Abstract
Most of the complex traits including aging phenotypes are caused by the interaction between genome and environmental conditions and the interface of epigenetics may be a central mechanism. Although modern technologies allow us high-throughput profiling of epigenetic patterns already at genome level, our understanding of genetic and environmental influences on the epigenetic processes remains limited. Twins are of special interest for genetic studies due to their genetic similarity and rearing-environment sharing. The classical twin design has made a great contribution in dissecting the genetic and environmental contributions to human diseases and complex traits. In the era of functional genomics, the valuable sample of twins is helping to bridge the gap between gene activity and the environments through epigenetic mechanisms unlimited by DNA sequence variations. We propose toextend the classical twin design to studythe aging–related molecular epigenetic phenotypes and link them with environmental exposures especially early life events. Different study designs and application issues will be highlighted and novel approaches introduced with aim at making uses of twins in assessing the environmental impact on epigenetic changes during development and in the aging process.
Keywords: twins, aging, epigenetics, environments, genomics
Introduction
The human life expectancy experienced a remarkable increase during the last century with an unprecedented gain in the developed world (Christensen et al. 2009) and similar improvement has been observed or expected in the developing countries. At the same time, researches have shown even larger improvement in health expectancy than in lifespan (Jeune and Brønnum-Hansen 2008; Robine 2006). These phenomena suggest the important role of improving environment in determining individual health status and survival. Although social-economic development and consequently advances in biomedical technology together with improved healthcare and disease treatment can be considered in explaining these changes, studying the biological mechanism of how environmental attributes affect individual health is of central importance to public health. In the literature, recent studies have shown that the impact of environmental factors can be acquired via the epigenome or genome in epigenetics (Fraga et al. 2005; Wong et al. 2005; Poulsen et al. 2007; Szyf et al. 2008; Ling and Groop 2009; Tan et al. 2010; Petronis 2010), one of the current topics in cancer and complex disease studies drawing active research. Although the molecular evidences are interesting and current techniques such as those offered by Affymetrix and Illumina allow genome-wide epigenetic (DNA methylation) profiling, identifying and understanding the epigenetic patterns under a given genetic predisposition and environmental exposures impose new challenges to traditional epidemiology both in experimental design and in methodological issues.
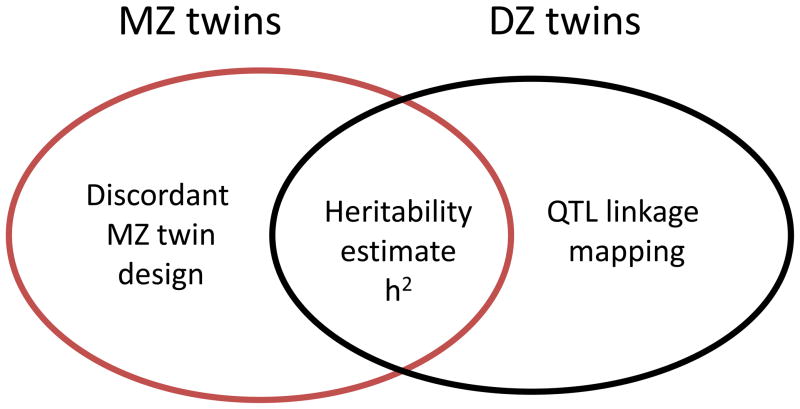
Twins are of special interest for genetic studies due to their genetic similarity and rearing-environmental sharing. The last century witnessed successful uses of twins in exploring the genetic and environmental contributions to human diseases and other complex traits like e.g. life span and aging. The twin design makes use of the unique genetic make-ups in twins and infers the genetic importance in human diseases or traits. By comparing phenotype correlation in identical or monozygotic (MZ) and fraternal or dizygotic (DZ) twin pairs, various genetic and environmental components can be assessed using the classical twin design. For example, the heritability for human lifespan was estimated about 25% using Danish twins (Herskind et al. 1996; Hjelmborg et al. 2006) meaning that about one quarter of the human life span variation can be accounted for by genetic factors. In our recent review (Tan et al. 2010), the use of twins in functional genomic studies of human complex diseases has been summarized and new approaches that expand the traditional twin design to molecular genetic studies proposed. Here, we emphasize the novel use of identical twins discordant in disease phenotypes in epigenetic studies which can be a powerful approach in linking disease with gene regulation patterns and environmental exposures. Likewise, the human aging phenotypes are complex phenotypes that can be affected by a large number of both genetic and environmental factors together with their interplay. With proper and efficient design, twins can offer remarkable opportunities for functional genomic studies of human aging and development. In this paper, we highlight the different twin study designs and application issues and summarize the newest development in making uses of twins in assessing the environmental impact on epigenetic changes during development and in the aging process (Figure 1).
Figure 1.
The uses of twins in epigenetic studies. To the left, MZ twin pairs discordant for one trait or phenotype can be sampled and analyzed for identifying loci under differential epigenetic modification. To the right, non-parametric quantitative trait loci linkage mapping can be applied to molecular epigenetic phenotypes in DZ twins to map genes showing trans- or cis- regulatory mechanisms. In the middle, with both MZ and DZ twins, the classical twin model can be extended to molecular epigenetic phenotypes for assessing the genetic and environmental contributions in epigenetic control over gene activities.
New technology and new opportunities
Right at conception, the genomic DNA sequence is identically fixed. However, the functional profile of a gene is determined not only by its sequence but also by the way in which it is regulated by epigenetic mechanisms including DNA methylation, histone modification, different species of non-coding RNAs, etc. It is anticipated that, perhaps the most significant change in the 21st century genetics will be the shift from structural genomics, where genes are regarded as a static concept, to functional genomics, where the dynamic patterns of gene activity are analyzed jointly from gene interaction to gene regulation and to functional genomics analysis (Peltonen & McKnusick, 2001). Among the various mechanisms, DNA methylation is the major form of epigenetic modification that is most robust and readily measurable using high throughput techniques. For example, the Affymetrix high-density tiling arrays using chromatin immunoprecipitation (ChIP-on-chip), Illumina Bead Arrays, and Illumina Solexa sequencing based DNA methylation analysis by sequencing bisulphite-treated DNA (Zhang and Jeltsch 2010). All these techniques enable massive epigenetic profiling at genome scale.
The epigenetic modification involves binding of small molecules to specific sites in DNA or chromatin (Foley et al. 2009) which acts as volume controls that up or down regulate a gene’s expression without changing its DNA sequence. For example, a gene is generally silenced by methylation at the 5′ position of the cytosine pyrimidine ring in DNA at a CpG (cytosine and guanine nucleotides linked by phosphate) dinucleotide, known as CpG islands, clustered in the promoter region of many genes. At certain times during development, specialized cellular machinery scours the genome and erases its epigenetic tags in order to restore a genetic “blank state” during which a small minority of genes make it through this process and pass their epigenetic tags unchanged from parent to offspring (Pál and Hurst, 2004). This phenomenon suggests that the molecular mechanisms of heritability may not be limited to DNA sequences. The inheritance of epigenetic patterns could help to explain, in part, the missing heritability in current GWAS studies on complex diseases or phenotypes (McCarthy and Hirschhorn, 2008).
Of important properties for epigenetic regulation of gene activities is that it is under control of both genetic and environmental factors. For example, individual variations at the methylenetetrahydrofolate reductase gene can vary the levels of DNA methylation (Castro et al. 2004; Friso et al. 2002). Dissecting the genetic and environmental components in the measured epigenetic status and linking it with disease conditions or the aging process imposes a new challenge to biostatisticians and bioinformaticians in health and aging research.
Modern use of a classical design
Aging is a natural process that everyone must undergo at his or her own time and pace. Although genetics may initially determine how a person ages, environment could over time play a higher role than the genes during aging.
Environments especially early life events are important modifiers of the aging process (Szyf 2012, 2009; Murgatroyd and Spengler, 2011). Since the genetic mechanisms behind aging are not controllable, understanding how environmental factors retard or accelerate the aging process is of more practical impact. For that purpose, it is essential to dissect the genetic and environmental components for which twins can contribute. In brief, the relative importance of genetic and environmental components in the variation of a phenotype can be assessed by comparing phenotype correlation within identical twin pairs who share their nuclear DNA with that within DZ twin pairs who share only 50% of DNA sequence on average. In the literature, the classical twin design has been applied to multiple aging phenotypes including for example, physical (Frederiksen et al. 2002) and cognitive (Greenwood et al. 2011; Reynolds et al. 2005) functions in the elderly. A similar twin method was applied to gene expression data from elderly Danish twins (Tan et al. 2005). This analysis gave relatively high heritability estimates for the activity levels of the top most active genes.
Recently, the twin method has been applied to studying global DNA methylation profiles in MZ and DZ twins (Kaminsky et al. 2009) with highly significant epigenetic differences reported in DZ twins as compared with that in MZ twins. The result suggests that both genetic and environmental factors influence epigenetic regulation of gene activities and provides a model for measuring their relative contributions. Note that DNA methylation levels are dynamic and change throughout an individual’s life course. However, both animal and human data suggest early life milieu (including intrauterine life) have a great impact on the epigenome which can be linked to the development of obesity (Lillycrop and Burdge, 2011), diabetes (Fradin and Bougneres, 2011), stress (Murgatroyd and Spengler, 2011) and late-onset mental illnesses (Szyf et al. 2007; McGowan and Szyf, 2010). This can be due to the fact that many of the differential gene expressions in determining the structural and functional differentiation of cells in a multicellular organism arise during development. Epigenetic changes triggered by early life environment offer a plausible mechanism by which early experiences could be integrated into the genome to program adult hormonal and behavioral responses, thus facilitating the adaptation of an organism to changing environment through alterations in gene activity. Of special interest in the classic twin model that contains additive genetic (A), common environmental (C) and unique environmental (E) effects, i.e. the ACE model, the C component reflects the common rearing environment in their early lives. When applied to epigenetic measurement data (e.g. treating the DNA methylation level as phenotype), the ACE model can be compared to its nested model, the AE model, to examine if the rearing or early-life environmental conditions contribute to the epigenetic regulations. This means that the ACE model meets nicely the need for linking early-life environmental exposure with later-life epigenetic status. The same model can be applied to the gene expression phenotypes as well with aim at assessing the genetic and environmental modifications on transcriptional activities afterwards.
Aging is accompanied by various altered phenotypes that might be linked to epigenetic change. A growing number of reports in the literature have demonstrated that the DNA methylation is an active process in post-mitotic cells and that the epigenome maturates continuously throughout lifespan with the effects of aging emerging gradually over time as a result of exposure to a variety of environmental factors during aging. As an example, it has been shown that changes in the epigenetic modification may form the molecular basis for cognitive aging (Peleg et al. 2010; Sweatt 2010).
Tan et al. (2008) reported the age-associated changes in gene expression in the CEPH families. Although the observed age-dependent changes in gene expression can be due to both epigenetic regulation and genetic alterations accumulated from DNA damage, it can be postulated that a considerable part of the changes can be accounted for by epigenetics. By treating the measured gene expression or DNA methylation levels as phenotypes of interest, the twin modelling can be applied at different ages to estimate age-associated changes in the genetic and environmental influences. Results from such studies could help to establish if age-associated epigenetic modification drives age-associated changes in gene expression and how genetic and environmental factors contribute to these changes.
Monozygotic twins: a unique source
Alterations in gene expression due to global epigenetic changes triggered by genetic, environmental and stochastic effects accumulate over time (Fraga et al. 2005) and can have substantial impact on susceptibilities to aging-associated diseases and health status (Gronniger et al. 2010; Gilbert 2009; Poulsen et al. 2007; Holiday 2010; Bocklandt et al. 2011), adding new evidence that nature and nurture are, in fact, inextricably linked. Elucidating the complex interplay between gene and environment that contributes to complex phenotypes poses a new challenge.
Identical twins serve as a good and valuable source for studying the epigenetics of complex phenotypes like aging because the genetic background is perfectly matched within each pair (Petronis 2006; Poulsen et al. 2007). Using the discordant MZ twins design (Figure 2), MZ twins discordant for a phenotype of interest are sampled from independent families with which intra-pair genome-wide epigenetic differences can be determined by high throughput techniques for measuring differential epigenetic modifications (Tan et al. 2010). In the discordant MZ twins design, the genetic influences on epigenetic changes are cancelled out so that differential DNA methylation triggered by environmental factors can be identified (Figure 3). In the literature, the design has been applied in gene expression analysis by Pietilainen et al. (2008) who studied the global transcript profiles of fat in MZ twins discordant for BMI and reported interesting findings suggesting the substantial roles of mitochondrial energy and amino acid metabolism behind acquired obesity and insulin resistance. The design has been applied in epigenetic studies of other complex traits as well including schizophrenia (Petronis et al. 2003), behavior traits (Kaminsky et al. 2008), and aging (Fraga et al. 2005; Gronniger et al. 2010; Wang et al. 2008). For example, using MZ twins who are derived from a single zygote and thus are considered genetically identical, Fraga et al. (2005) found that the young twins are epigenetically more similar than old twins suggesting epigenetic modification induced by accumulated stimulation by internal and external factors including environmental exposure.
Figure 2.
The discordant MZ twin design. With this design, identical twins discordant for one phenotype are collected and molecular epigenetic patterns compared to look for significant differential methylation sites which can then be linked to environmental exposures or conditions.
Figure 3.
Genetic and environmental influences on epigenetic regulation of gene expression behind human diseases and aging phenotype. Both internal genetic factors (cis- or trans- effects) and constant stimulation by environmental conditions can lead to altered epigenetic control over gene activities and the latter results in abnormal health status. With the discordant MZ twins design, the genetic part to the right is cancelled out so that increased power obtained for estimating the environmental effects on epigenetic responses.
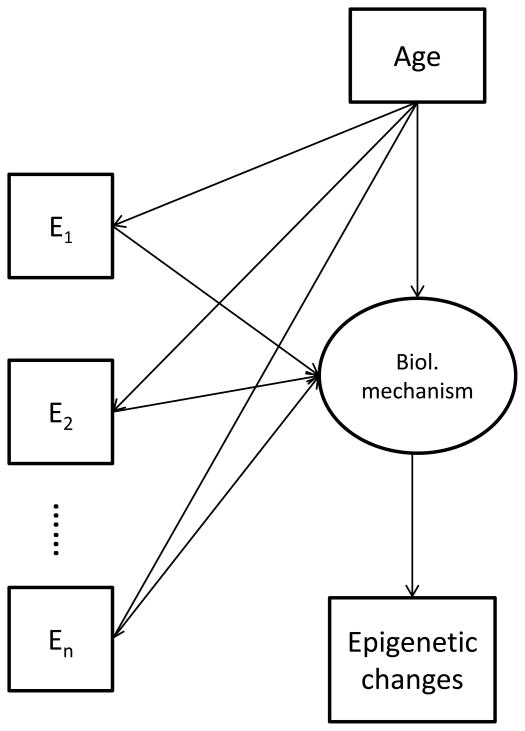
An identified site of significant epigenetic regulation can be a result of accumulated effect from multiple environmental stimuli that act through a shared biological mechanism. Both the exploratory (principal component analysis, PCA) and confirmatory (structural equation model, SEM) multivariate analyses are useful in analyzing such data. With the discordant MZ twins design, the SEM can be applied to link the multiple observed environmental factors with the measured molecular epigenetic phenotypes. In this model, age acts as a modifier for the environments and the latent biological mechanisms (Figure 4). Note that, in the diagram, the influences of age on the latent variable include stochastic effects accumulated over time during developmental stages or in the aging process. The model can be extended to cover clusters of environmental variables which can work through different biological mechanisms that all lead to altered patterns of epigenetic regulation.
Figure 4.
A structural equation model for linking multiple observed environmental factors with measured molecular epigenetic phenotypes. The model assumes there is a latent biological mechanism through which a group of environmental variables influence the epigenetic phenotypes. Note that, the model can be extended to include multiple groups of environmental variables each of specific nature and works through a specific biological pathway but all end up with the same epigenetic alterations.
DZ twins for QTL mapping
The fast development in high throughput SNP genotyping techniques using DNA microarray platforms, such as Illumina and Affymetrix, and more recently, the next generation sequencing technique, is revolutionizing the way we design and conduct genetic epidemiological studies of human complex traits or diseases. High resolution genome-wide scans enabled by the high density and informative SNP marker arrays makes it possible for researchers to map quantitative trait loci (QTL) that are responsible for human complex traits or diseases.
DZ twin pairs or fraternal twins are basically siblings. By comparing phenotype-dependent allele-sharing identical-by-decent (IBD) within a pair, the non-parametric linkage analysis can be conducted using regression or variance component analysis (Kruglyak et al. 1996). Instead of disease phenotypes, the measured molecular epigenetic status (e.g. DNA methylation levels) can be employed as the quantitative trait or phenotype of interest. We emphasize that linkage scans using DZ twins are advantaged by the fact that DZ twins are matched for pre-natal and childhood shared rearing environmental factors and especially perfectly matched for their ages. These factors could be potentially associated with the study phenotypes and thus need to be adjusted for in sib-pair based linkage analysis. The twin-based non-parametric QTL mapping of epigenetic phenotypes can be further advantaged by the low probability of non-paternity (MacGregor et al. 2000) that causes miscalculation of IBD probability and thus affects linkage results when parental genotype information is missing. This is especially relevant to aging studies because it is usually impossible to genotype the parents of old study participants. The DZ twins have been frequently used in linkage mapping of QTLs, especially in recent years (De Moor et al. 2007; Livshits et al. 2007; Perola et al. 2007; O’Connor et al. 2008). Likewise, sib-pair based methods for association mapping such as S-TDT (Spielman & Ewens, 1998) or QTDT (Abecasis et al. 2000; Ewens et al. 2008) can be applied to DZ twin data as well.
In the literature, both linkage (Schadt et al. 2003; Morley et al. 2004; Monks et al. 2004) and association (Cheung et al. 2005) approaches have been applied to map genomic regions that show epigenetic control over transcriptional activities of specific genes including cis (local) or trans (distant) acting regulations. Although the mRNA level can be correlated with molecular epigenetic status (e.g. DNA methylation levels), direct application of linkage and association mapping to a molecular epigenetic phenotype (DNA methylation, histone acetylation, ncRNA expression) should produce more efficient and specific results because mRNA levels can be affected by different epigenetic mechanisms involving different genetic regulations. Finally, both approaches can be applied to DZ twin samples of young, middle and old ages to examine how these regulation patterns change over time.
Twins and noncoding RNAs
The central dogma of “DNA makes RNA makes protein” is being challenged by recent observations about the extent of noncoding RNA (ncRNA) transcription in the higher eukaryotes and the range of RNA-directed genetic and epigenetic phenomena (Esteller 2011). The currently identified major species of ncRNAs include short micro-RNAs (miRNAs), small interfering RNA (siRNA), long intergenic ncRNAs (lincRNAs)(Mercer et al. 2009), and manyother, as yet, unknown ncRNAs. Among them, miRNAs are a major category in the ncRNAs that negatively regulate gene expression at the post -transcriptional level either by degrading the target messages or by inhibiting their translation. The regulatory RNAs play pivotal roles in a wide variety of cellular processes through up -regulation of specific groups of mi RNAs to suppress unwanted gene expressions and down-regulation of other miRNAs whose target genes’ expression is necessary for cellular function. Studies using model systems have shown that with age, there is a trend of up-regulation of miRNA expression which leads to down -regulation of target genes suggesting that epigenomic control of aging may be due in part to increased expression of unwanted miRNAs who in turn down-regulate their target genes (Liang et al. 2009). Recent genome-wide association studies have revealed that, a large ncRNA called ANRIL (antisense ncRNA in the INK4 locus) has shared association with coronary disease, intracranial aneurysm and also type 2 diabetes which in general suggest high implication in cellular aging (Pasmant et al. 2011; Gonzalo 2010). Overriding and dampening the activation of these ncRNAs may prove to be a new frontier for future researches with aim at delaying the aging process and extending health expectancy.
Current studies on ncRNAs are limited to expression analysis. In order to elucidate the genetic and environmental roles in the regulatory activity of ncRNAs, it is important to study the genetics of ncRNAs (Wapinski and Chang 2011). The different approaches in using twins for genetic studies of protein coding RNAs (Tan et al. 2010) apply to the genetics of ncRNAs (Figure 1). For example, the linkage analysis on DZ twins can be used to map genes that regulate the expression activity of an ncRNA which can be trans- or cis- regulations. Likewise, the discordant MZ twins design can help to associate observed phenotype variations with ncRNA expressions while controlling for genetic background (Figure 3). While the different analyses in Figure 1 can be performed at different ages or developmental stages to display the age-dependent patterns in ncRNA-based epigenetic regulations, the SEM approach in Figure 4 assesses directly the age effects on environmental exposure and on the latent unobserved biological mechanisms (including accumulation of stochastic events) that lead to epigenetic modifications.
Concluding remarks
Most of the complex traits including diseases and aging phenotypes are caused by the interaction between genome and environment through the interface of epigenetics. Although modern technologies allow us high-throughput profiling of epigenetic patterns already at genome level, our understanding of genetic and environmental influences on epigenetic processes remains limited. With proper design and analytical approaches, twins can help us with identifying epigenetic marks and link them with environmental exposures especially early life events for measuring their influences in epigenetic mechanisms at the population level using twin modeling, linkage mapping, discordant MZ twins design, etc. It can be expected that the valuable sample of twins is going to make new contributions in revealing and understanding the genetic and epigenetic mechanisms of human aging and development.
Highlights.
Summarize newest development in making use of twins in epigenetic studies on human aging
Introduce new extension of the classical twin design to studying molecular phenotypes
Propose novel approaches for finding loci under epigenetic regulation in human aging
Associate changes in the observed epigenetic phenotypes with environmental variables
Acknowledgments
This work was jointly supported by the Novo Nordisk Foundation 2010 research grant (principle investigator: Qihua Tan) and the Region of Southern Denmark 2010 research grant (principle investigator: Qihua Tan), the EU Seventh Framework Programme (FP7/2007-2011) under grant agreement n° 259679 and NIH/NIA grant P01 AG08761.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66(1):279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocklandt S, Lin W, Sehl ME, Sánchez FJ, Sinsheimer JS, et al. Epigenetic Predictor of Age. PLoS ONE. 2011;6(6):e14821. doi: 10.1371/journal.pone.0014821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R, Rivera I, Ravasco P, et al. 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C–>T and 1298A–>C mutations are associated with DNA hypomethylation. J Med Genet. 2004;41:454–458. doi: 10.1136/jmg.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VG, Spielman RS, Ewens KG, Weber TM, Morley M, Burdick JT. Mapping determinants of human gene expression by regional and genome-wide association. Nature. 2005;437(7063):1365–1369. doi: 10.1038/nature04244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moor MH, Spector TD, Cherkas LF, Falchi M, Hottenga JJ, Boomsma DI, De Geus EJ. Genome-wide linkage scan for athlete status in 700 British female DZ twin pairs. Twin Res Hum Genet. 2007;10(6):812–820. doi: 10.1375/twin.10.6.812. [DOI] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nature Review Genetics. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Ewens WJ, Li M, Spielman RS. A review of family-based tests for linkage disequilibrium between a quantitative trait and a genetic marker. PLoS Genet. 2008;4(9):e1000180. doi: 10.1371/journal.pgen.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley DL, Craig JM, Morley R, Olsson CJ, Dwyer T, Smith K, Saffery R. Prospects for Epigenetic Epidemiology. Am J Epidemiol. 2009;169(4):389–400. doi: 10.1093/aje/kwn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin D, Bougnères P. T2DM: Why Epigenetics? Journal of Nutrition and Metabolism. 2011:Article ID 647514. doi: 10.1155/2011/647514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genetic Epidemiology. 2002;23:110–122. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- Friso S, Choi SW, Girelli D, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SF. Ageing and cancer as diseases of epigenesist. J Biosci. 2009;34:601–604. doi: 10.1007/s12038-009-0077-4. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Beeri MS, Schmeidler J, Valerio D, Raventós H, Mora-Villalobos L, Camacho K, Carrión-Baralt JR, Angelo G, Almasy L, Sano M, Silverman JM. Heritability of cognitive functions in families of successful cognitive aging probands from the central valley of costa rica. J Alzheimers Dis. 2011;27(4):897–907. doi: 10.3233/JAD-2011-110782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S. Epigenetic alterations in aging. Journal of Applied Physiology. 2010;109(2):586–597. doi: 10.1152/japplphysiol.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronniger E, Weber B, Heil O, Peters N, Stab F, Wenck H, et al. Aging and chronic sun exposure cause distinct epigenetic Changes in Human Skin. PLoS Genet. 2010;6(5) doi: 10.1371/journal.pgen.1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97: 319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Hjelmborg JB, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL, Christensen K. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Holliday R. Perspectives in Aging and Epigenetics. Epigenetics of Aging. 2010;Part VII:447–455. doi: 10.1007/978-1-4419-0639-7_25. [DOI] [Google Scholar]
- Jeune B, Brønnum-Hansen H. Trends in health expectancy at age 65 for various health indicators, 1987–2005, Denmark. European Journal of Ageing. 2008;5:279–285. doi: 10.1007/s10433-008-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky Z, Petronis A, Wang SC, Levine B, Ghaffar O, Floden D, Feinstein A. Epigenetics of personality traits: an illustrative study of identical twins discordant for risk-taking behaviour. Twin Res Hum Genet. 2008;11(1):1–11. doi: 10.1375/twin.11.1.1. [DOI] [PubMed] [Google Scholar]
- Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, Feldcamp LA, Virtanen C, Halfvarson J, Tysk C, McRae AF, Visscher PM, Montgomery GW, Gottesman II, Martin NG, Petronis A. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009;41(2):240–245. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Lander ES. Complete multipoint sib-pair analysis of qualitative and quantitative traits. Am J Hum Genet. 1995;57:439–454. [PMC free article] [PubMed] [Google Scholar]
- Liang R, Bates DJ, Wang E. Epigenetic control of microRNA expression and aging. Curr Genomics. 2009;10:184–193. doi: 10.2174/138920209788185225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA, Burdge GC. Epigenetic changes in early life and future risk of obesity. Int J Obes (Lond) 2011;35(1):72–83. doi: 10.1038/ijo.2010.122. [DOI] [PubMed] [Google Scholar]
- Ling C, Groop L. Epigenetics: A molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718–2725. doi: 10.2337/db09-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshits G, Kato BS, Wilson SG, Spector TD. Linkage of genes to total lean body mass in normal women. J Clin Endocrinol Metab. 2007;92(8):3171–3176. doi: 10.1210/jc.2007-0418. [DOI] [PubMed] [Google Scholar]
- MacGregor AJ, Snieder H, Schork NJ, Spector TD. Twins. Novel uses to study complex traits and genetic diseases. Trends Genet. 2000;16(3):131–134. doi: 10.1016/s0168-9525(99)01946-0. [DOI] [PubMed] [Google Scholar]
- McCarthy MI, Hirschhorn JN. Genome-wide association studies: past, present and future. Hum Mol Genet. 2008;17 (R2):R100–R101. doi: 10.1093/hmg/ddn298. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol Dis. 2010;39(1):66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nature Review Genetics. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- Monks SA, Leonardson A, Zhu H, Cundiff P, Pietrusiak P, Edwards S, Phillips JW, Sachs A, Schadt EE. Genetic inheritance of gene expression in human cell lines. Am J Hum Genet. 2004;75(6):1094–1105. doi: 10.1086/426461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley M, Molony CM, Weber TM, Devlin JL, Ewens KG, Spielman RS, Cheung VG. Genetic analysis of genome-wide variation in human gene expression. Nature. 2004;430(7001):743–747. doi: 10.1038/nature02797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Spengler D. Epigenetics of early child development. Front in Psychiatry. 2011;16(2):1–15. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, Mahata SK, Mahata M, Wang L, Zhang K, Greenwood TA, Shih PA, Cockburn MG, Ziegler MG, Stridsberg M, Martin NG, Whitfield JB. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation. 2008;118(3):247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál C, Hurst LD. Epigenetic Inheritance and Evolutionary Adaptation. In: Hirt Robert P, Horner David S., editors. Organelles, genomes, and eukaryote phylogeny: an evolutionary synthesis in the age of genomics. CRC Press; 2004. pp. 347–364. [Google Scholar]
- Pasmant E, Sabbagh A, Vidaud M, Bièche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. The FASEB Journal. 2011;25(2):444–448. doi: 10.1096/fj.10-172452. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, et al. Altered Histone Acetylation Is Associated with Age-Dependent Memory Impairment in Mice. Science. 2010;328(5979):753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Peltonen L, McKusick VA. Genomics and medicine. Dissecting human disease in the postgenomic era. Science. 2001;291(5507):1224–1229. doi: 10.1126/science.291.5507.1224. [DOI] [PubMed] [Google Scholar]
- Perola M, Sammalisto S, Hiekkalinna T, et al. Combined genome scans for body stature in 6,602 European twins: evidence for common Caucasian loci. PLoS Genet. 2007;3(6):e97. doi: 10.1371/journal.pgen.0030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronis A, Gottesman II, Kan P, Kennedy JL, Basile VS, Paterson AD, Popendikyte V. Monozygotic twins exhibit numerous epigenetic differences: clues to twin discordance? Schizophr Bull. 2003;29(1):169–178. doi: 10.1093/oxfordjournals.schbul.a006988. [DOI] [PubMed] [Google Scholar]
- Petronis A. Epigenetics and twins: three variations on the theme. Trends Genet. 2006;22(7):347–350. doi: 10.1016/j.tig.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010;465:721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H, Suomalainen A, Götz A, Suortti T, Yki-Järvinen H, Oresic M, Kaprio J, Peltonen L. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 2008;5(3):e51. doi: 10.1371/journal.pmed.0050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P, Esteller M, Vaag A, Fraga MF. The epigenetic basis of twin discordance in age-related diseases. Pediatr Res. 2007;61(5 Pt 2):38R–42R. doi: 10.1203/pdr.0b013e31803c7b98. [DOI] [PubMed] [Google Scholar]
- Reynolds CA, Finkel D, McArdle JJ, Gatz M, Berg S, Pedersen NL. Quantitative genetic analysis of latent growth curve models of cognitive abilities in adulthood. Dev Psychol. 2005;41:3–16. doi: 10.1037/0012-1649.41.1.3. [DOI] [PubMed] [Google Scholar]
- Robine JM. Trends in population health. Aging Clinical and Experimental Research. 2006;18(5):1–3. doi: 10.1007/BF03324829. [DOI] [PubMed] [Google Scholar]
- Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, Colinayo V, Ruff TG, Milligan SB, Lamb JR, Cavet G, Linsley PS, Mao M, Stoughton RB, Friend SH. Genetics of gene expression surveyed in maize, mouse and man. Nature. 2003;422:297–302. doi: 10.1038/nature01434. [DOI] [PubMed] [Google Scholar]
- Spielman RS, Ewens WJ. A sibship test for linkage in the presence of association: the sib transmission/disequilibrium test. Am J Hum Genet. 1998;62(2):450–458. doi: 10.1086/301714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Epigenetics and Cognitive Aging. Science. 2010;328:701. doi: 10.1126/science.1189968. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reproductive Toxicology. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environmental and Molecular Mutagenesis. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- Szyf M. The early life environment and the epigenome. Biochimica et Biophysica Acta. 2009;1790:878–885. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Szyf M. The early life social environment and DNA methylation. Clin Genet. 2012 doi: 10.1111/j.1399–0004.2012.01843.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Tan Q, Christensen K, Christiansen L, Frederiksen H, Bathum L, Dahlgaard J, Kruse TA. Genetic dissection of gene expression observed in whole blood samples of elderly Danish twins. Hum Genet. 2005;117:267–274. doi: 10.1007/s00439-005-1308-x. [DOI] [PubMed] [Google Scholar]
- Tan Q, Zhao J, Li S, Christiansen L, Kruse TA, Christensen K. Differential and correlation analyses of microarray gene expression data in the CEPH Utah families. Genomics. 2008;92(2):94–100. doi: 10.1016/j.ygeno.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q, Ohm Kyvik K, Kruse TA, Christensen K. Dissecting complex phenotypes using the genomics of twins. Funct Integr Genomics. 2010;10(3):321–327. doi: 10.1007/s10142-010-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Oelze B, Schumacher A. Age-Specific Epigenetic Drift in Late-Onset Alzheimer’s Disease. PLoS ONE. 2008;3(7):e2698. doi: 10.1371/journal.pone.0002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wong AH, Gottesman II, Petronis A. Phenotypic differences in genetically identical organisms: the epigenetic perspective. Hum Mol Genet. 2005;14(Spec No 1):R11–8. doi: 10.1093/hmg/ddi116. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jeltsch A. The application of next generation sequencing in DNA methylation analysis. Genes. 2010;1:85–101. doi: 10.3390/genes1010085. [DOI] [PMC free article] [PubMed] [Google Scholar]