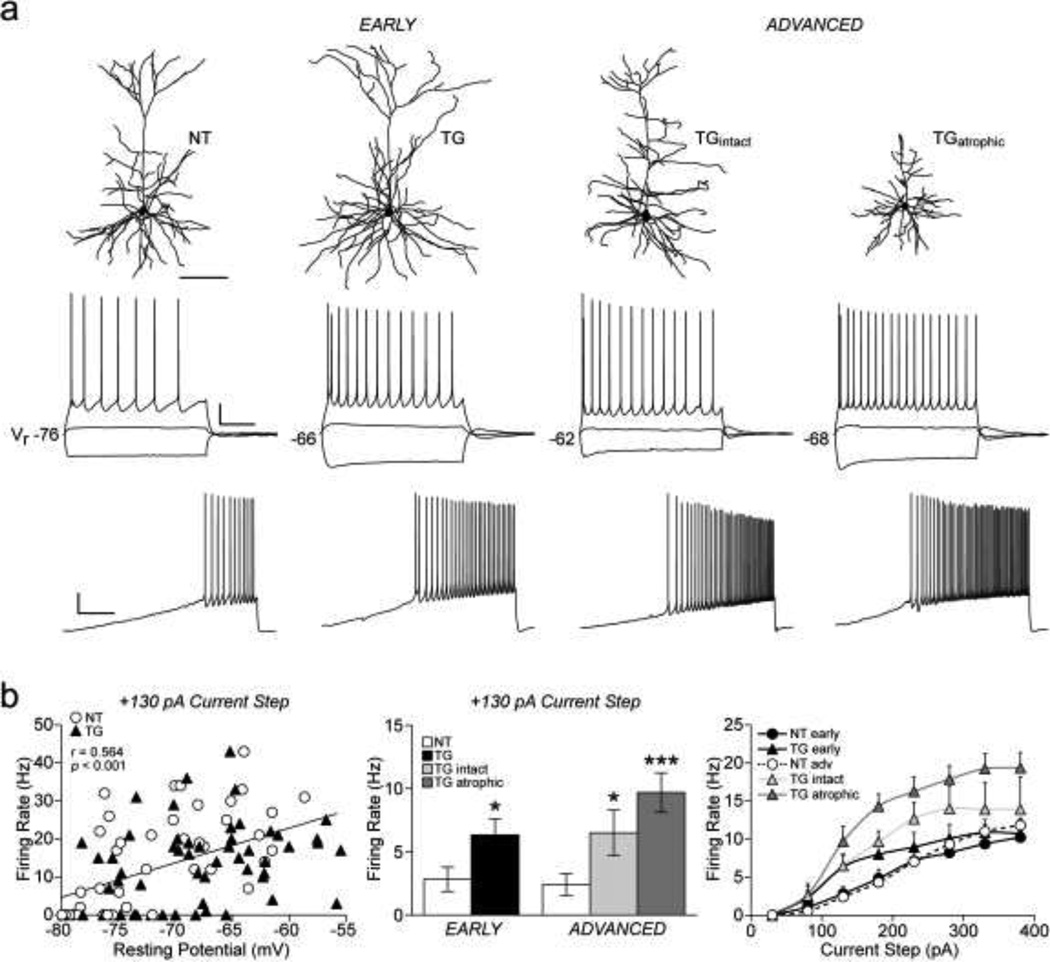
A multivariate ANOVA demonstrated that action potential (AP) amplitude (F(4, 83) = 2.5, p < 0.048) was significantly different between groups while AP threshold was the same. While AP amplitude did not differ between groups in early tauopathy, AP amplitude was significantly lower in TGatrophic compared to both NT (p = 0.020) and TGintact neurons (p = 0.013, Table 2). TGintact and NT neurons did not significantly differ with regard to AP amplitude. Exemplar traces of repetitive AP firing elicited by a 2 s depolarizing current step and a current ramp protocol are shown in Figure 4a (middle and bottom respectively). The significant positive linear relationship between AP firing rate (+130 pA current step) and Vr across all neurons (Pearson Product-Moment correlation; r (109) = 0.564, p < 0.001) is shown in Figure 4b (left). Graphs of mean AP firing rates at the +130 pA step and at each depolarizing current step are shown in Figure 4b (middle and right respectively). Firing rates differed between groups at depolarizing current steps greater than +80 pA (+130 to +380 pA; F(4,68) = 5.7, 6.4, 5.2, 5.5, 5.6, 5.1, p < 0.001). In early tauopathy, TG neurons had a significantly higher AP firing rate relative to NT neurons in response to the +130 pA depolarizing current step (p = 0.032). In advanced tauopathy, TGintact neurons exhibited increased firing rates at every step from +130 to +230 pA (p = 0.045, 0.033, 0.032) and TGatrophic neurons had an increased firing rate every step from +130 to +380 pA (p < 0.001 for each step).
Fig. 4. Action potential firing properties.
a) Top row: reconstructions of neurons from which recordings shown in bottom rows were obtained. Middle row: membrane voltage responses evoked by a family of 2 s current pulses (−170, +30 and +130 pA) in a representative NT neuron and in TG neurons from early and advanced tauopathy. TG neurons in both stages of tauopathy have depolarized resting membrane potentials (Vr) and higher firing rates. Bottom row: Repetitive action potential firing elicited by a 10 s depolarizing current ramp from 0 to 200 pA. b) Relationship of firing rate (+130 pA current step) to resting membrane potential for all NT and TG neurons; linear regression (black line) demonstrates a significant positive correlation (left). Graph of mean firing rates in response to a +130 pA current step (middle) and in response to a series of depolarizing current steps (right).
*p < 0.05; ***p < 0.001; Fisher’s LSD test; scale bars: a, top row- 100 µm; middle row- 20 mV/500 ms; bottom row- 20 mV/2 s; n: early- 22 NT, 18 TG neurons; advanced- 32 NT, 11 TGintact, 19 TGatrophic neurons.
Rheobase, the amount of current required to elicit an AP, was significantly different between groups (F(4, 83) = 6.2, p < 0.001). In early tauopathy, rheobase was significantly lower in TG relative to NT neurons (p = 0.010; Fig. 4a, bottom; Table 2). In advanced tauopathy, rheobase was significantly lower in TGatrophic versus NT neurons (p = 0.002; Fig. 4a, bottom; Table 2). While rheobase was also lower in TGatrophic compared to NT neurons, this did not reach statistical significance (p = 0.067; Fig. 4a, bottom; Table 2); TGintact and TGatrophic neurons did not significantly differ from one another with regard to rheobase.
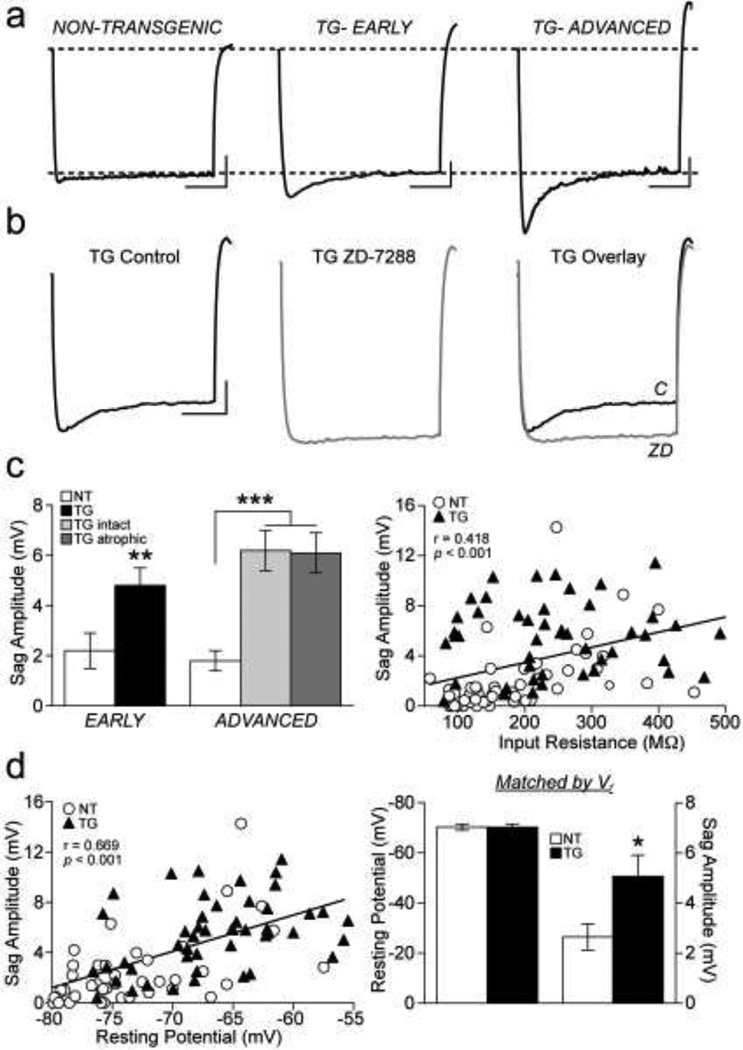
Depolarizing sag potential amplitude was significantly different between groups (F(4, 97) = 11.4, p < 0.001). This sag potential was significantly larger in amplitude in TG neurons in both early (TG vs. NT, p = 0.005) and advanced tauopathy groups (TGintact vs. NT, p < 0.001; TGatrophic vs. NT, p < 0.001; Fig. 5a; Fig. 5c, left) and was eliminated by HCN channel blocker ZD-7288 (Fig. 5b). There was a significant positive linear relationship between sag potential amplitude and Rn across all neurons (Pearson Product-Moment correlation; r (99) = 0.418, p < 0.001; Fig. 5c, right). There was also a significant positive linear relationship between sag potential amplitude and Vr across all neurons (Pearson Product-Moment correlation; r (101) = 0.669, p < 0.001; Fig. 5d, left). To determine if the greater sag potential amplitude was dependent upon the significantly depolarized Vr exhibited by TG neurons, sag potential amplitude was compared between TG and NT neurons that had the same Vr. When TG and NT neurons were matched by Vr,, the sag potential amplitude was still significantly greater in TG neurons (Student’s t-test, p = 0.016; Fig. 5d, right). Thus the difference in sag potential amplitude was not dependent on the difference in Vr.
Fig 5. Depolarizing sag potential properties.
a) Depolarizing sag potentials evoked by a −170 pA current pulse expanded to demonstrate differences in amplitude in NT (left) versus TG neurons from early (middle) and advanced (right) tauopathy. Dashed lines: baseline membrane potential (top) and membrane steady state level (bottom). b) Depolarizing sag potential in a representative TG neuron under control conditions (left) and in the presence of HCN channel blocker ZD-7288 (middle). Superimposed traces of the sag potential under control conditions (C) and following ZD-7288 (ZD) block (right). c) Graph of mean sag potential amplitudes of NT and TG neurons in early and advanced tauopathy (left). Relationship of sag amplitude to input resistance for all NT and TG neurons; linear regression (black line) demonstrates a significant positive correlation (right). d) Relationship of sag amplitude to resting potential for all NT and TG neurons; linear regression (black line) demonstrates a significant positive correlation (left). Graph of mean membrane potential and sag potential amplitudes of NT and TG neurons with the same resting membrane potential (right).
*p < 0.05; **p < 0.01; ***p < 0.001; Fisher’s LSD test (panel c, left) and Student’s t-test (panel d, right); scale bars: 5 mV/500 ms; n: early- 22 NT, 18 TG neurons; advanced- 32 NT, 11 TGintact, 19 TGatrophic neurons (panel c, left and right; panel d, left); n: NT- 21, TG- 20 neurons (panel d, right).
Changes in the amplitude distributions of spontaneous excitatory postsynaptic currents in early and advanced tauopathy
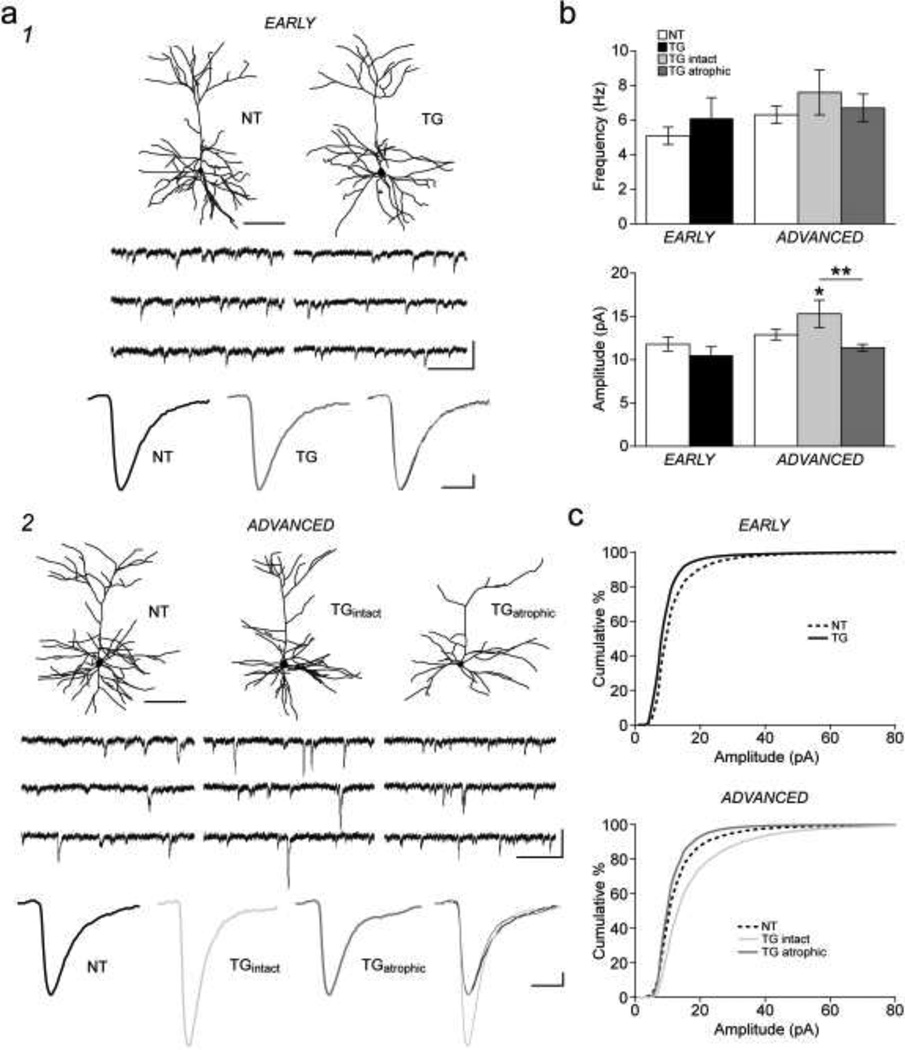
Electrophysiological traces of sEPSCs from representative neurons in early and advanced tauopathy are shown in Figure 6a1,2. The mean frequency of sEPSCs did not differ between groups at either the early or the advanced stage (Fig. 6b, Table 2). The rise and decay time constants of sEPSCs also did not significantly differ as a function of group (Fig. 6a1,2, Table 2). However, the mean sEPSC amplitude was significantly different between groups (F(4,93) = 3.8, p = 0.007). In early tauopathy, the mean sEPSC amplitude was the same in TG and NT neurons (Fig. 6b, Table 2). In advanced tauopathy, the mean amplitude of sEPSCs was significantly higher in TGintact neurons relative to both NT (p = 0.043) and TGatrophic neurons (p = 0.003; Fig. 6b, Table 2). On the other hand, TGatrophic and NT neurons did not significantly differ from one another with regard to mean sEPSC amplitude. Cumulative percentile histograms revealed an increased proportion of small amplitude events in TG relative to NT neurons in early tauopathy (K-S test, p < 0.001; Fig. 6c) and in TGatrophic relative to NT neurons in advanced tauopathy (p = 0.027; Fig. 6c). Conversely, TGintact neurons had an increased number of large amplitude events compared to NT (p = 0.013) and TGatrophic neurons (p < 0.001; Fig. 6c).
Fig. 6. Spontaneous excitatory postsynaptic currents.
a) (1) Top row: reconstructions of representative NT and TG neurons in early tauopathy from which sEPSC recordings (middle row) were obtained. Bottom row: averaged traces of sEPSCs typical of those used to assess current kinetics. Superimposed averaged traces (right) from the NT and TG neurons. (2) Top row: reconstructions of representative NT and TG neurons in advanced tauopathy from which sEPSC recordings (middle row) were obtained. Bottom row: averaged traces of sEPSCs typical of those used to assess current kinetics. Superimposed averaged traces (right) from the NT, TGintact and TGatrophic neurons. b) Bar graphs of mean frequency (top) and mean amplitude (bottom) of sEPSCs from NT and TG neurons in early and advanced tauopathy. c) Cumulative distribution histograms (1 pA bins) of sEPSC amplitudes from NT and TG neurons in early tauopathy (top) and advanced tauopathy (bottom).
*p < 0.05; **p < 0.01; Fisher’s LSD test; scale bars: a1,2, top row- 100 µm; middle row- 20 pA/250 ms; bottom row- 1 pA/10 ms; n: early- 20 NT, 15 TG neurons; advanced- 31 NT, 13 TGintact, 19 TGatrophic neurons.
Within genotype comparisons of parameters across the two age groups
Within genotype cross-age comparisons of structural and functional parameters are shown in Supplementary Tables 1 and 2. Notably, since differences in morphological and electrophysiological properties between early and advanced NT neurons were minor, most differences between early and advanced TG neurons were dependent upon the effects of tauopathy rather than development. However, spine density was significantly higher in early compared advanced NT neurons. When spine density of each TG group was expressed as a percentage of the corresponding NT mean value (thereby normalizing for differences due to development), early TG neurons still had a significantly greater spine density than advanced TG neurons (Student’s t-test, p < 0.001; data not shown) indicating that spine density did not decrease as a function of development alone, but more likely decreased as a function of tauopathy.
Suppression of the human mutant tau transgene prevents major structural and functional changes to TG neurons
To determine if structural and functional changes to neurons were dependent on expression of high levels of human mutant tau, electrophysiological and morphological properties of neurons from TG and NT mice that received lifelong dox treatment were examined (data not shown). These two groups of neurons were designated TGdox and NTdox respectively. Apical dendritic tufts of each well-filled TGdox neuron that met morphological (but not electrophysiological) inclusion criteria (n: 21 neurons) were qualitatively the same as those of normal, healthy NT neurons. A total of 9 TGdox and 6 NTdox neurons met both morphological and electrophysiological inclusion criteria and were further assessed with regard to detailed properties. Neuron reconstruction data confirmed that each TGdox neuron had an intact distal apical dendritic arbor (> 500 µm in length). Moreover, assessment of spine density in distal apical dendritic branches of TGdox and NTdox neurons revealed that spine density was not significantly different between the two groups. Finally, functional electrophysiological properties were indistinguishable between TGdox and NTdox neurons. In summary, the major structural and functional changes to TG neurons we observed were dependent on high levels of human mutant tau expression.
DISCUSSION
In this study, we assessed structural and functional changes to layer 3 frontal cortical pyramidal neurons from rTg4510 mice in early and advanced stages of tauopathy. The key findings are that: 1) apical dendritic tufts are unchanged in early tauopathy, but are either preserved (TGintact) or severely atrophic (TGatrophic) in advanced tauopathy; 2) Thioflavin-S positive NFTs are present (or are absent) in approximately equal proportions in TGintact and TGatrophic neurons; 3) some TGintact neurons exhibit proliferative sprouting of oblique branches of the apical trunk; 4) spine density is decreased due to a specific loss of mushroom spines, but filopodia are increased in advanced tauopathy; 5) TG neurons exhibit stage-independent depolarized resting membrane potentials, increased depolarizing sag potentials, and increased action potential firing rates; 6) the mean amplitude and the proportion of high amplitude sEPSCs is increased in TGintact neurons, while the proportion of low amplitude sEPSCs is greater in both early TG and TGatrophic neurons, and; 7) suppression of the human mutant tau transgene with lifelong dox treatment prevents structural and functional changes to TG neurons.
Dendritic regression
No major changes in dendritic morphology were observed at an early stage of tauopathy. This is consistent with observations in hippocampal neurons from early-stage tau-P301L mice [6] and in frontal cortical neurons from early-stage htau mice [10]. However, AAV-tauP301L infection in the hippocampus of mice [23] and overexpression of human tau in the lamprey neurons [29] result in early dendritic regression. Further, in early AD, dendritic regression precedes substantial tangle formation and neurodegeneration in the hippocampus [7]. Differences in experimental models, types and/or levels of tau species present or, in the case of AD, the potentially confounding interactions between tau- and amyloid-beta-mediated pathology may account for the apparent discrepancies between these findings.
By comparison to early tauopathy, changes to the dendritic morphology of many TG neurons in advanced tauopathy were dramatic. At this stage, TGatrophic neurons exhibited significant regression of both basal and apical dendritic arbors; the apical dendritic tuft was reduced to only two or three branches or was completely lost. These degenerative changes are reminiscent of pronounced regression and dystrophy of distal apical dendrites and loss of basal dendrites of hippocampal neurons in AD [33,7]. Jaworski and coworkers [23] reported dystrophy and loss of apical dendrites of hippocampal neurons from AAV-tauP301L-infected mice that were particularly pronounced distally. Similarly, successive tau-mediated loss of microtubules and subsequent dendritic degeneration of first distal and then more proximal dendrites was observed in lamprey neurons [29]. In both of these models of tauopathy, as with rTg4510 mice, particularly high levels of tau may substantially contribute to the observed dendritic degeneration.
What causes dendritic degeneration during tau pathology? Since in healthy neurons tau binds to and promotes the stabilization and assembly of microtubule primarily in the axon, it is unlikely that tau loss-of-function is exclusively responsible for dendritic degeneration in tauopathies. It is plausible that a gain-of-function for pathological tau may lead to dendritic retraction by indirectly contributing to microtubule destabilization in dendrites. Loss of microtubules occurs in dendrites into which tau is missorted, suggesting a mechanistic link between the presence of abnormal tau species and degenerative changes to dendrites [59,29]. Indeed, previous studies have shown that hyperphosphorylated tau sequesters endogenous tau and other microtubule-binding proteins, including those responsible for dendritic microtubule stabilization [21,1,53]. Microtubule destabilization alone would be expected to lead to perturbations in intracellular microtubule-dependent transport, but there is evidence that abnormal tau may cause transport deficits by other mechanisms as well. These proposed mechanisms have been generated in large part from studies focusing on transport deficits in axons during tau pathology. However, many of these mechanisms may also underlie impaired transport in dendrites, as has been shown to occur in tau-transfected hippocampal neurons [56], and has been proposed as a principal underlying cause for early synaptic dysfunction in rTg4510 mice [19]. For example, there is evidence that excess tau binding to microtubules displaces motor proteins [11] and may prevent access of motors to microtubules [52,56]. In addition, high levels of tau may cause microtubule bundling leading to transport blockage [56]. Given that tau itself is transported as a kinesin cargo, increased levels of tau may lead to increased competition between tau and other kinesinbinding cargoes [11]. Pathological tau may also play a role in preventing the successful docking of cargoes to molecular motors [22]. Thus, as a direct consequence of impaired transport, dendrites (as well as axons) are likely deprived of proteins, vesicles and organelles that support maintenance of their structural integrity. Lastly, given that ~50% of neurons are lost in 9-month-old rTg4510 mice [49], surviving neurons examined in the present study are likely significantly deafferented; this loss of presynaptic inputs may exacerbate dendritic regression [14,24].
Dendritic proliferation
One of the most intriguing findings of this study was that almost half of the advanced-stage TG neurons examined possessed intact dendritic arbors. It is theoretically possible that these TGintact neurons do not express the mutant (P301L) tau transgene or contain hyperphosphorylated tau, however the latter is unlikely given that they commonly contained NFTs. Future studies will attempt to identify the mechanisms underlying selective vulnerability or preservation of TG neurons in progressive tauopathy. In addition to preservation of the apical tuft, half of the TGintact neurons demonstrated proliferative sprouting of oblique apical dendritic branches. A similar phenomenon was previously reported for layer 3 frontal cortical neurons from htau mice that show increased proximal apical dendritic length and complexity [10]. The dendritic proliferation seen in some neurons in these mouse models of tauopathy are reminiscent of those that have been described in normal human aging [8,13] and in a variety of neuropathological conditions including AD (review [55]), Huntington’s disease [12], and epilepsy [34]. Dendritic sprouting in tauopathies could plausibly be triggered by deafferentation [20,54] or by reactivation of developmentally regulated programs (review [3]).
Spine density and subtypes
There is an extensive literature demonstrating alterations in dendritic spines in neurodegenerative disease (review [38,31]). In the present study we found that in early tauopathy, spine density was not different in TG compared to NT neurons. This finding is consistent with a previous study showing that postsynaptic density (PSD) 95 levels are the same in PSD fractions of forebrain lysates prepared from young (4.5-month-old) rTg4510 relative to control mice [19]. Further, densities of spines are not different in PSD95-labeled primary hippocampal neurons from rTg4510 mice or in DsRed-labeled rat primary hippocampal neurons transfected with P301L human mutant tau compared to control neurons [19]. In addition, a recent study by Kremer and colleagues [26] showed that spine density is not significantly different in the cortex of young (1- to 2-month-old) Tau.P301L mice, but is increased at a later age (4- to 6-month-old) versus age-matched control mice. Taken together, these findings provide supporting evidence that spine density is not reduced in these tau P301L mutant mice at relatively early stages of disease pathogenesis. Our spine classification analyses revealed that the relative proportions of mushroom-, stubby-, thin-, and filopodia-type spines were the same in TG versus NT neurons in early-stage tauopathy, although subtle changes in spine morphology (such as head diameter or neck length) have been reported in early stage Tau.P301L mouse neurons [26]. In advanced tauopathy, a ~20–30% reduction in dendritic spine density occurred in TG relative to NT neurons [reported here and in: 43,9], and to a greater extent in the TGatrophic than in the TGintact neurons. Spine loss in surviving neurons in advanced tauopathy may be due to a combination of tau-mediated deficits in the transport of synaptic cargoes to pre- and postsynaptic targets and loss of afferent inputs due to substantial neuronal loss. Here and in previous work [9], we found that the relative proportion of mushroom spines was reduced, while the relative proportion of filopodia is increased in advanced TG neurons. In the present study, we demonstrated that these changes occur in both TGintact and TGatrophic populations. We propose that the increase in filopodia in TG neurons at the advanced stage may be a compensatory response to decreased excitatory input [9]. Indeed, previous studies in hippocampal [39] and neocortical [40] neurons have shown that proliferation of filopodia occurs following functional deafferentation.
Excitable properties
We have previously demonstrated that cortical pyramidal neurons in ~9-month-old rTg4510 mice exhibit markedly increased excitability that is primarily a consequence of the significant depolarization of these neurons [9,43]. An unexpected finding of the present study was that this increase in excitability was also prominent in the early stage of tauopathy, prior to NFT deposition and neuronal death. Thus at both the early and the advanced stage, TG neurons exhibited a significantly depolarized resting membrane potential, increased depolarizing sag potential and increased evoked action potential firing rates. Threshold was unchanged at both stages of tauopathy, and therefore was not a major determinant of increased excitability in TG neurons. However, there was a reduction in rheobase in TG neurons, which reached the significance level for early TG and TGatrophic groups.
From a mechanistic standpoint the large increase in depolarizing sag potential in TG neurons is particularly intriguing. This sag potential is due to the activation of the mixed cationic H-current, which is carried through hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. The H-current is active at rest and causes a tonic 4 to 8 mV depolarization of the membrane potential [42]. Thus the increased sag potential seen in TG neurons may underlie the depolarized resting potential and increased action potential firing rates in these neurons. By what mechanism might the sag potential be increased in TG neurons? HCN channels are rapidly trafficked along microtubule and actin cytoskeletal networks in dendrites [36] and are particularly targeted to the distal dendritic arbor [30,32]. These networks are disrupted in neurodegenerative tauopathies (review [51]), and trafficking abnormalities occur early in the time course of the disease in rTg4510 mice [25]. Early and persistent impairment of HCN channel trafficking may result in accumulation of HCN channels at the soma and resultant increased sag potential and depolarization. Given the significantly increased sag potential, an unchanged or even increased input resistance in TG neurons is unexpected. While an increase in HCN channels may lead to a decrease in input resistance, findings from previous studies suggest that this may not always be the case. For example, in olfactory bulb mitral cells an increase in the amplitude of the sag potential is correlated with an increase in input resistance [2], while HCN channel block in CA3b pyramidal neurons does not lead to alterations in input resistance [18].
The early and persistent increase in excitability that occurs in this mouse model may be an initial pathological event that leads to downstream alterations in neuronal structure on the one hand and/or may represent early functional changes that occur as a consequence of altered trafficking of ion channels in these neurons. Understanding the time course and mechanisms underlying increased excitability during progressive tauopathy is important given evidence for reduced seizure threshold in human neurodegenerative disease [37,35,58].
Excitatory postsynaptic currents
In early tauopathy, glutamatergic spontaneous excitatory postsynaptic currents (sEPSCs) were unchanged in mean frequency, amplitude and kinetics in TG neurons. However, while the mean amplitude of sEPSCs did not differ, there were a significantly higher proportion of small amplitude events in TG neurons at this stage. This difference in amplitude distribution suggests that changes in glutamatergic transmission occur prior to spine loss. Indeed, a recent study by Hoover et al. [19] demonstrated that reduced surface expression of postsynaptic GluR1 AMPA receptors, related to mislocalization of hyperphosphorylated tau to spines, is associated with impaired basal synaptic transmission and LTP in the hippocampus of early stage rTg4510 mice.
In advanced tauopathy, neither the mean frequency nor the kinetics of sESPCs of TGintact or TGatrophic neurons differed from those of NT neurons. Thus, it is possible that surviving neurons at this stage may compensate for decreased input from dying neurons within the cortical network by increasing insertion or turnover of AMPA receptors at postsynaptic sites and/or by increasing presynaptic neurotransmitter release or the number of presynaptic terminals [9]. Indeed, we previously reported that in addition to increased firing rates, TG neurons from 9-month-old mice exhibit sprouting of new axonal boutons and filopodia; evidence for the formation of new, albeit shorter, excitatory synapses was also observed in TG frontal cortical neuropil [9]. These small synapses could account for the significantly higher proportion of small amplitude sEPSCs observed in TGatrophic versus NT neurons. Further, the mean amplitude of sEPSCs is significantly higher in TGintact neurons versus NT neurons and cumulative distribution histograms reveal a significantly higher proportion of large amplitude events in TGintact neurons compared to all other groups. This phenomenon could be due to the increased number of oblique apical branches observed in these neurons. Previous studies have shown that in healthy neurons, oblique dendrites are responsible for ~20% of the total area of the apical dendritic shaft of layer 2/3 neurons [27] and that these dendrites, together with basal branches, contribute ~2/3 of the total number of spines for an individual neuron [28]. The increased number of apical oblique dendritic branches and their relatively close proximity to the soma could result in less attenuation and greater summation of synaptic currents along the main apical dendritic trunk. Thus, dendritic sprouting may be a mechanism by which neurons in advanced tauopathy maintain synaptic gain for a time during progressive tauopathy. Additionally, a larger proportion of axodendritic versus axospinous synapses would be expected to lead to larger amplitude events in TGintact neurons. While we previously found no evidence for an increase in the proportion of axodendritic synapses in TG neurons from 9-month-old mice, it is possible that axodendritic synapses could increase with disease progression, and/or that TGintact neurons in particular exhibit increased axodendritic synapses. Studies are currently underway to determine if this is the case.
Conclusion
The principal findings of this study were that TG neurons exhibit increased excitability both in early and advanced stages of tauopathy, while dendrite and spine retraction occurs only late in disease progression. Initial changes in electrophysiological properties of TG neurons are likely due to impaired trafficking of ion channels, such as the HCN channel, whereas slower subsequent changes to morphology may be a consequence of cytoskeletal destabilization, deafferentation, and neuronal death. Whether early electrophysiological alterations contribute to subsequent morphological changes and/or to neuron death, or whether these events are independent of one another remains to be determined.
Supplementary Material
Acknowledgements
The authors declare no competing financial interests. This work was supported by NIH/NIA grants R01-AG025062. The authors are grateful to Dr. Jada Lewis for generously providing us with rTg4510 mice, Joseph Amatrudo for assistance with data collection, Dr. Christina Weaver for assistance with statistical analyses, Dr. Maria Medalla for careful reading of the manuscript, and Dr. Tara Spires-Jones for helpful discussions throughout the course of this project.
REFERENCES
- 1.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U S A. 1997;94(1):298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelo K, Margrie TW. Population diversity and function of hyperpolarization-activated current in olfactory bulb mitral cells. Sci Rep. 2011;1:50. doi: 10.1038/srep00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendt T. Alzheimer's disease as a disorder of mechanisms underlying structural brain self-organization. Neuroscience. 2001;102(4):723–765. doi: 10.1016/s0306-4522(00)00516-9. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 5.Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27(14):3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, Ramakers GJ, de Vos K, Krugers H, Tomiyama T, Mori H, Joels M, van Leuven F, Lucassen PJ. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26(13):3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak E, Braak H. Alzheimer's disease: transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon's horn. Acta Neuropathol. 1997;93(4):323–325. doi: 10.1007/s004010050622. [DOI] [PubMed] [Google Scholar]
- 8.Buell SJ, Coleman PD. Dendritic growth in the aged human brain and failure of growth in senile dementia. Science. 1979;206(4420):854–856. doi: 10.1126/science.493989. [DOI] [PubMed] [Google Scholar]
- 9.Crimins JL, Rocher AB, Peters A, Shultz P, Lewis J, Luebke JI. Homeostatic responses by surviving cortical pyramidal cells in neurodegenerative tauopathy. Acta Neuropathol. 2011;122(5):551–564. doi: 10.1007/s00401-011-0877-0. [DOI] [PubMed] [Google Scholar]
- 10.Dickstein DL, Brautigam H, Stockton SD, Jr, Schmeidler J, Hof PR. Changes in dendritic complexity and spine morphology in transgenic mice expressing human wild-type tau. Brain Struct Funct. 2010;214(2–3):161–179. doi: 10.1007/s00429-010-0245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey M, Chaudhury P, Kabiru H, Shea TB. Tau inhibits anterograde axonal transport and perturbs stability in growing axonal neurites in part by displacing kinesin cargo: neurofilaments attenuate tau-mediated neurite instability. Cell Motil Cytoskeleton. 2008;65(2):89–99. doi: 10.1002/cm.20243. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante RJ, Kowall NW, Richardson EP., Jr Proliferative and degenerative changes in striatal spiny neurons in Huntington's disease: a combined study using the section-Golgi method and calbindin D28k immunocytochemistry. J Neurosci. 1991;11(12):3877–3887. doi: 10.1523/JNEUROSCI.11-12-03877.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flood DG, Buell SJ, Defiore CH, Horwitz GJ, Coleman PD. Age-related dendritic growth in dentate gyrus of human brain is followed by regression in the 'oldest old'. Brain Res. 1985;345(2):366–368. doi: 10.1016/0006-8993(85)91018-2. [DOI] [PubMed] [Google Scholar]
- 14.Flood DG, Coleman PD. Dendritic regression dissociated from neuronal death but associated with partial deafferentation in aging rat supraoptic nucleus. Neurobiol Aging. 1993;14(6):575–587. doi: 10.1016/0197-4580(93)90042-a. [DOI] [PubMed] [Google Scholar]
- 15.Fox LM, William CM, Adamowicz DH, Pitstick R, Carlson GA, Spires-Jones TL, Hyman BT. Soluble tau species, not neurofibrillary aggregates, disrupt neural system integration in a tau transgenic model. J Neuropathol Exp Neurol. 2011;70(7):588–595. doi: 10.1097/NEN.0b013e318220a658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60(9):1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41(1):17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 18.Hemond P, Migliore M, Ascoli GA, Jaffe DB. The membrane response of hippocampal CA3b pyramidal neurons near rest: Heterogeneity of passive properties and the contribution of hyperpolarization-activated currents. Neuroscience. 2009;160(2):359–370. doi: 10.1016/j.neuroscience.2009.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoy RR, Nolen TG, Casaday GC. Dendritic sprouting and compensatory synaptogenesis in an identified interneuron follow auditory deprivation in a cricket. Proc Natl Acad Sci U S A. 1985;82(22):7772–7776. doi: 10.1073/pnas.82.22.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal K, Alonso Adel C, Grundke-Iqbal I. Cytosolic abnormally hyperphosphorylated tau but not paired helical filaments sequester normal MAPs and inhibit microtubule assembly. J Alzheimers Dis. 2008;14(4):365–370. doi: 10.3233/jad-2008-14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ittner LM, Ke YD, Gotz J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J Biol Chem. 2009;284(31):20909–20916. doi: 10.1074/jbc.M109.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaworski T, Lechat B, Demedts D, Gielis L, Devijver H, Borghgraef P, Duimel H, Verheyen F, Kugler S, Van Leuven F. Dendritic degeneration, neurovascular defects, and inflammation precede neuronal loss in a mouse model for tau-mediated neurodegeneration. Am J Pathol. 2011;179(4):2001–2015. doi: 10.1016/j.ajpath.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones WH, Thomas DB. Changes in the dendritic organization of neurons in the cerebral cortex following deafferentation. J Anat. 1962;96:375–381. [PMC free article] [PubMed] [Google Scholar]
- 25.Kopeikina KJ, Carlson GA, Pitstick R, Ludvigson AE, Peters A, Luebke JI, Koffie RM, Frosch MP, Hyman BT, Spires-Jones TL. Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer's disease brain. Am J Pathol. 2011;179(4):2071–2082. doi: 10.1016/j.ajpath.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kremer A, Maurin H, Demedts D, Devijver H, Borghgraef P, Van Leuven F. Early improved and late defective cognition is reflected by dendritic spines in Tau.P301L mice. J Neurosci. 2011;31(49):18036–18047. doi: 10.1523/JNEUROSCI.4859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larkman A, Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci. 1990;10(5):1407–1414. doi: 10.1523/JNEUROSCI.10-05-01407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkman AU. Dendritic morphology of pyramidal neurones of the visual cortex of the rat: III. Spine distributions. J Comp Neurol. 1991;306(2):332–343. doi: 10.1002/cne.903060209. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Kim W, Li Z, Hall GF. Accumulation of vesicle-associated human tau in distal dendrites drives degeneration and tau secretion in an in situ cellular tauopathy model. Int J Alzheimers Dis. 2012;2012:172837. doi: 10.1155/2012/172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5(11):1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- 31.Luebke JI, Weaver CM, Rocher AB, Rodriguez A, Crimins JL, Dickstein DL, Wearne SL, Hof PR. Dendritic vulnerability in neurodegenerative disease: insights from analyses of cortical pyramidal neurons in transgenic mouse models. Brain Struct Funct. 2010;214(2–3):181–199. doi: 10.1007/s00429-010-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18(19):7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKee AC, Kowall NW, Kosik KS. Microtubular reorganization and dendritic growth response in Alzheimer's disease. Ann Neurol. 1989;26(5):652–659. doi: 10.1002/ana.410260511. [DOI] [PubMed] [Google Scholar]
- 34.Naegele J. Epilepsy and the plastic mind. Epilepsy Curr. 2009;9(6):166–169. doi: 10.1111/j.1535-7511.2009.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nance MA, Myers RH. Juvenile onset Huntington's disease--clinical and research perspectives. Ment Retard Dev Disabil Res Rev. 2001;7(3):153–157. doi: 10.1002/mrdd.1022. [DOI] [PubMed] [Google Scholar]
- 36.Noam Y, Zha Q, Phan L, Wu RL, Chetkovich DM, Wadman WJ, Baram TZ. Trafficking and surface expression of hyperpolarization-activated cyclic nucleotide-gated channels in hippocampal neurons. J Biol Chem. 2010;285(19):14724–14736. doi: 10.1074/jbc.M109.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66(4):435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrak LJ, Harris KM, Kirov SA. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J Comp Neurol. 2005;484(2):183–190. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- 40.Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23(18):7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25(46):10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 43.Rocher AB, Crimins JL, Amatrudo JM, Kinson MS, Todd-Brown MA, Lewis J, Luebke JI. Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs. Exp Neurol. 2010;223(2):385–393. doi: 10.1016/j.expneurol.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez A, Ehlenberger D, Kelliher K, Einstein M, Henderson SC, Morrison JH, Hof PR, Wearne SL. Automated reconstruction of three-dimensional neuronal morphology from laser scanning microscopy images. Methods. 2003;30(1):94–105. doi: 10.1016/s1046-2023(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3(4):e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez A, Ehlenberger DB, Hof PR, Wearne SL. Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nat Protoc. 2006;1(4):2152–2161. doi: 10.1038/nprot.2006.313. [DOI] [PubMed] [Google Scholar]
- 47.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- 49.Spires TL, Orne JD, SantaCruz K, Pitstick R, Carlson GA, Ashe KH, Hyman BT. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol. 2006;168(5):1598–1607. doi: 10.2353/ajpath.2006.050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spires-Jones TL, Kopeikina KJ, Koffie RM, de Calignon A, Hyman BT. Are tangles as toxic as they look? J Mol Neurosci. 2011;45(3):438–444. doi: 10.1007/s12031-011-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32(3):150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156(6):1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E, D'Hooge R, Alzheimer C, Mandelkow EM. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J Neurosci. 2011;31(7):2511–2525. doi: 10.1523/JNEUROSCI.5245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tailby C, Wright LL, Metha AB, Calford MB. Activity-dependent maintenance and growth of dendrites in adult cortex. Proc Natl Acad Sci U S A. 2005;102(12):4631–4636. doi: 10.1073/pnas.0402747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teter B, Ashford JW. Neuroplasticity in Alzheimer's disease. J Neurosci Res. 2002;70(3):402–437. doi: 10.1002/jnr.10441. [DOI] [PubMed] [Google Scholar]
- 56.Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci. 2007;27(11):2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wearne SL, Rodriguez A, Ehlenberger DB, Rocher AB, Henderson SC, Hof PR. New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neuroscience. 2005;136(3):661–680. doi: 10.1016/j.neuroscience.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 58.Weiner MF, Hynan LS, Parikh B, Zaki N, White CL, 3rd, Bigio EH, Lipton AM, Martin-Cook K, Svetlik DA, Cullum CM, Vobach S, Rosenberg RN. Can alzheimer's disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16(4):245–250. doi: 10.1177/0891988703258671. [DOI] [PubMed] [Google Scholar]
- 59.Zempel H, Mandelkow EM. Linking amyloid-beta and tau: amyloid-beta induced synaptic dysfunction via local wreckage of the neuronal cytoskeleton. Neurodegener Dis. 2011;10(1–4):64–72. doi: 10.1159/000332816. [DOI] [PubMed] [Google Scholar]
- 60.Zhang K, Peng BW, Sanchez RM. Decreased IH in hippocampal area CA1 pyramidal neurons after perinatal seizure-inducing hypoxia. Epilepsia. 2006;47(6):1023–1028. doi: 10.1111/j.1528-1167.2006.00574.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.