Abstract
Objective
There is significant evidence for a central role of inflammation in the development of Alzheimer’s disease (AD). Epidemiological studies indicate that chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) reduces the risk of developing AD in healthy aging populations. As NSAIDs inhibit the enzymatic activity of the inflammatory cyclooxygenases COX-1 and COX-2, these findings suggest that downstream prostaglandin signaling pathways function in the pre-clinical development of AD. Here, we investigate the function of prostaglandin E2 (PGE2) signaling through its EP3 receptor in the neuroinflammatory response to Aβ peptide.
Methods
The function of PGE2 signaling through its EP3 receptor was examined in vivo a model of subacute neuroinflammation induced by administration of Aβ42 peptides. Our findings were then confirmed in young adult APPSwe-PS1 ΔE9 transgenic mice.
Results
Deletion of the PGE2 EP3 receptor in a model of Aβ42 peptide-induced neuroinflammation reduced pro-inflammatory gene expression, cytokine production, and oxidative stress. In the APPSwe-PS1 ΔE9 model of Familial AD, deletion of the EP3 receptor blocked induction of pro-inflammatory gene and protein expression and lipid peroxidation. In addition, levels of Aβ peptides were significantly decreased, as were BACE-1 and β-CTF levels, suggesting that generation of Aβ peptides may be increased as a result of pro-inflammatory EP3 signaling. Finally, deletion of EP3 receptor significantly reversed the decline in pre-synaptic proteins seen in APPSwe-PS1 ΔE9 mice.
Interpretation
Our findings identify the PGE2 EP3 receptor as a novel pro-inflammatory, pro-amyloidogenic, and synaptotoxic signaling pathway, and suggest a role for COX-PGE2-EP3 signaling in the development of AD.
Keywords: Alzheimer’s disease, NSAIDs, cyclooxgenases, prostaglandins, COX-2, PGE2, EP3 receptor, Aβ amyloid, and innate immunity
INTRODUCTION
The pre-clinical development of AD is now believed to span years to decades, with progressive increases in amyloid β accumulation, tau phosphorylation, and synaptic injury that are well underway by the time of initial diagnosis. Importantly, these cardinal features of pathology develop in the context of progressive inflammation, manifested by glial activation, generation of cytokines and chemokines, complement proteins, inflammatory enzymes, and oxidative stress 1. Epidemiologic data indicate that inhibition of the inflammatory cyclooxygenases COX-1 and COX-2 by non-steroidal anti-inflammatory drugs (NSAIDs) can prevent the development of AD in normal aging populations 2–7. A primary action of NSAIDs is enzymatic inhibition of both COX-1 and COX-2 activity leading to a reduction of downstream prostaglandin signaling. In addition, selected NSAIDs can have cyclooxygenase-independent effects including the modulation of γ-secretase activity 8, activation of PPAR-γ receptors 9, and inhibition of NF-kB 10. Although NSAID administration to healthy aging populations delays the onset and risk of developing AD, administration of NSAIDs or selective COX-2 inhibitors does not benefit patients with mild cognitive impairment (MCI) or symptomatic AD 11, 12, and may accelerate early cognitive impairment in patients who are at early stages of cognitive decline 7, 13–15. Thus, inhibition of COX enzymatic activity by NSAIDs appears to have different consequences depending on the timing of AD evolution, and inhibition of cyclooxygenase may be beneficial in preventing disease onset in healthy aging individuals but ineffectual or deleterious once symptoms of cognitive dysfunction begin 7, 16. Given the expanding population of aging individuals and the anticipated rise in AD cases, understanding the molecular mechanisms by which NSAIDs prevent AD has taken on significant urgency.
A primary pharmacologic effect of NSAIDs is the inhibition of the COX-1 and COX-2 enzymes that catalyze the first committed step in the synthesis of the prostanoids PGE2, PGD2, PGI2, PGF2a, and TXA2. These lipid signaling molecules activate specific G-protein-coupled receptors designated EP (for E-prostanoid receptor), FP, DP, IP, and TP, respectively 17. PGE2 in particular is of interest in the development of AD, as it is initially significantly elevated in patients with very early stage or probable AD 18, 19 but then declines with disease progression 19. PGE2 binds four distinct E-prostanoid receptors (EP1–4) that have differing downstream signaling cascades and cellular distributions 20. The PGE2 EP2 receptor in particular has been extensively studied, and in vivo mediates pro-inflammatory effects in models of innate immunity 21, AD 22, and amyotrophic lateral sclerosis 23, and suppresses clearance of toxic substances such as amyloid beta peptides 24, 25 and α-synuclein 26. In this study, we investigate the function of the related PGE2 EP3 receptor in a model of Aβ peptide-induced neuroinflammation and in young aging APPSwe-PS1 ΔE9 mice, and we determine that the EP3 receptor exerts significant inflammatory, amyloidogenic, and synaptotoxic effects in vivo.
METHODS
Detailed methods are available in Supplementary Materials
Human brain tissue
Temporal cortex from control, MCI, and AD patients (ADRC, University of Washington, Seattle, WA) was derived from subjects aged 79–88 with a post-mortem delay of 2.5h to 8h.
Animals
This study was conducted in accordance with NIH guidelines and protocols were approved by the Institutional Animal Care and Use Committee at Stanford University. C57BL/6J APPSwe-PS1 ΔE9 mice 27 were serially crossed to C57BL/6J EP3−/− and EP3+/+ mice 28, 29 and aged to 8 mos.
Immunostaining
Immunostaining of brain sections was carried out as previously described 23 using anti-human EP3 (1/100; Cayman Chemicals, Ann Arbor, MI), Neu N (1/1000; Chemicon, Temacula, CA), anti-Iba 1 (1/500; Wako, Richmond, VA), and anti-GFAP (1/2000; Dako, Carpenteria, CA).
Intracerebroventricular (ICV) injections of Aβ42
Oligomeric and fibrillar Aβ peptide species were generated as previously described 30 and Aβ peptides (40 pmol) or vehicle (PBS) were administered to EP3+/+ and EP3−/− mice ICV as previously described 31. Stereotaxic injections were placed at the following coordinates from the bregma: mediolateral: −1.0 mm; anteroposterior: −0.3mm; dorsoventral: −2.5mm. Following injection, each mouse recovered spontaneously on a heated pad. The reliability of injection sites was validated by injecting trypan blue dye (Life Technologies, Grand Island, NY) in separate cohorts of mice and observing staining in the cerebral ventricles. 7 days after injection, mice were euthanized and brains were rapidly harvested.
Quantitative Real-Time PCR
Quantitative real-time PCR (qPCR) using the QuantiTect SYBR Green PCR kit (Qiagen) was carried out as previously described 32. Primers (Integrated DNA Technologies, Coralville, IA) are listed in Supplementary Table 1.
Western analysis and ELISA
Quantitative Western analysis was performed as previously described 32, 33 and proteins were normalized to GAPDH or actin on the same blot. TNF-α, MIP-1α (R&D Systems, Minneapolis, MN) and IL-1β (BD Biosciences, San Diego, CA) were measured by ELISA.
Measurement of F2-isoprostanes and F4-neuroprostanes
F2-isoprostanes and F4-neuroprostanes were measured by GC/MS as previously described 32.
Aβ peptide quantification
Levels of total guanidine-extracted Aβ40 and Aβ42 peptides were measured by ELISA as previously described 22, 34. Quantification of Congo Red and 6E10 antibody stained sections was carried out as previously described 22.
Statistical analysis
Student’s T test or one-way and two-way ANOVAs were used. Newman-Keuls post-hoc test was applied to significant main effects and interactions to estimate differences between particular sets of means. All data are plotted as mean +/− SEM.
RESULTS
EP3 receptor expression in brain
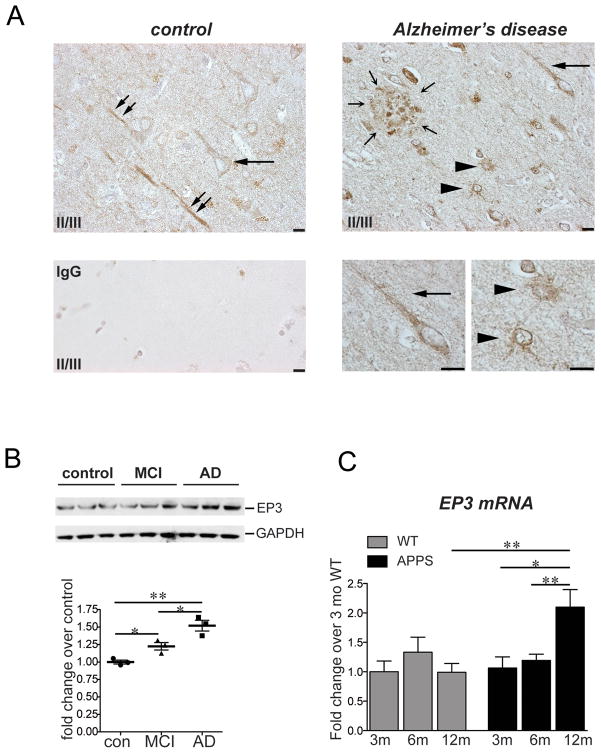
Expression of the EP3 receptor was examined in temporal cortex of control, mild cognitive impairment (MCI), and AD brain (Figure 1). In AD brain, EP3 immunostaining using an antibody directed against the human EP3 receptor was prominent throughout the cortical rim in multiple cell types, including neurons (Figure 1A, horizontal arrow) and astrocytes (arrowheads) surrounding amyloid plaques (outlined by small arrows). Strong EP3 staining was localized within amyloid deposits, potentially corresponding to dystrophic neurites. Staining was present at much lower levels in neurons in control temporal cortex, and EP3 stained glial cells were not visualized. Quantitative Western analysis demonstrated a modest but significant increase in EP3 receptor expression in MCI samples compared to control samples, with a further increase in AD samples (Figure 1B). In mouse, staining of primary neuronal, astrocytic, and microglial cells demonstrated specific EP3 receptor staining (Supplementary Fig 1), however examination of EP3 receptor expression in mouse brain sections yielded high background in both EP3+/+ and EP3−/− mice despite multiple staining strategies (not shown). Quantitative PCR (qPCR) however indicated a significant increase in levels of hippocampal EP3 mRNA in aging male APPSwe-PS1 ΔE9 (APPS) mice as compared to wild type C57B6 age-matched control mice (Figure 1C). Taken together, these data indicate that the EP3 receptor is present in brain in neuronal and glial cell types, and levels increase in human samples with MCI and AD and in mouse transgenic APPS mice between 6 and 12 mo, a period in which inflammatory and oxidative injury, amyloid deposition, and synaptotoxicity begin and progress rapidly.
Figure 1. The PGE2 EP3 receptor is expressed in AD and in mouse brain.
(A)400X magnification of human control and AD temporal cortex, layers II/III, demonstrates EP3 receptor expression in multiple cell types. In control temporal cortex, EP3 receptor is expressed at low levels in neuronal soma (horizontal arrow), and at higher levels in apical dendrites (diagonal double arrows). In AD temporal cortex, EP3 receptor is expressed in neurons and apical dendrites (horizontal arrow), as well as in cells morphologically consistent with astrocytes (arrowheads) surrounding an amyloid plaque (small arrows in a circle). Enlargements (bottom right) show EP3 receptor present in a perinuclear distribution and in a neuronal apical dendrite (arrow), and in astrocytes (arrowheads; scale bar=10μm). Negative control sections showed no background staining (bottom left panel). (B) Quantitative Western analysis of temporal cortex of control, MCI, and AD post-mortem tissues (n=3 per group) demonstrates increased expression of EP3 receptor in MCI and a further increase in AD (*p<0.05; **p<0.01). (C) qPCR of hippocampal EP3 receptor mRNA derived from aging 3 mo, 6 mo, and 12 mo non-transgenic (WT) and APPSwe-PS1 ΔE9 (APPS) mouse hippocampus demonstrates a significant induction in expression between 6 mo and 12 mo in APPS but not wild type mice (n=6 per group, *p<0.05; **p<0.01).
EP3 signaling is pro-inflammatory in response to exogenous Aβ peptides
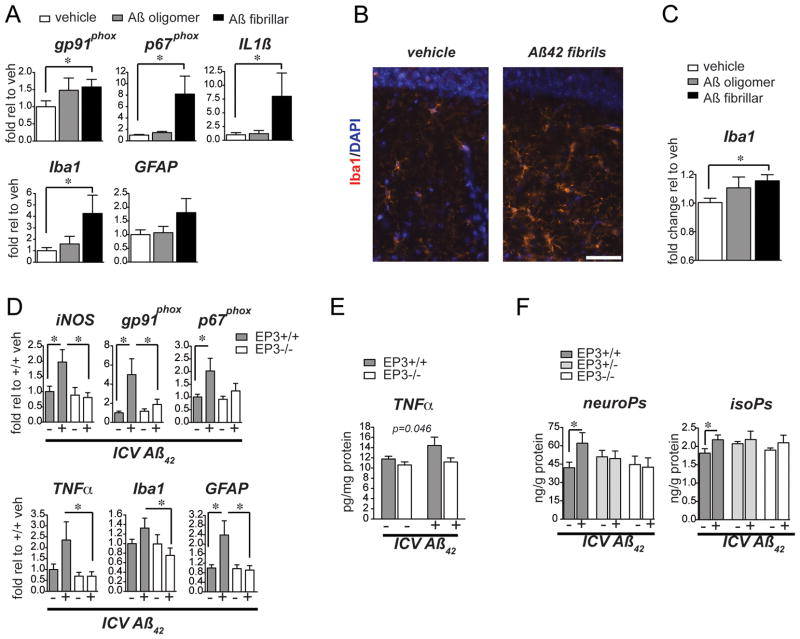
To investigate the function of PGE2 signaling in the development of AD pathology, we tested whether the EP3 receptor might promote an inflammatory response to Aβ peptides in vivo. A model of Aβ42 peptide-induced neuroinflammation was developed, in which a subacute inflammatory response is induced that persists for many days after a single intracerebroventricular (ICV) injection of Aβ42 peptides. This strategy has been previously used to examine effects of aggregated Aβ40 31, 35 or Aβ42 peptides 36. Initial experiments compared the inflammatory responses of vehicle, oligomeric Aβ42 peptide, and fibrillar Aβ42 peptide ICV injections to identify the form of Aβ peptide that would yield the most robust inflammatory response (Figure 2). qPCR of hippocampal mRNA demonstrated a significant induction of inflammatory markers 7 days after ICV injection of fibrillar Aβ42 peptides that was not present following ICV injection of oligomeric Aβ42 peptide or vehicle (Figure 2A; p<0.05). Quantification of immunofluorescent Iba1 in the dentate gyrus of hippocampus revealed a significant increase in microglial Iba1 expression following ICV injection of Aβ42 fibrils, consistent with activation of microglia in this inflammatory model (Figure 2B and C).
Figure 2. EP3 signaling mediates a pro-inflammatory oxidative response to ICV injection of fibrillar Aβ42.
(A) qPCR analysis of pro-inflammatory gene expression in wild type 3 mo male C57B6 mice subjected to a single ICV injection of vehicle, Aβ42 oligomers, or Aβ42 fibrils demonstrates induction of pro-inflammatory genes, including gp91phox, p67phox, IL1β, and Iba1 in hippocampal mRNA 7 days after injection (*p<0.05). (B) Immunofluorescent staining of the hilus in the dentate gyrus (dg) at 7 days after ICV injection demonstrates a morphological change towards activation in Iba1-stained microglia with Aβ42 fibrillar injection (scale bar=48 μm). (C) Quantification of immunofluorescent signal demonstrates a significant increase in Iba1 staining with ICV Aβ42 injection (p<0.05; n=5 mice per group). (D) qPCR of hippocampal mRNA demonstrates an induction of pro-inflammatory gene expression 7 days after ICV of Aβ42 fibrils that is abolished with deletion of EP3 (n=5–8 per group; *p<0.05). (E) ELISA of TNFα demonstrates a block of induced expression in ICV Aβ42-treated EP3−/− as compared to EP3+/+ mice (2 way ANOVA [F(1,23)=4.461, p<0.05, effect of EP3 genotype]; n=5–8 per group). (F) GC/MS measurement of lipid peroxidation products demonstrates lack of induction of lipid peroxidation in EP3+/− and EP3−/− mice administered ICV Aβ42 fibrils. F2-isoprostanes (neuroPs), which are free radical-generated isomers of prostaglandin PGF2a, and F4-neuroprostanes (isoPs), which are products of neuronal docosohexanoic acid (DHA) oxidation are increased in the setting of inflammatory oxidative stress (*p<0.05, n=3–7 per group).
We then tested whether deletion of EP3 receptor would impact on the neuroinflammatory response to Aβ42 fibrils in this model. Cohorts of 13–16 month old EP3+/+ and EP3−/− mice were subjected to a single ICV injection of fibrillar Aβ42 and examined 7 days later (Figure 2D). Hippocampal mRNA revealed a broad induction of pro-inflammatory gene expression in EP3+/+ mice in response to Aβ42, as expected, however this was completely abolished in EP3−/− mice (Figure 2D). Induction of genes encoding pro-oxidant inflammatory enzymes, including gp91phox, p67phox, and iNOS was reduced in EP3−/− mice treated with Aβ42 peptide, as was induction of the pro-inflammatory cytokine TNFα. Activation markers for microglia as well as astrocytes, Iba1 and GFAP, respectively, were also significantly downregulated with deletion of EP3 receptor. ELISA measurements of cerebral cortical levels of TNFα were decreased in EP3−/− mice stimulated with Aβ42 ICV (Figure 2E). The induction of lipid peroxidation in EP3+/+ cerebral cortex at 7 days was absent with deletion of one or both EP3 alleles. (Figure 2F). Taken together, these data suggest that EP3 receptor signaling promotes an inflammatory oxidative response to exogenous Aβ42 peptides.
EP3-mediated inflammation in the APPSwe-PS1 ΔE9 model
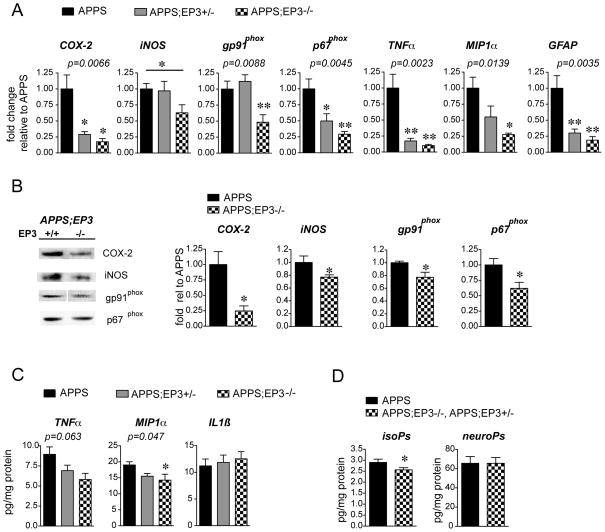
We next tested whether EP3 signaling elicited similar pro-inflammatory and pro-oxidative responses in young adult APPSwe-PS1 ΔE9 (APPS) mice (Figure 3). This mouse model of AD does not develop spatial memory deficits in the water maze until after 10 months of age 34, 37–39. Here, we selected a time point of 8 mo to examine early inflammatory changes in response to EP3 signaling, a period during which levels of EP3 receptor are rapidly increasing (see Figure 1C). Hippocampal mRNA was analyzed in 8 mo female C57B6 APPS;EP3+/+ lacking one or both EP3 alleles (Figure 3A). qPCR demonstrated that deletion of one or both EP3 alleles blocked the induction of inflammatory gene expression in a gene dose-response manner in APPS mice. Moreover, quantitative Western analyses demonstrated that global deletion of EP3 receptor reduced protein expression of COX-2, iNOS, and the gp91phox and p67phox subunits of NADPH oxidase and MIP1α with a trend towards decreased TNF-α by ANOVA (Figure 3B and 3C). Measurements of lipid peroxidation demonstrated a modest but significant decrease in levels of F2-isoprostanes with deletion of EP3, but no difference in F4-neuroprostanes. Thus, deletion of EP3 in 8 mo APPS mice reduced expression of pro-inflammatory proteins, most notably COX-2, and reduced lipid peroxidation.
Figure 3. Deletion of EP3 receptor reduces inflammation and oxidative stress in 8 mo APPS mice.
(A) qPCR demonstrates reduction in inflammatory gene expression in APPS mice with deletion of one or both EP3 receptor alleles by one-way ANOVA (n=5–8 8 mo female littermates per genotype; post-hoc test *p<0.05, **p<0.01); iNOS gene-dose response was significant by t-test between APPS and APPS;EP3−/− (*p<0.05). (B) Quantitative Western analysis demonstrates downregulation of protein expression for pro-inflammatory proteins in parietal cortex with global deletion of EP3 receptor (n=5–6 per genotype; *p<0.05). (C) Cerebral cortical levels of TNFα and MIP-1α, but not IL1β, decrease with deletion of EP3 receptor in 8 mo female mice (n= 5–7 per genotype; ANOVA p=0.047 for MIP1-α, post-hoc APPS vs APPS;EP3−/− is *p<0.05). (D) The F2-isoprostane level is reduced with deletion of EP3 receptor (n=8 per group; *p<0.05), while the F4- neuroprostane level is unchanged.
Effect of EP3 signaling on Aβ peptide levels
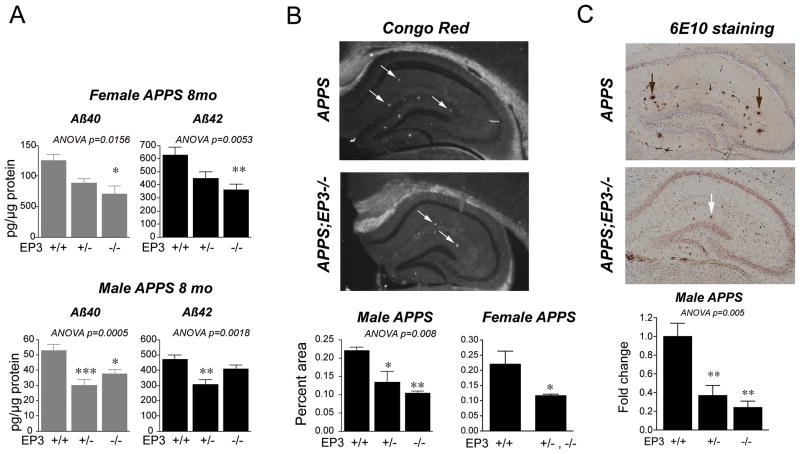
Increased neuroinflammation and oxidative stress are associated in models of AD with increased levels of Aβ peptides. Examination of total Aβ40 and Aβ42 peptides in APPS;EP3+/+, APPS;EP3+/−, and APPS;EP3−/− genotypes (Figure 4) revealed a significant decrease in total Aβ40 and Aβ42 levels in female and male 8 mo cohorts lacking one or both EP3 receptor alleles (Figure 4A). At this age, female APPS mice exhibit increases in total Aβ peptide loads compared to male APPS mice, a finding we and others have observed with this specific transgenic line. The basis for differences in Aβ peptide levels between female and male APPS cohorts is not clear, but could be due to developmental and/or hormonal differences. The effect of deletion of the EP3 receptor however was preserved, with decreases in levels of Aβ peptides in both genders. Hippocampal amyloid plaque deposition was also reduced, as assayed by Congo Red staining to identify β-pleated sheet cores (Figure 4B). Quantification of Congo Red fluorescence demonstrated a significant reduction in β-pleated sheet Aβ deposits with deletion of one or both EP3 alleles in 8 mo male cohorts (ANOVA p<0.01 for all three genotypes) and in female 8 mo cohorts (p<0.05; APPS;EP3+/− and APPS;EP3−/− genotypes combined, with n=4 APPS;EP3+/− and n=2 APPS;EP3−/− mice). Quantification of 6E10 immunostaining, representative of amyloid plaque load, also demonstrated a significant reduction in a gene-dose response manner in 8 mo APPS male cohorts with deletion of one or both EP3 alleles (Figure 4C; ANOVA p<0.01).
Figure 4. Deletion of EP3 receptor in APPS mice reduces levels of Aβ40 and Aβ42 peptides.
(A) Levels of total Aβ40 and Aβ42 were measured in female and male 8 mo APPS;EP3 cohorts. Total Aβ40 and Aβ42 levels were significantly lower in APPS mice lacking one or both alleles of EP3 in both genders (females: n=10–11 per group, ANOVA p<0.05 for Aβ40 and p<0.01 for Aβ42 levels; males: n=9–15 per group; ANOVA p<0.001 for Aβ40 and p<0.01 for Aβ42 levels). (B) Staining of hippocampus with Congo Red (left panels, diagonal arrows) and quantification of Congo red positive percent area demonstrates a lower level of Aβ peptide β-pleated sheet in 8 mo APPS;EP3+/− and APPS;EP3−/− male mice (n=3–4 mice per genotype), and in 8 mo combined APPS;EP3+/− with APPS;EP3−/− female mice (n=4 APPS;EP3+/− mice combined with n=2 APPS;EP3−/− mice; n=8 APPS mice; *p<0.05 and ** p<0.01). (C) Quantification of 6E10 immunostaining in hippocampus demonstrates significant decreases in amyloid plaque deposition (vertical arrows) in 8 mo male APPS mice lacking one or both alleles of EP3 (n=3–6 per group for males ANOVA p<0.01, with post-hoc p<0.05 and **p<0.01 relative to APPS genotype).
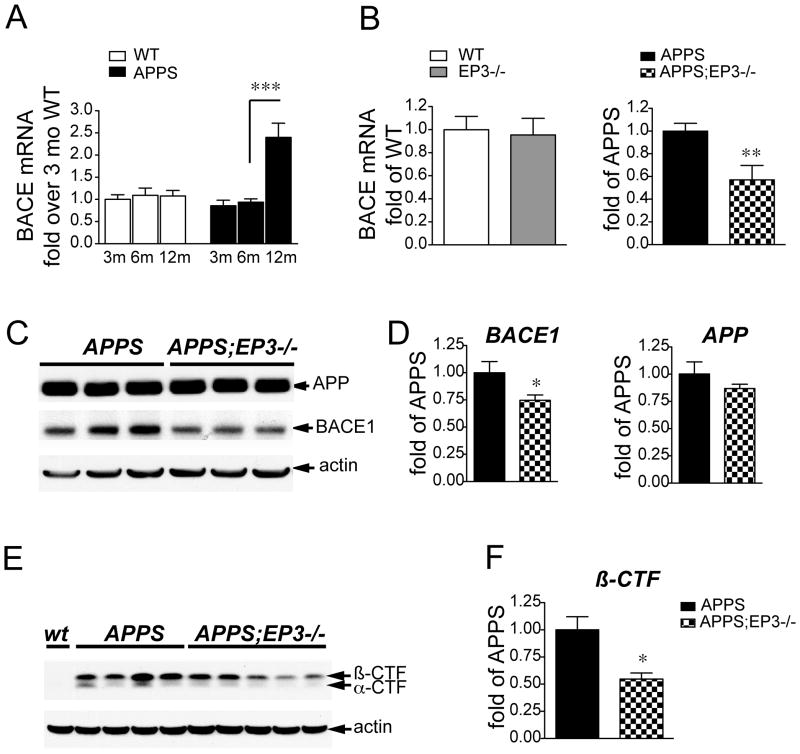
APP is normally processed by α-secretase to yield soluble APPsα. In genetic and sporadic AD, APP is preferentially cleaved by β-secretase (BACE1) to form APPsβ and the β C-terminal fragment (β-CTF), which is then cleaved by the γ-secretase complex to yield Aβ40 and Aβ42 peptides. BACE1 expression levels and enzymatic activity increase in contexts of oxidative stress and inflammation 40, 41, and particularly in aging brain and AD 42–45. Since loss of EP3 signaling reduced the inflammatory and oxidative responses in the APPS model, we tested whether deletion of EP3 in APPS mice might be associated with diminished BACE1 levels and BACE1 generation of β-CTF (Figure 5). A time course examining ages 3 mo to 12 mo APPS and non-transgenic cohorts demonstrated that BACE mRNA levels increased significantly between the ages of 6mo and 12 mo in APPS mice and not in non-transgenic mice (Figure 5A). At 8 mo of age, levels of hippocampal BACE1 mRNA were unchanged in wild type versus EP3−/− mice, however in hippocampus of APPS;EP3−/− 8 mo female mice, BACE mRNA levels were significantly reduced by 53% (Figure 5B; p<0.01) and BACE1 protein was reduced by 26% in APPS;EP3−/− cerebral cortex (Figure 5C and 5D; p<0.05) while levels of total APP protein were not different between APPS and APPS;EP3−/− genotypes (Figure 5C and 5D). BACE1 activity was quantified by measuring levels of its cleavage product of APP, the peptide β-CTF 40, 41. There was a marked decrease in levels of β-CTF in APPS;EP3−/− cerebral cortex as compared to APPS controls (Figure 5E and F) suggesting that inflammatory EP3 signaling can modulate BACE1 expression and cleavage of APP. Examination of BACE1 protein levels at 3 mo of age, a time point before the onset of inflammatory and oxidative changes in this model 22, did not demonstrate differences between genotypes (Supplementary Figure 2).
Figure 5. Deletion of EP3 receptor in APPS mice decreases levels of BACE enzyme and its product β-CTF.
(A) Time course of mRNA expression in hippocampus of aging wild type (WT) and APPS male mice demonstrates a significant increase in BACE1 mRNA expression between 6mo and 12mo of age (***p<0.001; n=6 per group). (B) Level of BACE1 mRNA is unchanged in 8 mo female EP3−/− versus EP3+/+ hippocampus, however is significantly reduced in hippocampus of 8 moAPPS;EP3−/− female mice (n=5–8 per genotype; **p<0.01). (C) Representative Western blot of APP and BACE1 in APPS;EP3+/+ and APPS;EP3−/− brain lysates demonstrates no change in APP levels between genotypes, but a reduction in BACE1 levels in APPS;EP3−/− mice. (D) Quantification of protein levels of BACE1 in APPS;EP3+/+ and APPS;EP3−/− mice (left panel; n=5–6 per genotype;*p<0.05) demonstrates reduction in levels of BACE1 protein with deletion of EP3. Quantification of levels of APP in APPS;EP3+/+ and APPS;EP3−/− mice is unchanged (right panel; n=5–6 per genotype). (E) Levels of β CTF, the product of BACE1 cleavage of APP, are reduced in 8 mo female APPS;EP3−/− mice as compared to APPS control, and are quantified in (F) (n=4–5 per genotype; *p<0.05).
Deletion of EP3 receptor rescues loss of pre-synaptic proteins in APPS mice
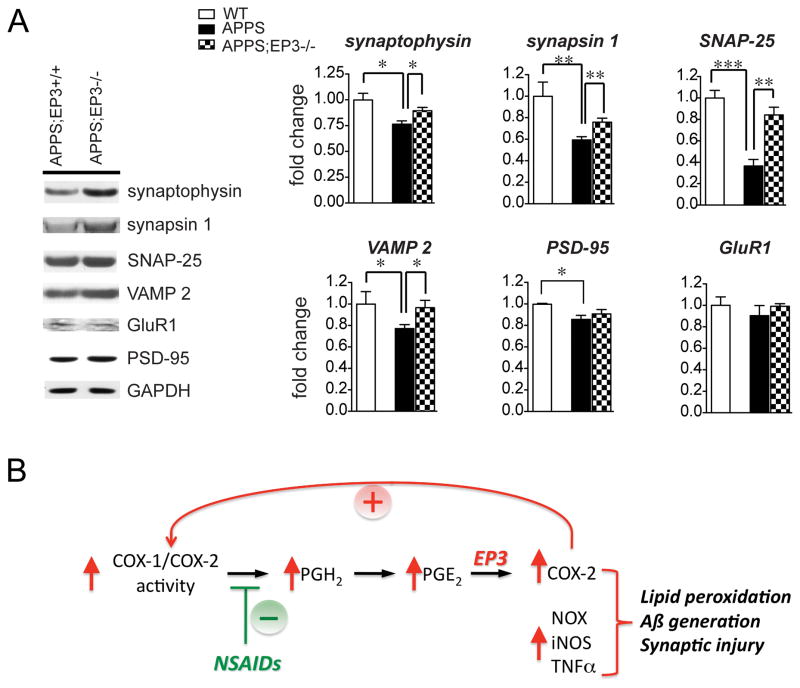
Although at 8 mo these mice to not demonstrate deficits in spatial memory34, 37–39, Aβ peptides can directly and indirectly impact on synaptic proteins prior to onset of spatial memory deficits. We quantified levels of pre-synaptic proteins including synaptophysin, synapsin 1, SNAP-25, and VAMP 2, as well as post-synaptic markers PSD-95 and GluR1 in cerebral cortex of 8 mo female wild type (WT), APPS, and APPS;EP3−/− mice. All four pre-synaptic markers tested demonstrated significant decreases in levels between WT and APPS genotypes at this age, with a significant rescue of levels with deletion of the EP3 receptor (Figure 6A). Changes in levels of post-synaptic proteins in wild type versus APPS mice were less robust, with a mild but significant loss only for PSD-95 but not GluR1. Deletion of the EP3 receptor did not significantly rescue levels of PSD-95 in APPS mice. Thus, at this early age, deletion of EP3 receptor has a beneficial effect on levels of pre-synaptic proteins in particular.
Figure 6. Deletion of EP3 receptor in APPS mice reverses loss of pre-synaptic proteins.
(A) Synaptophysin and synapsin 1 levels, as well as levels of SNARE complex proteins SNAP-25 and VAMP2 were examined in 8 mo female wild type (WT), APPS;EP3+/+, and APPS;EP3−/− cerebral cortical lysates. Values are normalized to wild type controls. APPS mice demonstrated significant decreases in all pre-synaptic proteins as well as post-synaptic PSD-95 as compared to WT controls. Deletion of EP3 receptor in APPS mice rescued the loss of all four pre-synaptic proteins but did not rescue loss of PSD-95 (n=3 WT and n=5–6 APPS;EP3+/+ and APPS;EP3−/− genoptypes; *p<0.05; **p<0.01; ***p<0.001).. (B) Model proposed for the downstream action of EP3 signaling in the early development of AD pathology. Induced cyclooxygenase activity increases levels of PGE2 observed in the development of AD 19, 52. PGE2 signaling through the EP3 receptor further induces expression of COX-2, which through generation of more PGE2 potentiates pro-inflammatory EP3 signaling; EP3 signaling induces expression of additional pro-inflammatory and oxidative proteins, including NADPH oxidase (NOX), iNOS, and toxic cytokines such as TNFα, leading to the development of lipid peroxidation, Aβ peptide accumulation, and synaptic injury. NSAID administration would be predicted to reduce COX generation of PGE2, and reduce effects of PGE2 signaling through the EP3 receptor.
DISCUSSION
The present study demonstrates a novel pro-inflammatory function of PGE2 signaling through its EP3 receptor in two distinct models of neuroinflammation involving Aβ peptide, a highly immunogenic peptide that induces an innate inflammatory response that contributes to disease progression in AD.
In control human temporal cortex, EP3 receptor was expressed basally at low levels in neurons, however the distribution of cellular EP3 expression expanded in AD brain to include glial cells in addition to neurons; this was particularly the case in the immediate areas surrounding amyloid plaques. Protein levels of EP3 receptor increased moderately but significantly with progression from control to MCI and from MCI to AD states, suggesting that progression of disease is associated with further increases in expression of this deleterious receptor, a situation that could create a feed-forward circuit of EP3-mediated inflammatory injury. Progressive increase of EP3 receptor levels was also noted in aging APPS mice between 6 and 12 months of age. From separate studies examining the onset and progression of inflammatory responses in the APPS model (Johansson, Woodling, et al., unpublished), we have determined that the time point of 8 mo is coincident with the onset of inflammatory gene expression as well as that of the EP3 receptor. Therefore, the relatively early time point of 8 mo of age was selected in this study to examine the effects of EP3 signaling on early inflammatory changes.
We first tested whether EP3 signaling participated in exogenous Aβ peptide-induced neuroinflammation in vivo. Intracerebroventricular administration of fibrillar Aβ42 elicited a robust microglial inflammatory response characterized by induction of pro-inflammatory gene expression, cytokine generation, and lipid peroxidation. This is consistent with the vigorous innate immune response observed in the brain by Aβ peptides in previous studies 46, 47. Deletion of EP3 receptor significantly blunted all aspects of this toxic inflammatory response, suggesting that EP3 signaling functions in the inflammatory response to exogenous Aβ peptide. Examination of young adult APPS mice in the EP3+/+ or EP3−/− backgrounds further confirmed a critical function for EP3 signaling in early pro-inflammatory gene and protein expression. In particular, the induction of proteins capable of increasing oxidative injury, including iNOS, components of the NADPH oxidase complex, and COX-2 was markedly reduced with ablation of EP3 signaling. The increased inflammatory and oxidative response in APPS;EP3+/+ mice was associated with significantly higher levels of Aβ peptide accumulation, which decreased in a gene-dose dependent manner in APPS;EP3+/− and APPS;EP3−/− mice. Interestingly, loss of just one allele of EP3 receptor resulted in marked decreases in levels of inflammatory gene expression as well as Aβ peptides, suggesting that partial inhibition of this receptor might be sufficient to elicit an anti-inflammatory and anti-amyloidogenic effect.
This anti-amyloidogenic effect of EP3 deletion could be due to either decreased generation of Aβ peptides from APP or increased clearance of Aβ peptides by microglia. Because of the association between increased oxidative stress and inflammation and increased BACE1 expression and activity 40, 41, we investigated whether EP3 signaling might indirectly increase generation of Aβ peptides. BACE expression levels were found to significantly increase in APPS mice after 6 mo of age. No changes in BACE levels were detected in EP3−/− versus EP3+/+ mice, or in very young 3mo APPS mice long before the onset of inflammatory and oxidative changes. However, at 8 mo of age, levels of both BACE1 mRNA and protein were significantly decreased with loss of EP3 receptor, and BACE1 activity, as measured by quantification of its cleavage product β-CTF, was also markedly reduced in the EP3−/− background. This would suggest that EP3 signaling, via its promotion of inflammatory oxidative stress, potentiates the generation of Aβ peptide in this model. Deletion of EP3 receptor in the APPS genotype had the additional benefit of reversing loss of pre-synaptic proteins, in particular synaptophysin and synapsin 1 as well as representative components of the SNARE complex, SNAP-25 and VAMP2, that mediate vesicle fusion. Loss of the pre-synaptic protein synaptophysin as a marker of synaptic injury is well established in mutant APP models and in clinical AD.
Other that its well-established function in regulating the febrile response 48, 49, the function of EP3 in the innate immune response is relatively unexplored, and in particular its relevance to neurodegenerative disorders where innate immune responses play a critical role in disease progression. Given the fact that elevated PGE2 levels appear to coincide with very early AD 18, 19, a future question to explore is whether NSAIDs, by reducing levels of PGE2, may dampen PGE2 pro-inflammatory signaling through the EP3 receptor in aging brain, thus delaying onset and progression of pathology. It is interesting to note that COX-2 mRNA and protein expression were markedly decreased with deletion of EP3 in APPS mice; this would suggest that PGE2 EP3 signaling engages and amplifies a feed-forward cycle of further increases in COX-2-driven PGE2 signaling through the EP3 receptor, leading to increased production of inflammatory proteins (Figure 6B).
Chronic inhibition of the COX-2 isoform of cyclooxygenase is deleterious, as it inhibits not only toxic, but also beneficial prostaglandin signaling pathways, notably the vasodilatory and anti-thrombotic prostacyclin signaling cascade 50, 51 and potentially other protective prostaglandin pathways as well 20, 33, leading to serious vascular complications. Because non-selective NSAIDs inhibit COX-2 but also COX-1, the main cyclooxygenase in platelets required for the generation of thromboxane, NSAIDs have not been as strongly associated with thrombotic vascular sequellae. Nevertheless, selective targeting of toxic prostaglandin receptors, such as the EP3 receptor, may be more advantageous than global inhibition of prostanoid synthesis with NSAIDs. Of the other PGE2 EP receptors, the EP2 receptor in particular potentiates toxic inflammation and oxidative stress in several models of neurodegeneration, including the APPS model of AD and related models of inflammatory neurodegeneration 21–23. The property of toxic or beneficial prostaglandin signaling may underlie the opposing effects of COX inhibition in AD prevention versus treatment. Although long term follow up studies of the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) trial suggest a preventive effect of NSAIDs on development of AD after the first 2 years of preventive treatment 7, 16, consistent with the epidemiology 4, 6, NSAIDs may be deleterious in cognitively impaired subjects, presumably because of inhibition of beneficial prostaglandin signaling pathways.
In summary, we have identified the PGE2 EP3 receptor as a critical pro-inflammatory signaling pathway in the early development of AD pathology in young adult APPSwe-PS1 ΔE9 mice. Current efforts in treating AD have focused for the most part on symptomatic relief in patients with MCI or AD, where there is already discernible synaptic and neuronal injury. The study of prostaglandin signaling at early stages in models of AD may provide important insights into mechanisms of early pre-clinical changes, and result in the identification of nodal receptors like the EP3 receptor that could be targeted preventively.
Supplementary Material
Acknowledgments
This work was supported by the American Federation for Aging Research (KA), RO1AG030209 (KA), R21AG033914 (KA), Alzheimer’s Association (KA), National Science Foundation (NW), NIH NRSA (NW), AG24011 (TJM), P50 AG05136 (TJM). The authors thank Aimee Schantz and Angela Wilson for their generous assistance and Frank Longo for use of equipment.
References
- 1.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000 May-Jun;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szekely CA, Thorne JE, Zandi PP, et al. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease: a systematic review. Neuroepidemiology. 2004 Jul-Aug;23(4):159–69. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–32. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 4.in t′ Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345(21):1515–21. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 5.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996 Aug;47(2):425–32. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 6.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008 May 6;70(19):1672–7. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitner JC, Baker LD, Montine TJ, et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011 Jul;7(4):402–11. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–6. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997 Feb 7;272(6):3406–10. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 10.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. Faseb J. 2001 Oct;15(12):2057–72. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 11.Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama. 2003 Jun 4;289(21):2819–26. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 12.Soininen H, West C, Robbins J, Niculescu L. Long-term efficacy and safety of celecoxib in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(1):8–21. doi: 10.1159/000096588. [DOI] [PubMed] [Google Scholar]
- 13.Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005 Jun;30(6):1204–15. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 14.Lyketsos CG, Breitner JC, Green RC, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007 May 22;68(21):1800–8. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 15.Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008 Jul;65(7):896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leoutsakos JM, Muthen BO, Breitner JC, Lyketsos CG. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: findings from the randomized controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial. Int J Geriatr Psychiatry. 2011 May 10; doi: 10.1002/gps.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 18.Montine TJ, Sidell KR, Crews BC, et al. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53(7):1495–8. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- 19.Combrinck M, Williams J, De Berardinis MA, et al. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006 Jan;77(1):85–8. doi: 10.1136/jnnp.2005.063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat. 2010 Apr;91(3–4):104–12. doi: 10.1016/j.prostaglandins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montine TJ, Milatovic D, Gupta RC, Valyi-Nagy T, Morrow JD, Breyer RM. Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem. 2002 Oct;83(2):463–70. doi: 10.1046/j.1471-4159.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 22.Liang X, Wang Q, Hand T, et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005 Nov 2;25(44):10180–7. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang X, Wang Q, Shi J, et al. The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol. 2008 Sep;64(3):304–14. doi: 10.1002/ana.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shie FS, Breyer RM, Montine TJ. Microglia lacking E Prostanoid Receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005 Apr;166(4):1163–72. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keene CD, Chang RC, Lopez-Yglesias AH, et al. Suppressed accumulation of cerebral amyloid {beta} peptides in aged transgenic Alzheimer’s disease mice by transplantation with wild-type or prostaglandin E2 receptor subtype 2-null bone marrow. Am J Pathol. 2010 Jul;177(1):346–54. doi: 10.2353/ajpath.2010.090840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin J, Shie FS, Liu J, et al. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007;4:2. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001 Jun;17(6):157–65. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 28.Jewell ML, Breyer RM, Currie KP. Regulation of calcium channels and exocytosis in mouse adrenal chromaffin cells by prostaglandin EP3 receptors. Mol Pharmacol. 2011 Jun;79(6):987–96. doi: 10.1124/mol.110.068569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Liang X, Wang Q, Breyer RM, McCullough L, Andreasson K. Misoprostol, an anti-ulcer agent and PGE(2) receptor agonist, protects against cerebral ischemia. Neurosci Lett. 2008 Jun 20;438(2):210–5. doi: 10.1016/j.neulet.2008.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T, Knowles JK, Lu Q, et al. Small molecule, non-peptide p75 ligands inhibit Abeta-induced neurodegeneration and synaptic impairment. PLoS One. 2008;3(11):e3604. doi: 10.1371/journal.pone.0003604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piermartiri TC, Figueiredo CP, Rial D, et al. Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-beta(1–40) administration in mice: evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol. 2010 Dec;226(2):274–84. doi: 10.1016/j.expneurol.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010 Jun 15;184(12):7207–18. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang X, Lin L, Wang Q, Woodling NS, Anacker C, Pan T, Merchant M, Andreasson K. Neuronal and vascular protection by the prostaglandin E2 EP4 receptor in a mouse model of cerebral ischemia. J Clin Invest. 2011;121(11):4362–71. doi: 10.1172/JCI46279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melnikova T, Savonenko A, Wang Q, et al. Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer’s disease in a sex-dimorphic pattern. Neuroscience. 2006 Sep 1;141(3):1149–62. doi: 10.1016/j.neuroscience.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Passos GF, Figueiredo CP, Prediger RD, et al. Involvement of phosphoinositide 3-kinase gamma in the neuro-inflammatory response and cognitive impairments induced by beta-amyloid 1–40 peptide in mice. Brain Behav Immun. 2010 Mar;24(3):493–501. doi: 10.1016/j.bbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Kim DH, Jung WY, Park SJ, et al. Anti-amnesic effect of ESP-102 on Abeta(1–42)-induced memory impairment in mice. Pharmacol Biochem Behav. 2010 Dec;97(2):239–48. doi: 10.1016/j.pbb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Yoshiike Y, Kimura T, Yamashita S, et al. GABA(A) receptor-mediated acceleration of aging-associated memory decline in APP/PS1 mice and its pharmacological treatment by picrotoxin. PLoS One. 2008;3(8):e3029. doi: 10.1371/journal.pone.0003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savonenko A, Xu GM, Melnikova T, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005 Apr;18(3):602–17. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Minkeviciene R, Ihalainen J, Malm T, et al. Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice. J Neurochem. 2008 May;105(3):584–94. doi: 10.1111/j.1471-4159.2007.05147.x. [DOI] [PubMed] [Google Scholar]
- 40.Tamagno E, Bardini P, Obbili A, et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002 Aug;10(3):279–88. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- 41.Apelt J, Bigl M, Wunderlich P, Schliebs R. Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. Int J Dev Neurosci. 2004 Nov;22(7):475–84. doi: 10.1016/j.ijdevneu.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002 Sep;59(9):1381–9. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 43.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002 Jun;51(6):783–6. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 44.Yang LB, Lindholm K, Yan R, et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003 Jan;9(1):3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 45.Borghi R, Patriarca S, Traverso N, et al. The increased activity of BACE1 correlates with oxidative stress in Alzheimer’s disease. Neurobiol Aging. 2007 Jul;28(7):1009–14. doi: 10.1016/j.neurobiolaging.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Letiembre M, Liu Y, Walter S, et al. Screening of innate immune receptors in neurodegenerative diseases: A similar pattern. Neurobiol Aging. 2007 Sep 28; doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Walter S, Letiembre M, Liu Y, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem. 2007;20(6):947–56. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 48.Lazarus M, Yoshida K, Coppari R, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007 Sep;10(9):1131–3. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 49.Ushikubi F, Segi E, Sugimoto Y, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395(6699):281–4. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 50.Egan KM, Lawson JA, Fries S, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004 Dec 10;306(5703):1954–7. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 51.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007 Nov;50(5):470–9. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 52.Montine TJ, Sidell KS, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, Morrow JD. Elevated cerebrospinal fluid prostaglandin E2 levels in patients with probable Alzheimer’s disease. Neurology. 1998;53:1495–8. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.