Abstract
Glioblastoma (GBM) is the most common primary brain tumor in adults and has a dismal prognosis despite multimodality treatment. Given the resistance of glioma stem cells (GSC) to chemotherapy and radiation therapy, their eradication could prevent tumor recurrence. We sought to evaluate the antitumor activity of MV derivatives against GSC. We generated neurosphere cultures from patient derived primary tumor GBM xenografts, and we characterized them for the GSC markers CD133, SOX2, Nestin, ATF5 and OLIG2. Using the MV-strains MV-GFP, MV-CEA and MV-NIS we demonstrated infection, viral replication and significant cytopathic effect in vitro against GSC lines. In tumorigenicity experiments, GBM 44 GSC were infected with MV in vitro and subsequently implanted into the right caudate nucleus of nude mice: significant prolongation of survival in mice implanted with infected GSC was observed, compared to mock infected controls (p=0.0483). In therapy experiments in GBM6 and GBM12 GSC xenograft models, there was significant prolongation of survival in MV-GFP treated animals compared to inactivated virus treated controls (GBM6 p=0.0021, GBM12 p=0.0416). Abundant syncytia and viral replication was demonstrated in tumors of MV treated mice. Conclusion: Measles virus derivatives have significant antitumor activity against glioma derived stem cells in vitro and in vivo.
Keywords: measles virus, gliomas, stem cells, MV-CEA, MV-NIS
Introduction
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults and has a dismal prognosis despite aggressive use of multimodality treatment including surgery, chemotherapy and radiation therapy.1 One potentially significant hurdle to overcome in the treatment of GBM pertains to eradication of glioma cells with stem-cell like properties (GSC); these are cells with self-renewing properties, able to differentiate into neurons, oligodendrocytes and astrocytes, initiate brain tumors in vivo, and have been associated with the promotion of angiogenesis development of resistance to radiation and chemotherapy.2–4 Our laboratory has previously demonstrated that derivatives of the measles virus (MV) Edmonston’s strain have significant activity against glioma lines and orthotopic xenografts,5–7 and a phase I trial of the measles derivative MV-CEA is currently ongoing in recurrent GBM patients. Assessing the ability of MV-strains to infect and eradicate glioma cells with stem-cell like properties represents an important parameter which could potentially affect the antitumor activity of this novel virotherapy platform and impact on the development of combinatorial strategies. Here we show that GSC overexpress the measles virus receptor CD46. Futhermore, MV-strains of the Edmonston vaccine lineage have significant antitumor activity against glioma stem cells in vitro and in vivo, and can decrease their tumorigenicity; these findings can have important translational implications in glioma treatment.
Results
Viral strains
Antitumor activity of the MV-strains MV-CEA, MV-NIS, and MV-GFP against GSC was examined. Their construction has been previously described.8–10 A schematic representation of the strains used is included in Figure 1.
Figure 1.
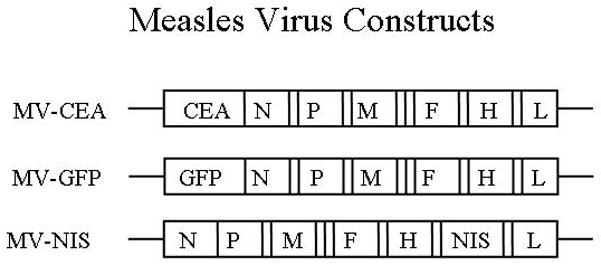
Schematic representation of the viral strains: MV-CEA: MV strain expressing the human carcinoembryonic antigen; MV-NIS: MV strain expressing the sodium iodine symporter. N, nucleoprotein gene; MV-GFP: MV strain expressing the enhanced Green Fluorescent Protein; L, large protein gene; P, phosphoprotein gene; M, matrix protein gene; F, fusion protein gene; H, hemagglutinin gene; CEA, human carcinoembryonic antigen gene; NIS, sodium iodine symporter gene.
Characterization of stem cell marker expression in neurosphere lines
GBM xenografts were developed in BALB/c nude mice from GBM patients as previously described.11, 12 Tumors were excised from mice bearing the GBM xenografts GBM 6, 10, 12, 14, 23, 34, 38, 39, 43, and 44, mechanically disrupted and grown in NeuroCult medium formation of neurospheres was observed within 2–6 weeks depending on the cell line. Neurosphere lines used in vitro and in vivo experiments were further characterized for the expression of the glioma stem cell markers CD133, Nestin, Sox 2, ATF5, and OLIG 2. All neurosphere lines tested expressed at least 3/5 stem cell markers. Representative results for three of the neurosphere lines are shown in Figure 2.
Figure 2.
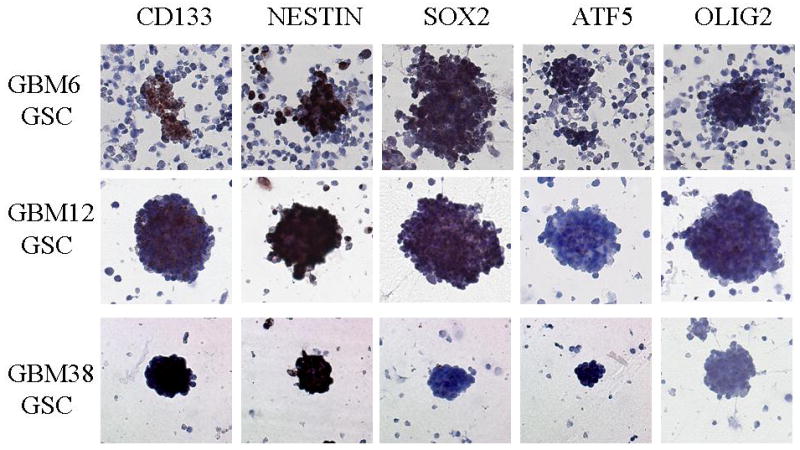
Neurospheres derived from GSC xenograft cultures express stem-cell markers (CD133, Nestin, SOX2, ATF5 and OLIG2). Representative data for GBM6, GBM12 and GBM38 glioma stem cell lines are shown.
Expression of measles virus receptor CD46 in neurosphere lines
CD46 expression in neurosphere lines was tested by FACS and immunohistochemistry overexpression was confirmed in all 10 lines tested. Representative results are shown for GBM34 and GBM22 (Figure 3).
Figure 3.
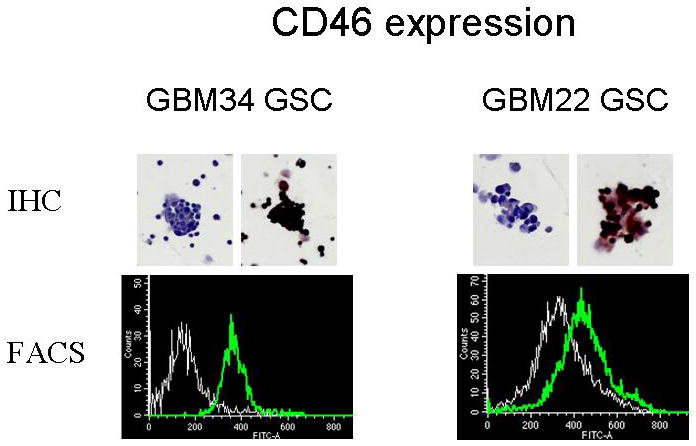
Expression of measles virus receptor CD46 was examined by IHC and flow cytometry. Significant expression of CD46 was observed in all neurosphere lines tested. Representative ICH and FACS data in GBM34 and GBM22 GSC lines are presented.
Assessment of cytopathic affect of MV strains against GSC
In order to access antitumor activity of measles virus strains against GSCs neurosphere lines were infected with the MV strains, MV-NIS, MV-GFP and MV-CEA at an MOI of 0.1 or 1.0 and trypan blue exclusion assays were performed at multiple timepoints. Results were presented as percentage of the uninfected controls. Measles virus derivatives had significant antitumor activity against GSCs in vitro, which was dependent on MV strain and MOI (Figure 4).
Figure 4.
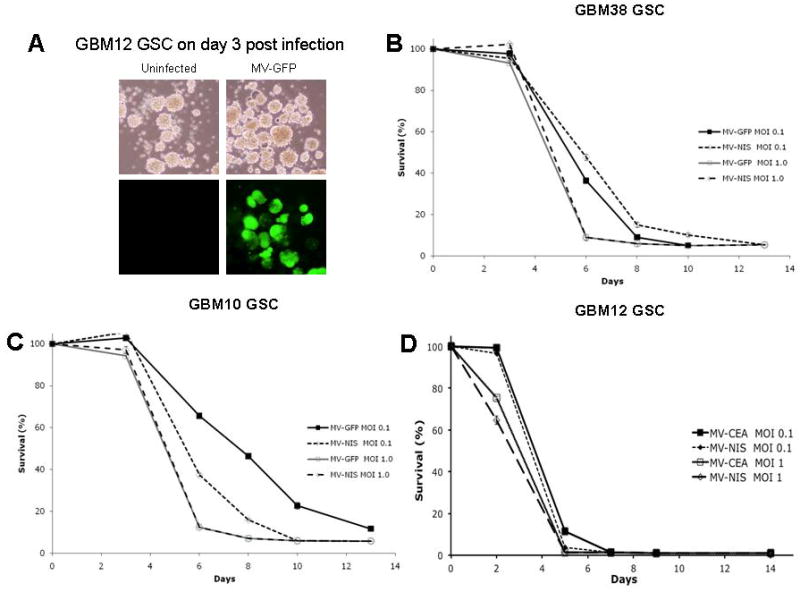
A Infection with MV-GFP resulted in abundant GFP expression in infected GBM12 neurospheres. B, C, D: The cytopathic effect of MV-GFP, MV-NIS and MV-CEA was assessed in GSC neurosphere lines using trypan blue exclusion assays. Viral MOIs of 0.1 and 1 were used. Results are presented as percentage of uninfected controls. Representative results are shown for GBM 38 (B), GBM10 (C) and GBM12 (D). There was MOI dependent cytotoxicity with almost complete eradication of neurosphere cultures by day 6 post infection.
Assessment of viral replication
Virus replication was assessed using one-step viral growth curves as previously described.7 Neurosphere cultures supported robust growth of the MV-NIS, MV-GFP and MV-CEA virus (Figure 5).
Figure 5.
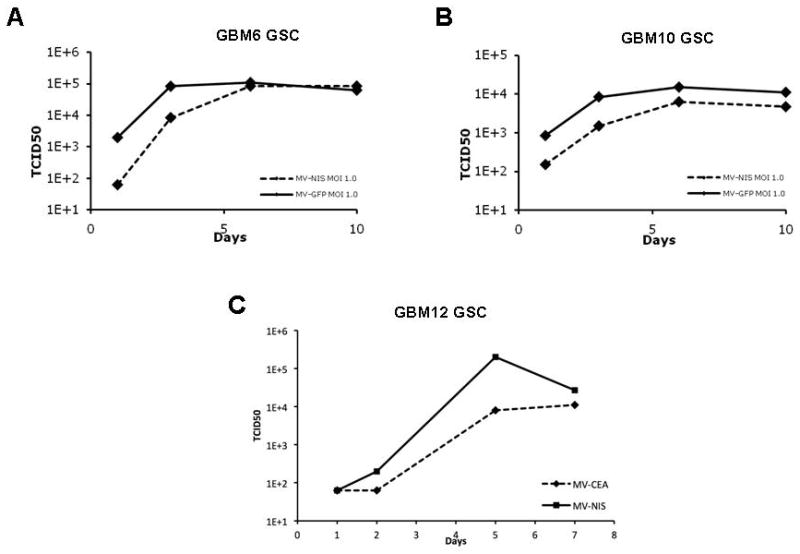
Viral replication was assessed by one-step viral growth curves. Representative results in GSC deriving from GBM 6, 10 and 12 are shown. Cells were infected with MV-NIS, MV-GFP or MV-CEA at an MOI of 1, harvested at different time points and titered in Vero cells. Results are expressed as TCID50 of harvested virus. All glioma stem cell lines effectively supported viral replication.
Assessment of tumorigenicity of infected neurospheres
GSC cells obtained from GBM44 xenografts were infected with either MV-NIS (MOI of 10) or mock infected control and 3 × 105 cells were implanted into the right caudate nucleus of BALB/C nude mice. There was significant prolongation of survival in mice implanted with infected neurospheres as compared to mice implanted with mock infected neurospheres (p=0.04) indicating that infection of GSC in vitro can modify their aggressive biologic behavior following orthotopic implantation (Figure 6).
Figure 6.
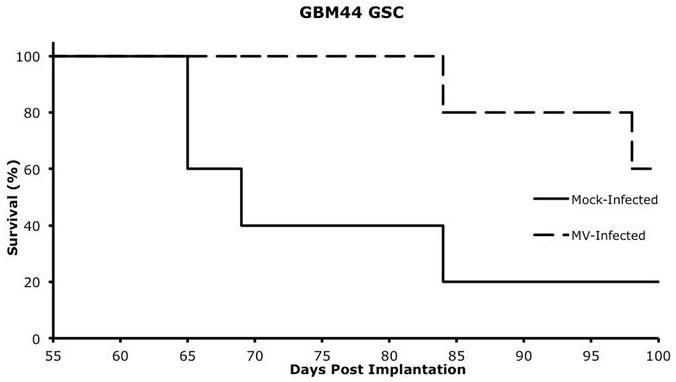
GBM44 GSC were either infected with MV-NIS (MOI 10) or mock-infected, and 3×105 GSC cells were implanted into the right caudate nucleus of nude mice. Animals were followed for survival. There was significant prolongation of survival in mice which were implanted with cells infected with MV-NIS (p=0.04) with median survival of 68 days in the control group versus not having been reached at 100 days in mice implanted with infected GSC.
As Figure 6 illustrates, some of the mice implanted with infected neurospheres developed tumors. Examination of these tumors at the time of euthanasia showed lack of syncytia, indicating that the tumors likely derived from non-infected cells. Immunofluorescence for the natural killer cell lectin-like receptor (KLRG1) and the macrophage marker CD68, sohowed lack of a significant immune infiltrate (Supplemental Figure 1), suggesting a low likelihood that innate immune response driven viral inactivation was responsible for tumor cell repopulation in this model.
Assessment of therapeutic efficacy in vivo
In order to assess the antitumor activity of MV strains against neurosphere derived xenografts, GBM6 or GBM12 derived glioma stem cells were orthotopically implanted into the right caudate nucleus of nude mice. Animals were treated with a total dose of either 9 × 105 TCID50 (GBM12) or 1.8 × 106 TCID (GBM6) of MV-GFP. Control animals received UV-inactivated virus. Significant prolongation of survival of the treated animals was observed in both models (p=0.0416 and p=0.0021 respectively) (Figure 7). In a parallel experiment treated and control animals bearing orthotopic GBM6 GSC xenografts (a CD133 positive neurosphere line) were euthanized either on day 3 post treatment completion or when moribund. Abundant syncytia were observed in MV-GFP treated mice. Syncytia expressed CD133 (Figure 7D) confirming activity of the measles virus derivatives against GSC derived tumors in vivo. In situ hybridization for the measles virus N (nucleoprotein)-mRNA was strongly positive (Figure 7F) indicating viral replication in the GSC derived syncytia.
Figure 7.
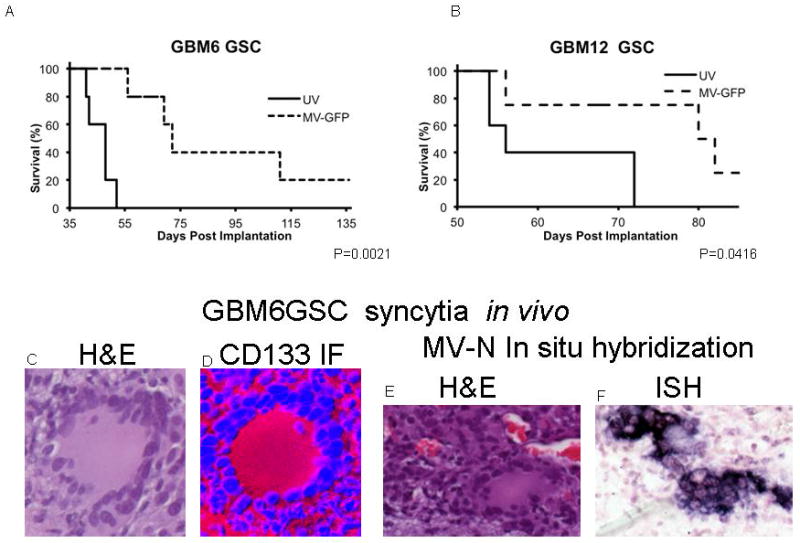
GBM6 (A) or GBM12 (B) derived GSC were orthotopically implanted into the right caudate nucleus of nude mice. Animals were treated with a total dose of 1.8×106or 9×105 TCID50 MV-GFP, respectively, or UV inactivated virus. In both orthotopic treatment models, significant prolongation in survival was observed (p=0.0021 and p=0.0416 respectively). Abundant syncytia were observed in MV-GFP treated mice in H and E stain (C, E). Syncytia expressed the stem cell marker CD133 (D). Viral replication was confirmed by in situ hybridization for measles virus N mRNA (F).
Discussion
Glioblastoma (GBM) remains incurable with a median survival of 15–16 months, despite the use of multimodality treatments including surgery, chemotherapy, and radiation therapy.13 Brain tumor stem cells are believed to play a key role in mediating glioma resistance to chemotherapy and radiation therapy, in part due to their ability to more efficiently repair damaged DNA3, 14–16 as compared to tumor cells without stem cell properties. Treatment of GBM with temozolomide, an agent commonly used in glioma treatment, leads to preferential survival of a cell population, enriched in the stem cell marker16 CD133, and glioblastomas that recur following chemoradiation are characterized by a stem-cell related gene signature.17 Stem cell resistance to chemotherapy agents such as temozolomide, carboplatin, paclitaxel and etoposide appears to be induced through elevated expression of antiapoptotic genes and 0–6 methylguanine-methyltransferase (MGMT), a DNA repair enzyme that inhibits the activity of DNA methylating agents such as nitrosoureas and temozolomide.18 These cells have the capacity for self renewal and multilineage differentiation and efficiently initiate tumor xenografts in vivo.3
Development of approaches that can effectively eradicate stem cells has therefore the potential to significantly improve outcome for glioblastoma multiforme patients. Although small molecule inhibitors of key pathways in glioma stem cells survival, such as the notch pathway, are currently in early clinical testing;19 their clinical efficacy to date has been modest and the development of additional approaches targeting glioma cells with stem cell-like properties is needed. Oncolytic viruses might represent such an approach. DNA viruses such as the Delta-24-RGD adenovirus19 and oncolytic HSV strains20, 21 have been reported to have antitumor activity against glioma stem cells. There is no information to date, however, regarding activity of oncolytic RNA viruses.
We have demonstrated that engineered strains of measles virus, a negative strain RNA virus of the paramyxovirus family, deriving from the Edmonston vaccine lineage have significant activity against glioma cells with stem-like properties. These cells allow efficient viral propagation as indicated by one-step viral growth curves and were effectively killed even in MOIs as low as 0.1. In addition, infection prior to orthotopic implantation of human GBM xenograft derived glioma stem cells modified the aggressive biologic behavior of these cells as demonstrated by the significant prolongation of survival of animals treated with infected cells. Treatment of orthotopic xenografts established by glioma stem cells using two different models, GBM6 and GBM12, also led to therapeutic benefit and significant prolongation of median survival. The characteristic cytopathic effect with formation of syncytia was observed in these CD133 positive orthotopic xenografts. In situ hybridization for measles virus mRNA confirmed in vivo replication of the virus.
Sensitivity of glioma cells to measles infection can at least in part be explained by overexpression of measles virus receptor CD46 in neurosphere lines. In contrast to the wild type MV strains which enter the cells predominantly via the SLAM receptor (expressed in B-, T-cells and macrophages)22, 23, measles virus oncolytic vaccine strains of the Edmonston vaccine lineage have been shown to enter cells predominantly via the ubiquitous expressor CD46.24, 25 CD46 or membrane cofactor protein is overexpressed in different tumors, including gliomas26 and protects them from complement mediated lysis.27–29 We have tested levels of CD46 expression by both immunohistochemistry and FACS in ten different neurosphere lines deriving from patient originated xenografts and observed high expression in all ten neurosphere lines tested, indicating the glioma stem cells represent a good target for CD46 targeted therapeutics such as MV strains. In an ongoing trial of intratumoral and resection cavity administration of the measles derivative MV-CEA in recurrent GBM patients, we are in the process of investigating the correlation between CD46 expression in tumors and viral replication/response to oncolytic measles virotherapy.30
It is of note that recently a third measles receptor, the adherens junction protein nectin-4, has been identified.31 The new receptor, the adherens junction protein nectin-4, is expressed in the respiratory epithelium.31 It is also expressed in ovarian, breast, and lung cancer.32–34 Although members of the nectin family, such as nectin-1 and -3 appear to play an important role in the function of brain synopses,35 the role of nectin-4 is not well characterized in brain tumors or normal brain. FACS analysis in 4 different measles permissive glioma stem cell lines (GBM6, GBM12, GBM102, GBM143) showed no nectin-4 expression in GSC (Allen and Galanis, unpublished data), indicating a low likelihood that nectin-4 expression represent an important parameter determining the efficacy of MV based therapeutics against GSC. We are in the process of expanding this analysis in a larger panel of GSC, however.
Our results, demonstrating the efficacy of MV strains against glioma cells with stem cell like properties, suggest that combinatorial strategies of oncolytic measles strains with agents representing the standard of care in GBM treatment, such as RT or temozolomide, represent a direction worth exploiting since they have the potential to improve outcome in response to these conventional treatment strategies by eradicating resistance mediating cells with stem cell-like properties.
Methods
Viral construction
The construction of recombinant measles virus strains which contain the soluble extracellular domain of human CEA (MV-CEA), green fluorescent protein-eGFP (MV-GFP) or the sodium-iodide symporter gene (NIS, MV-NIS) has been described elsewhere8–10 (Figure 7).
Cell lines
Subcutaneous xenografts were developed in BALB/c nude mice from GBM patients as previously described;36 when orthotopically implanted these xenografts have been demonstrated to maintain the histologic characteristics and invasiveness of the primary tumor they derived from. Tumors were excised from mice bearing the GBM xenografts GBM6, 10, 12, 14, 22, 34, 38, 39, 43, and 44 and each was mechanically disrupted with a sterile blade and grown at 37°C in a humidified environment, 5% CO2 in either regular media (DMEM with 10% FBS, 1X Penn/Strep) or NeuroCult® media (Stemcell Technologies, Vancouver, BC, CA).
Immunohistochemistry to assess expression of GSC markers, CD133, SOX2, Nestin, ATF5, OLIG2, and the MV receptor CD46 in neurospheres
Neurospheres from primary GBM xenograft cultures were harvested, expanded in vitro, fixed in acetone, and examined by IHC for the expression of: a) GSC markers, CD133, SOX2, Nestin, ATF5, OLIG2, and MV receptor CD46. Primary antibodies to test marker expression were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and IHC was performed as per manufacturer’s instructions. In summary, slides were blocked in PBS containing 10% goat or donkey serum, 1% BSA and 0.025% Triton-X-100 for one hour and incubated overnight at 4°C with 1:100 dilution of primary antibody. Primary antibody was omitted in negative controls. The slides were rinsed thrice for 5 min in PBS and exposed to a 1:500 dilution of either biotinylated goat anti-mouse or donkey anti-goat for one hour and visualized with DAB substrate staining followed by VECTASTAIN Elite ABC kit (Vector Laboratories, INCl, Burlingame, CA) and counterstained with ACCUSTAIN (Sigma-Aldrich INC., St. Louis, MO).
CD46 flow cytometry
Primary GSC cultures deriving from GBM 6, 10, 12, 14, 22, 34, 38, 39, 43, and 44 xenografts (5×105 cells), were incubated for 45 minutes on ice with 10μg of FITC labeled mouse anti-human CD46 (BD Pharmingen, San Jose, CA) in DPBS containing 0.5% BSA, FITC labeled mouse IgG2a, κ serving as the isotype control. The cells were washed twice, fixed in DPBS containing 0.5% paraformaldehyde and were analyzed using a Becton Kickinson FACScan Plus cytometer with Cell Quest software.
Assessment of cytotoxicity of MV strains against GSC
Neurospheres were enzymatically and mechanically disrupted in TrypleE (Invitrogen, Carlsbad, CA) with a 10 ml pipette, 5 × 106 cells were plated into 6-well dishes and infected 24 hours later at an MOI of 0.1 or 1.0 with MV-GFP, MV-CEA, or MV-NIS, with uninfected cells serving as controls. Cytotoxicity of MV-strains against GSC was evaluated by counting the number of viable cells by trypan blue exclusion assay as previously described7 and compared against uninfected controls.
Assessment of viral growth in GSC
Viral growth was assessed by one step viral growth curves as previously described.10 In summary, neurospheres were infected with MV-NIS, MV-GFP or MV-CEA at an MOI of 1. Cells were harvested at different time points and viral titer was determined by 50% end point dilution assay on Vero cells, as previously described.37
Tumorigenicity experiments
Neurospheres derived from GBM44 xenografts were infected with MV-NIS in vitro at a MOI of 10 TCID50. Mock infected neurospheres were used as controls. Six hours later the cells were harvested, mechanically disrupted and subsequently 3 × 105 cells were orthotopically implanted into the right caudate nucleus of 5–6 week old BALB/c mice (n=5 per group). These mice were followed daily and euthanized when neurologic symptomatology or >10% weight loss was observed. All animal experimental protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Assessment of therapeutic efficacy in vivo
GBM6 and GBM12 orthotopic models were established by implantation of 3 × 106 GSC into the right caudate nucleus of five week old BALB/c mice, using the small animal stereotactic frame (ASI Instruments, Warren, MI) and a 26-gauge Hamilton syringe. Treatment was initiated at either 1 wk or 2 wks post implantation respectively. Mice received either MV-GFP (N=5) or UV-inactivated virus as control (N=5) using the same coordinates as for implantation. MV-GFP treated mice received 1 × 105 (GBM12 GSC model) or 2 × 105 (GBM6 GSC model) TCID50 per dose three times per week over a three-week period for a total dose of 9 × 105 or 1.8 × 106 TCID50 respectively. Mice were observed daily and were euthanized when neurologic symptomatology or >10% weight loss was observed.
In a parallel experiment, designed to perform correlative studies in treated tumors, 3 × 106 GBM6 derived GSC (a line expressing the stem cell marker CD133) were implanted into the right caudate nucleus of 5–6 weeks old BALB/c mice as previously described. One group of animals (n=10) received MV-GFP, 1.5 × 105 TCID50 per dose every second day for a total of 3 doses starting at 2 weeks following implantation and a second group treated with UV inactivated virus served as the control (n=5). Treated mice and corresponding controls were euthanized either at 3 days following completion of viral treatment (n=5) or when mice became moribund (n=5). Animal brains were formalin fixed, stained with H and E and examined by immunofluorescence for CD133 expression and in situ hybridization, for viral N mRNA.
Immunofluorescence for CD133 expression in vivo
Formalin fixed brains were examined for CD133 expression by immunofluorescence: following heat mediated sodium citrate antigen retrieval, slides were blocked in PBS containing 10% goat serum, 1% BSA and 0.025% Triton-X-100 for one hour and incubated overnight at 4°C with primary antibody (AbCam, Inc., Cambridge, MA, 1:200 dilution) or without primary antibody control. The slides were rinsed three times for 5 min in PBS and exposed to Alexa Fluor 647 Goat Anti-Rabbit (Invitrogen, Eugene, OR). Slides were counterstained with DAPI and examined with a Zeiss LSM510 confocal microscope.
In situ hybridization for detection of measles virus N (nucleoprotein)-mRNA
A custom designed digoxigenin (DIG)-labeled oligonucleotide probe (GeneDetect) was used for ISH. The samples were treated with Proteinase K (20 mg/mL) for 20 minutes at 37°C, washed twice with phosphate-buffered saline/Glycine and prehybridized for 2 hours at 39.4°C. The sections were then washed and incubated overnight with the DIG-labeled oligonucleotide probe (250 ng) at 39.4°C in a humidified chamber. The next day the slides were washed in SSC/DTT followed by the detection step performed using Anti-DIG-AP, Fab fragments (Roche Diagnostics, Indianapolis, IN) at 4°C in a humidified chamber, followed by serial washes, NBT/BCIP detection and counterstaining with Nuclear fast red (Sigma).
Immunofluorescence for natural killer cell lectin-like receptor (KLRG1) and CD68
Five –micron sections were cut from formalin fixed, paraffin embedded mouse brains, deparaffinized with Citrisolv (ThermoFisher Scientific) and then rehydrated in graded ethanol baths with a final three minute immersion in water. Antigen retrieval was performed with Antigen Retrieval Citra (Biogenex, San Ramon, CA) by boiling for twenty minutes. Sections were subsequently rinsed in water and then PBS. Blocking was performed in PBS containing 10% goat serum, 1% BSA and 0.025% Triton X-100 for two hours. Sections were incubated with a 1:100 dilution of either anti-KLRG1 (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-CD68 (AbD serotec, Raleigh, NC) for one hour, primary antibody being omitted in the negative control sections. Mouse spleen sections were used as positive control. Sections were washed three times, five minutes each, in PBS and exposed to goat anti-rabbit or goat anti-rat alexa-fluor-647 respectively (Life Technologies, Grand Island, NY) for 45 minutes and subsequently washed in PBS for 35 minutes. Slides were dehydrated in graded ethanol baths, dried and mounted with UltraCruz mounting media (Santa Cruz Biotechnology, Santa Cruz, CA) and examined using a Zeiss LSM510 confocal microscope using a 42X objective (Carl Zeiss Incorporated, North America).
Supplementary Material
Acknowledgments
Funding support provided by NIH P50 CA 108961 and R01 CA 154348.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary information is available at Gene Therapy's website.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer cell. 2009;15(1):45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer research. 2006;66(16):7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 4.Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer cell. 2010;17(4):362–75. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Allen C, Paraskevakou G, Liu C, Iankov ID, Msaouel P, Zollman P, et al. Oncolytic measles virus strains in the treatment of gliomas. Expert opinion on biological therapy. 2008;8(2):213–20. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, Peng KW, et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15(4):677–86. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- 7.Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer research. 2003;63(10):2462–9. [PubMed] [Google Scholar]
- 8.Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641–6. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 9.Msaouel P, Iankov ID, Allen C, Morris JC, von Messling V, Cattaneo R, et al. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate. 2009;69(1):82–91. doi: 10.1002/pros.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng KW, Facteau S, Wegman T, O'Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nature medicine. 2002;8(5):527–31. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 11.Carlson BL, Pokorny JL, Schroeder MA, Sarkaria JN. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. In: Enna SJ, editor. Current protocols in pharmacology. Unit 14. Chapter 14. 2011. p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7 (2):164–76. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 14.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer research. 2007;67(9):4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 15.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer cell. 2006;10(6):454–6. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Nguyen DH, Dong Q, Shitaku P, Chung K, Liu OY, et al. Molecular properties of CD133+ glioblastoma stem cells derived from treatment-refractory recurrent brain tumors. Journal of neuro-oncology. 2009;94(1):1–19. doi: 10.1007/s11060-009-9919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26(18):3015–24. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 18.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9 (11):882–92. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, et al. Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene. 2011;30(31):3454–67. doi: 10.1038/onc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai R, Wakimoto H, Martuza RL, Rabkin SD. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(11):3686–96. doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakimoto H, Kesari S, Farrell CJ, Curry WT, Jr, Zaupa C, Aghi M, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer research. 2009;69 (8):3472–81. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhiman N, Jacobson RM, Poland GA. Measles virus receptors: SLAM and CD46. Rev Med Virol. 2004;14(4):217–29. doi: 10.1002/rmv.430. [DOI] [PubMed] [Google Scholar]
- 23.Yanagi Y, Takeda M, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87(Pt 10):2767–79. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- 24.Schneider U, von Messling V, Devaux P, Cattaneo R. Efficiency of measles virus entry and dissemination through different receptors. J Virol. 2002;76(15):7460–7. doi: 10.1128/JVI.76.15.7460-7467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagi Y. The cellular receptor for measles virus. Uirusu. 2001;51:201–8. [PubMed] [Google Scholar]
- 26.Ulasov IV, Tyler MA, Zheng S, Han Y, Lesniak MS. CD46 represents a target for adenoviral gene therapy of malignant glioma. Hum Gene Ther. 2006;17(5):556–64. doi: 10.1089/hum.2006.17.556. [DOI] [PubMed] [Google Scholar]
- 27.Adams EM, Brown MC, Nunge M, Krych M, Atkinson JP. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. Journal of immunology. 1991;147(9):3005–11. [PubMed] [Google Scholar]
- 28.Fishelson Z. Complement C3: a molecular mosaic of binding sites. Molecular immunology. 1991;28(4–5):545–52. doi: 10.1016/0161-5890(91)90169-k. [DOI] [PubMed] [Google Scholar]
- 29.Oglesby TJ, White D, Tedja I, Liszewski K, Wright L, Van den Bogarde J, et al. Protection of mammalian cells from complement-mediated lysis by transfection of human membrane cofactor protein and decay-accelerating factor. Transactions of the Association of American Physicians. 1991;104:164–72. [PubMed] [Google Scholar]
- 30.Galanis E, O'Neill BP, Piepgras D, Meyer FB, Uhm JH, Marks R, et al. Phase I intratumoral and resection cavity administration of a measles virus derivative expressing the human carcionembroyonic antigen (CEA) in patients with recurrent glioblastoma multiforme (Abstract MA-15) Neuro-Oncology. 2008;10 (5):819. [Google Scholar]
- 31.Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480(7378):530–3. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, Lopez M, et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol. 2010;134(5):835–45. doi: 10.1309/AJCPGXK0FR4MHIHB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabre-Lafay S, Garrido-Urbani S, Reymond N, Goncalves A, Dubreuil P, Lopez M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J Biol Chem. 2005;280(20):19543–50. doi: 10.1074/jbc.M410943200. [DOI] [PubMed] [Google Scholar]
- 34.Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009;69(16):6694–703. doi: 10.1158/0008-5472.CAN-09-0016. [DOI] [PubMed] [Google Scholar]
- 35.Togashi H, Sakisaka T, Takai Y. Cell adhesion molecules in the central nervous system. Cell Adh Migr. 2009;3(1):29–35. doi: 10.4161/cam.3.1.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncology. 2005;7 (2):164–76. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Msaouel P, Iankov ID, Allen C, Aderca I, Federspiel MJ, Tindall DJ, et al. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(12):2041–8. doi: 10.1038/mt.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


