Abstract
Animals subjected to maternal separation stress during the early stages of development display behavioural, endocrine and growth factor abnormalities that mirror the clinical findings in anxiety/depression. In addition, maternal separation has been shown to exacerbate the behavioural deficits induced by 6-hydroxydopamine (6-OHDA) in a rat model of Parkinson's disease. In contrast, voluntary exercise reduced the detrimental effects of 6-OHDA in the rat model. The beneficial effects of exercise appeared to be largely due to compensation in the non-lesioned hemisphere. The aim of the present study was to investigate whether voluntary exercise for 3 weeks could reverse the effects of maternal separation in rats challenged with the neurotoxin 6-OHDA infused into the medial forebrain bundle after 1 week of exercise, at postnatal day 60 (P60). The rats were killed 2 weeks later, at P74. Their brains were dissected and the hippocampus rapidly removed for proteomic analysis - isobaric tagging (iTRAQ) and quantification of peptides by matrix-assisted laser desorption/ionization tandem mass spectrometry (MALDI-MS/MS). Maternal separation up-regulated hippocampal proteins functionally involved in energy metabolism (nucleoside diphosphate kinase B, enolase, triosephosphate isomerase) and synaptic plasticity (alpha-synuclein, tenascin-R, Ba1-667, brevican and neurocan core protein) in the non-lesioned hemisphere. Exercise reversed many of these changes by down-regulating the levels of hippocampal proteins functionally associated with energy metabolism (nucleoside diphosphate kinase B, enolase, triosephosphate isomerase) and synaptic plasticity (alpha-synuclein, tenascin-R, Ba1-667, brevican and neurocan core protein) in the non-lesioned hemisphere of rats subjected to maternal separation. Exercise and maternal separation therefore appeared to have opposing effects on the hippocampus in the non-lesioned hemisphere of the rat brain. Exercise seemed to partially reverse the effects of maternal separation stress on these proteins in the non-lesioned hemisphere. The partial reversal of maternal separation-induced proteins by exercise in the non-lesioned side sheds some insight into the mechanism by which exercise alters the molecular role players involved in determining the consequences of early life stress.
Keywords: Maternal separation, Exercise, Proteomics
Introduction
Early life adversity has been associated with adult psychopathologies including mood- and anxiety-related behavioural disorders which have been suggested to result from maladapted neurobiological responses to stress (Heim and Nemeroff, 2001). The maternal separation paradigm produces an animal model that has been successfully used to study the long term effects of early life trauma. Animals subjected to stress early in life display behavioural, endocrine and growth factor abnormalities that mirror clinical findings in patients with anxiety/depressive disorders. Animals' subjected to maternal separation spent less time swimming and more time immobile in the Porsolt forced swim test, a well-established test for depression-like behaviour (Marais et al. 2008; Ryu et al. 2009). Similar to humans reporting childhood maltreatment (Heim and Nemeroff, 2001; Harkness et al. 2011), rats subjected to maternal separation early in life displayed altered stress hormone levels in adulthood (Marais et al. 2008; van Heerden et al. 2010).
Rats that were repeatedly subjected to maternal separation (3 h/day for 14 days) during the early stages of development were shown to have a blunted response to dopamine receptor activation, decreased apomorphine-induced locomotor activity (Faure et al. 2009) and increased 6-hydroxydopamine (6-OHDA)-induced locomotor deficits (Pienaar et al. 2008; Mabandla and Russell, 2010), implicating the dopaminergic system. These behavioural changes are reminiscent of neurological and psychiatric disorders such as Parkinson's disease, depression and anxiety disorders. Evidence suggests that the behavioural consequences of maternal separation are not caused by one specific alteration in the brain, but rather a complex pattern of changes in downstream signaling pathways that regulate the release of many neurotransmitters and neurotrophins that in turn affect the expression of a variety of signaling proteins. It was therefore considered that an intensive investigation of the differential expression of cytosolic protein levels may provide further insight into the molecular mechanisms that drive maternal separation-induced pathogenesis.
Interestingly, some of these behavioural abnormalities could be reversed by exercise (Marais et al. 2009; Mabandla and Russell, 2010). We have previously shown that voluntary exercise has a neuroprotective effect in both non-separated and maternally separated rats presenting with either parkinsonian-like locomotor deficits (Mabandla et al. 2004; 2009; Howells et al. 2005; Mabandla and Russell, 2010) or depressive-like behaviour (Marais et al. 2009). Maternal separation increased forelimb akinesia and forelimb use asymmetry in a 6-OHDA lesioned animal (Pienaar et al. 2008) and reduced the beneficial effects of exercise on forelimb akinesia (Mabandla and Russell, 2010). In agreement with reports that maternal separation increased the 6-OHDA-induced loss of dopamine terminals in the striatum (Pienaar et al. 2008), there was a small increase in dopamine neuron loss when expressed as a percentage of the non-lesioned hemisphere, however no difference in dopamine cell number, suggesting that exposure to maternal separation did not exacerbate dopamine cell loss (Mabandla and Russell, 2010). The marked effects of maternal separation on forelimb use asymmetry following 6-OHDA lesion of dopamine neurons, contrasts with the small effect on dopamine neuron survival suggesting that dopamine regulation of behavioural networks may not be the only system affected by exercise and maternal separation, a role for other proteins needs to be investigated.
The aim of the present study was to investigate whether voluntary exercise for 3 weeks could reverse the effects of maternal separation in rats challenged with the neurotoxin 6-OHDA infused into the medial forebrain. In order to achieve this, hippocampal tissue was collected for proteomic analysis, from rats that had been subjected to maternal separation, exercise and 6-OHDA infusion into the medial forebrain bundle (MFB) at 60 days of age (P60) and killed 2 weeks later, at P74.
Materials and Methods
Ethical Approval
The study was approved by the Faculty of Health Sciences Animal Research Ethics Committee of the University of Cape Town and adhered to international guidelines according to the South African National Standard: The Care and Use of Animals for Scientific Purposes (2008). Twelve male Sprague-Dawley (Rattus Norvegicus) rats weighing between 180g-280g were used in the study. Animals were housed in a temperature-controlled room (23 ± 2°C) in the Satellite Animal Facility, at the University of Cape Town and had ad libitum access to standard rat chow and water. The housing facility was maintained on a 12h/12h light/dark cycle (lights on at 06h00). On postnatal day 1 (P1), rats were sexed and culled to 8 males per litter. If the litter had less than 8 males, female littermates were added to standardize litter size and to allow for equal suckling from the dam.
Drugs
Ascorbic acid, desipramine and 6-OHDA were purchased from Sigma, St Louis, MO, U.S.A. Halothane was supplied by Lakato.
Maternal Separation
Maternal separation was performed as described by Daniels et al. (2004). Pups were separated from the dam for a period of 3 hours per day (09h00 – 12h00) from P2 - P14. The maternal separation procedure involved physically removing the dam from the home cage and placing her in a separate clean plexiglass cage with clean bedding and introducing a little bedding from the home cage in order to decrease the stress of the mother. The temperature in the separation room was maintained at 32 ± 1°C to prevent hypothermia. After the 3-hour separation period the pups were returned to the housing facility and reunited with their dams. The home cage was cleaned every fourth day. To ensure that the dam recognized the odour of both her cage and her litter when returned to the home cage, only half of the bedding was changed (the half on which the pups were not lying). During cleaning, great care was taken not to disturb or handle the pups and to minimize other factors (smells, sounds) that could cause distress to the pups. After the separation protocol, the litters were left with their dams from P14 until P21, when the litters were weaned. The non-maternally separated group (control group) was left undisturbed until weaning. From P21 to P48 the rats from both maternally separated and non-maternally separated groups were housed under normal conditions.
Voluntary Exercise
On P48 male rats were transferred to a room with a 12h/12h light/dark cycle (lights off at 11h00) and temperature maintained at 23 ± 2°C. On P54, maternally separated male rats and non-maternally separated male rats were weighed and further divided into runners and non-runners. Maternally separated rats (stressed runners (SR)) and non-maternally separated (non-separated runners (NSR)) were allowed to exercise voluntarily by housing them in cages that had running wheels attached with counters that recorded the number of revolutions. The number of revolutions was recorded daily between 10h00 and 11h00 just before the onset of their dark cycle. There was no difference in average distance traveled by stressed (SR) and non-separated (NSR) rats in the running wheels. The distance increased from 847 ± 197 and 669 ± 243 m/day, respectively, to an average of 4400 m/day after 17 days. The remaining maternally separated rats (stressed non-runners (SNR)) and non-maternally separated rats (non-separated non-runners (NSNR)) were placed individually in plexiglass cages and had no access to running wheels.
Stereotaxic Surgery
Stereotaxic surgery was performed according to the procedure described by Mabandla et al. (2004; 2009) and Mabandla and Russell (2010) which produced a 70% lesion of dopamine neurons in the ipsilateral substantia nigra of the midbrain of non-separated, non-exercised male Sprague-Dawley rats. Midbrain dopamine neurons project to several forebrain areas, including the striatum, prefrontal cortex, amygdala and hippocampus. Injection site accuracy was ascertained prior to the study. On P60 NSNR, SNR, NSR and SR rats were weighed and transported to the laboratory for surgery. Desipramine (15 mg/kg), a norepinephrine reuptake blocker, was injected intraperitoneally 30 min before surgery to limit the effect of 6-OHDA to dopamine neurons. Rats were deeply anaesthetized with a mixture of oxygen and halothane administered by means of a calibrated Blease Vaporiser (DATUM). Both maternally separated and non-separated rats received 4 μl 6-OHDA (5 μg/4 μl 0.02% ascorbic acid in saline) infused unilaterally at 0.4 μl/min into the left medial forebrain bundle (MFB) (4.7 mm anterior to lambda, 1.6 mm lateral to the midline and 8.4 mm ventral to dura, according to the Rat Brain Atlas of Paxinos and Watson, 1986).
The number of revolutions made by the rats in cages with attached running wheels was recorded for a further 14 days after the surgery. On P74 rats were sacrificed, their brains rapidly removed from the skull, their hippocampi dissected out, snap-frozen in liquid nitrogen, and stored at -80° C until proteomic analysis.
Isobaric tagging for relative and absolute quantitation (iTRAQ) of the proteome
Left and right hippocampal tissue was analyzed separately. Tissue collected from 3 rats per group (NSNR, NSR, SNR, SR) was pooled and sonicated in 1M triethylammonium bicarbonate (TEAB) buffer. After centrifugation at 19,000 rpm for 30 min at 4°C, the supernatant was collected and used for isobaric tagging for relative and absolute quantitation (iTRAQ) of proteins using matrix-assisted laser desorption/ionization tandem mass spectrometry (MALDI-MS/MS) analysis by the Centre for Proteomic and Genomic Research (CPGR) at the University of Cape Town. Each sample was incubated with trypsin and labeled with an 8-plex iTRAQ reagent kit. All 8 samples were modified with iTRAQ tags. After mixing the samples, the iTRAQ reagents were removed from the mixture using a strong cation exchange solid phase extraction device. The sample mixture was submitted to 1 dimensional liquid chromatography using a Pepmap C18 reverse phase column with gradient elution. The chromatogram confirmed the complete digestion and suitable signal intensity to proceed to mass spectrometric analysis. The samples collected from the chromatographic separation were mixed with MALDI matrix through an inline T connector and spotted on the MALDI source plate. The matrix was spiked with a 5-point internal calibration mixture. Internal calibration showed that the mass spectrometer was functioning within specifications.
Statistics
The MS/MS spectra were analyzed with ProteinPilot software (ProteinPilot™ Software 3.0, Applied Biosystems, MDS Analytical Technologies) using the Ratus ratus database. A Paragon Algorithm was used to determine differentially expressed proteins by calculating the average ratio of sample protein to reference protein, for each protein, along with the associated p-value and error factors. The p-value for each protein was derived from a t-test, where the sample size (n) was the number of peptides contributing to the identification of a specific protein and calculated as
where Log Ratio = log (ratio of specific protein in test sample to specific protein in reference sample)
and Log Bias = log (sample bias)
Proteins detected with > 95 % confidence and those that differed significantly between the experimental groups (p < 0.05) are reported as fold change with respect to the appropriate reference sample. Bonferroni correction for multiple comparisons i.e. lesioned vs non-lesioned; NR vs R; NS vs S for each protein was p < 0.0166.
Results
The effect of the lesion on hippocampal protein levels
The neurotoxin, 6-OHDA, increased mitochondrial energy-related enzymes (ATP synthase, creatine kinase), neurotransmitter–related proteins (aspartate aminotransferase) and nucleophosmin in the ipsilateral hippocampus of non-separated rats (NSNR lesioned (L) compared to non-lesioned (R) hemisphere), while it decreased triosephosphate isomerase and structural proteins (actin, RNA binding motif protein, Table 1). 6-OHDA caused a similar decrease in cytoskeletal proteins (tenascin-R, spna2 protein) in maternally separated rats (SNR). However, in contrast to non-separated rats, the neurotoxin caused a decrease in energy metabolism (nucleoside diphosphate kinase, malate dehydrogenase), neurotransmission (vesicle-fusing ATPase), brain-specific angiogenesis inhibitor, myelin basic protein S and transcription factor Psip 1 protein, in maternally separated rats. It would appear that maternal separation caused changes in the brain that prevented the 6-OHDA-induced increase in energy- and neurotransmission-related proteins.
Table 1.
Effect of the 6-OHDA lesion hippocampal proteins in left (L) and right (R) non-separated non-runners (NSNR), maternally separated non-runners (SNR) and exercised rats (NSR, SR).
| Accession no. | %Cov(95) | Peptides | NSNR(L):NSNR(R) | p-value | |
|---|---|---|---|---|---|
| Lesion effect on NSNR | |||||
| Energy metabolism | |||||
| ATP synthase subunit alpha, mitochondrial | sp|P15999| | 10.490 | 5 | 1.43 | 0.0301 |
| Creatine kinase, mitochondrial 1, ubiquitous | tr|Q5BJT9| | 5.980 | 2 | 1.26 | 0.0099* |
| Triosephosphate isomerase | sp|P48500| | 40.560 | 13 | 0.69 | 0.0311 |
| Neurotransmission / Signalling | |||||
| Aspartate aminotransferase, cytoplasmic | sp|P13221| | 11.620 | 4 | 1.79 | 0.0135* |
| Cytoskeletal /Structural | |||||
| Actin, cytoplasmic 2 | sp|P63259| | 18.400 | 5 | 0.69 | 0.0343 |
| Other | |||||
| Nucleophosmin | sp|P13084| | 2.397 | 1 | 1.37 | 0.0429 |
| RNA binding motif protein, X-linked | tr|Q4V898| | 6.920 | 2 | 0.57 | 0.0243 |
|
| |||||
| Accession no. | %Cov(95) | Peptides | SNR(L):SNR(R) | p-value | |
|
| |||||
| Lesion effect on SNR | |||||
| Energy metabolism | |||||
| Nucleoside diphosphate kinase B | sp|P19804| | 35.530 | 5 | 0.5 | 0.0346 |
| Malate dehydrogenase, mitochondrial | sp|P04636| | 19.530 | 7 | 0.75 | 0.0114* |
| Neurotransmission / Signalling | |||||
| Vesicle-fusing ATPase | sp|Q9QUL6| | 3.760 | 3 | 0.9 | 0.0072* |
| Cytoskeletal /Structural | |||||
| Tenascin-R | sp|Q05546| | 5.240 | 5 | 0.57 | 0.003* |
| Spna2 protein | tr|Q6IRK8| | 7.140 | 15 | 0.61 | 0.0016* |
| Other | |||||
| Brain-specific angiogenesis inhibitor 1-associated protein 2 | sp|Q6GMN2| | 4.490 | 2 | 0.5 | 0.0071* |
| Isoform 14kDa of Myelin basic protein S | sp|P02688-4| | 14.060 | 3 | 0.74 | 0.0498 |
| Psip 1 protein | tr|Q566D6| | 5.740 | 2 | 0.8 | 0.0098* |
|
| |||||
| Accession no. | %Cov(95) | Peptides | NSR(L):NSR(R) | p-value | |
|
| |||||
| Lesion effect on NSR | |||||
| Neurotransmission / Signalling | |||||
| Aspartate aminotransferase, cytoplasmic | sp|P13221| | 11.62 | 4 | 2.92 | 0.0024* |
| Alpha-synuclein | sp|P37377| | 37.86 | 5 | 0.44 | 0.0094* |
| Dihydropyrimidinase-related protein 2 | sp|P47942| | 14.34 | 7 | 0.93 | 0.0348 |
| Other | |||||
| Haptoglobin | sp|P06866| | 6.34 | 2 | 0.79 | 0.0373 |
|
| |||||
| Accession no. | %Cov(95) | Peptides | SR(L):SR(R) | p-value | |
|
| |||||
| Lesion effect on SR | |||||
| Energy metabolism | |||||
| Nucleoside diphosphate kinase B | sp|P19804| | 35.53 | 5 | 0.55 | 0.0282 |
| Glyceraldehyde-3-phosphate dehydrogenase | sp|P04797| | 15.92 | 5 | 0.68 | 0.0350 |
| Neurotransmission / Signalling | |||||
| Syntaxin-binding protein 1 | sp|P61765| | 2.02 | 1 | 0.64 | 0.0228 |
| Vesicle-fusing ATPase | sp|Q9QUL6| | 3.763 | 3 | 0.65 | 0.0392 |
| 2′,3′-cyclic-nucleotide 3′-phosphodiesterase | sp|P13233| | 8.333 | 3 | 0.66 | 0.0263 |
| Mu-crystallin homolog | sp|Q9QYU4| | 4.792 | 1 | 0.75 | 0.0189 |
| Cytoskeletal / Structural | |||||
| Receptor-type tyrosine-protein phosphatase zeta | sp|Q62656| | 1.42 | 2 | 1.56 | 0.0285 |
| Tenascin-R | sp|Q05546| | 5.236 | 5 | 0.55 | 0.0006* |
| Tubulin alpha-1B chain | sp|Q6P9V9| | 28.6 | 11 | 0.66 | 0.0255 |
| Actin, cytoplasmic 2 | sp|P63259| | 18.4 | 5 | 0.75 | 0.0055* |
| Other | |||||
| Alpha-1-inhibitor 3 | sp|P14046| | 2.505 | 4 | 0.64 | 0.0281 |
The % Cov (95) refers to the number of amino acids in the identified peptides that match the protein with a confidence of 95% or higher, divided by the total number of amino acids in the protein and expressed as a percentage. Peptides refer to the number of peptides in the protein that have been identified with a confidence of 95% or greater.
Significant with Bonferroni correction (p < 0.0166).
Similar to non-exercised rats, the lesion increased hippocampal aspartate aminotransferase, important for glutamate synthesis, in exercised rats (NSR). This effect of exercise was not seen in maternally separated rats (SNR and SR). In exercised rats (NSR) the lesion resulted in decreased alpha-synuclein, dihydropyrimidase-related protein 2 and haptoglobin and, in contrast to all other groups, did not result in decreased structural proteins. Similar to non-exercised maternally separated rats, the 6-OHDA lesion decreased energy metabolism (nucleoside diphosphate kinase, glyceraldehyde-3-phosphate dehydrogenase), neurotransmission-related proteins (syntaxin-binding protein, vesicle-fusing ATPase, 2′,3′-cyclic-nucleotide 3′-phosphodiesterase, Mu-crystallin homolog) and cytoskeletal proteins (tenascin-R, tubulin, actin) in exercised rats that had been maternally separated (SR, Table 1).
The effects of maternal separation on hippocampal protein levels
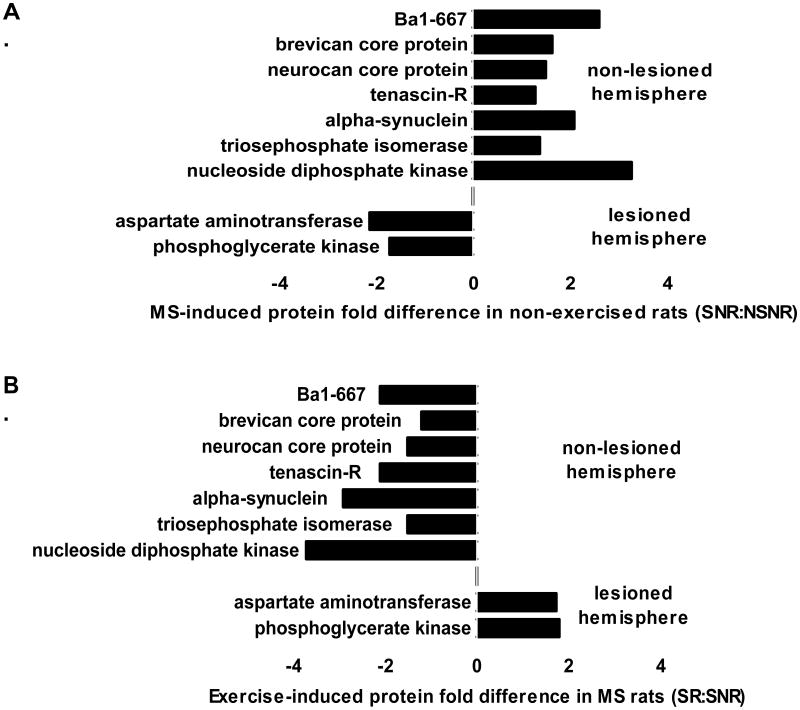
Maternal separation caused similar effects on protein levels in the hippocampus of both lesioned and non-lesioned hemispheres (SNR compared to NSNR, Table 2). In the lesioned hemisphere, maternal separation increased energy-related proteins (triosephosphate isomerase, phosphofructokinase, enolase, malate dehydrogenase) while decreasing phosphoglycerate kinase and ATP synthase. Maternal separation decreased neurotransmitter/signaling-related proteins (aspartate aminotransferase, dihydropyrimidase-related protein 2, phosphatidylethanolamine-binding protein), while it increased vesicle associated proteins (adaptin ear-binding coat-associated protein), cytoskeletal proteins (neurocan core protein) and antioxidants (peroxiredoxin-1, ferric iron binding protein Ba1-667) and the RNA binding motif protein X-linked.
Table 2.
Effect of maternal separation on hippocampal proteins in the 6-OHDA lesioned and non-lesioned hemispheres of non-runners.
| Accession no. | % Cov(95) | Peptides | SNR:NSNR | p-value | |
|---|---|---|---|---|---|
| Lesion | |||||
| Energy metabolism | |||||
| Triosephosphate isomerase | sp|P48500| | 40.560 | 13 | 1.86 | 0.0000* |
| Phosphofructokinase | tr|Q52KS1| | 5.000 | 4 | 1.55 | 0.0483 |
| Enolase | tr|Q5EB49| | 20.050 | 8 | 1.49 | 0.0327 |
| Malate dehydrogenase, mitochondrial | sp|P04636| | 19.530 | 7 | 1.23 | 0.0390 |
| Phosphoglycerate kinase 1 | sp|P16617| | 5.516 | 2 | 0.58 | 0.0493 |
| ATP synthase subunit alpha, mitochondrial | sp|P15999| | 10.490 | 5 | 0.69 | 0.0229 |
| Neurotransmission / Signalling | |||||
| Aspartate aminotransferase, cytoplasmic | sp|P13221| | 11.620 | 4 | 0.47 | 0.0066* |
| Dihydropyrimidase-related protein 2 | sp|P47942| | 144.340 | 7 | 0.75 | 0.0179 |
| Phosphatidylethanolamine-binding protein 1 | sp|P31044| | 35.290 | 9 | 0.77 | 0.0425 |
| Adaptin ear-binding coat-associated protein 1 | sp|P69682| | 9.386 | 2 | 1.66 | 0.0204 |
| Cytoskeletal /Structural | |||||
| Neurocan core protein | sp|P55067| | 1.671 | 3 | 1.67 | 0.0124* |
| Other | |||||
| Peroxiredoxin-1 | sp|Q63716| | 16.580 | 3 | 1.41 | 0.007* |
| Ba1-667 | tr|Q7TP24| | 4.082 | 4 | 2.05 | 0.0064* |
| RNA binding motif protein, X-linked | tr|Q4V898| | 6.92 | 2 | 2.35 | 0.0351 |
| Non-lesion | |||||
| Energy metabolism | |||||
| Nucleoside diphosphate kinase B | sp|P19804| | 35.530 | 5 | 3.27 | 0.0058* |
| Aconitrate hydratase, mitochondrial | sp|Q9ER34| | 3.974 | 3 | 1.69 | 0.0475 |
| Triosephosphate isomerase | sp|P48500| | 40.560 | 13 | 1.37 | 0.0111* |
| Neurotransmission / Signalling | |||||
| Alpha-synuclein | sp|P37377| | 37.860 | 5 | 2.09 | 0.0448 |
| Adaptin ear-binding coat-associated protein1 | sp|P69682| | 9.386 | 2 | 1.97 | 0.0158* |
| Protein kinase C and casein kinase substrate in neurons protein | sp|Q9Z0W5| | 4.082 | 2 | 1.94 | 0.0135* |
| Cytoskeletal /Structural | |||||
| Spna2 protein | tr|Q61RK8| | 7.137 | 15 | 1.7 | 0.0030* |
| Brevican core protein | sp|P55068| | 3.058 | 4 | 1.63 | 0.0186 |
| Neurocan core protein | sp|P55067| | 1.671 | 3 | 1.5 | 0.0032* |
| Tenascin-R | sp|Q05546| | 5.236 | 5 | 1.29 | 0.0506 |
| Other | |||||
| Ba1-667 | tr|Q7TP24| | 4.082 | 4 | 2.6 | 0.0023* |
| Brain-specific angiogenesis inhibitor 1-associated protein 2 | sp|Q6GMN2| | 4.486 | 2 | 1.92 | 0.0048* |
| Elongation factor 1-alpha 1 | sp|P62630| | 7.792 | 4 | 0.73 | 0.0175 |
The % Cov (95) refers to the number of amino acids in the identified peptides that match the protein with a confidence of 95% or higher, divided by the total number of amino acids in the protein and expressed as a percentage. Peptides refer to the number of peptides in the protein that have been identified with a confidence of 95% or greater.
Significant with Bonferroni correction (p < 0.0166).
SNR = maternally separated non-runners, NSNR = non-separated non-runners.
In the non-lesioned hemisphere, maternal separation increased energy-related proteins (nucleoside diphosphate kinase B, aconitate hydratase, triosephosphate isomerase), vesicle associated proteins (alpha-synuclein, adaptin ear-binding coat-associated protein, protein kinase C and casein kinase substrate), cytoskeletal proteins (spna2 protein, brevican core protein, neurocan core protein, tenascin-R) and antioxidants (ferric iron binding protein Ba1-667). Maternal separation also inhibited angiogenesis (brain-specific angiogenesis inhibitor 1-associated protein 2) and decreased protein synthesis (elongation factor 1-alpha 1) in the non-lesioned hemisphere of non-runners.
The effect of exercise on hippocampal protein levels
In the lesioned hemisphere of non-separated rats, exercise reversed the 6-OHDA-induced decrease in energy-related proteins (triosephosphate isomerase) and structural proteins (dynactin, spectrin, RNA binding motif protein) and the 6-OHDA-induced increase in ATP synthesis by decreasing pyruvate kinase (decreased synthesis of ATP from glycolytic metabolism of glucose to pyruvate). Exercise caused a further increase in glutamate synthesis/neurotransmission (aspartate aminotransferase). Exercise also increased hippocampal chaperones (78 kDa glucose-regulated protein, heat shock cognate 71 kDa protein, stress-induced-phosphoprotein1), structural proteins (tyrosine-protein phosphatase zeta) and antioxidants (Ba1-667) in the lesioned hemisphere (Table 3). Exercise produced very few hippocampal changes in the non-lesioned hemisphere of non-separated rats (NSR compared to NSNR), predominantly an increase in nucleoside triphosphate metabolism (nucleoside diphosphate kinase, Table 3).
Table 3.
Effect of exercise on hippocampal proteins in the 6-OHDA lesioned and non-lesioned hemispheres of non-separated rats.
| Accession no. | % Cov(95) | Peptides | NSR:NSNR | p-value | |
|---|---|---|---|---|---|
| Lesion | |||||
| Energy metabolism | |||||
| Triosephosphate isomerase | sp|P48500| | 40.56 | 13 | 1.52 | 0.0212 |
| Pyruvate kinase isozyme M1/M2 | sp|P11980| | 12.81 | 7 | 0.74 | 0.0492 |
| Neurotransmission / Signalling | |||||
| Aspartate aminotransferase, cytoplasmic | sp|P13221| | 11.62 | 4 | 1.81 | 0.0124* |
| Cytoskeletal /Structural | |||||
| Dynactin subunit 2 | sp|Q6AYH5| | 9.701 | 4 | 1.35 | 0.0039* |
| Non-erythroid spectrin beta | tr|Q6XD99| | 4.707 | 11 | 1.21 | 0.0060* |
| Receptor-type tyrosine-protein phosphatase zeta | sp|Q62656| | 1.425 | 2 | 1.59 | 0.0208 |
| Isoform 14kDa of myelin basic protein S | sp|P02688-4| | 14.06 | 3 | 0.68 | 0.0374 |
| Other | |||||
| 78 kDa glucose-regulated protein | sp|P06761| | 12.54 | 7 | 1.38 | 0.0199 |
| RNA binding motif protein, X-linked | tr|Q4V898| | 6.923 | 2 | 1.89 | 0.0039* |
| Heat shock cognate 71 kDa protein | sp|P63018| | 11.46 | 6 | 1.36 | 0.0408 |
| Stress-induced-phosphoprotein1 | sp|O35814| | 9.945 | 5 | 1.19 | 0.0005* |
| Haptoglobin | sp|P06866| | 6.34 | 2 | 1.16 | 0.0402 |
| Ba1-667 | tr|Q7TP24| | 4.08 | 4 | 1.82 | 0.0062* |
| Non-lesion | |||||
| Energy metabolism | |||||
| Nucleoside diphosphate kinase B | sp|P19804| | 35.53 | 5 | 1.22 | 0.0251 |
| Other | |||||
| Hemoglobin alpha, adult chain 2 | tr|B1H216| | 51.41 | 6 | 1.44 | 0.0214 |
The % Cov (95) refers to the number of amino acids in the identified peptides that match the protein with a confidence of 95% or higher, divided by the total number of amino acids in the protein and expressed as a percentage. Peptides refer to the number of peptides in the protein that have been identified with a confidence of 95% or greater.
Significant with Bonferroni correction (p < 0.0166).
NSR = non-separated runners, NSNR = non-separated non-runners.
The combined effects of maternal separation and exercise on hippocampal protein levels
In the lesioned hemisphere of maternally separated rats, exercise (SR compared to SNR) increased energy-, neurotransmission- and structure-related proteins (phosphoglycerate kinase, aspartate aminotransferase, tenascin-R) as well as increased proliferation of glia (glial fibrillary acidic protein). Exercise also increased plasticity (decreased microtubule-associated protein 1a, important for regulating microtubule stability) and neuroprotective chaperones (mitochondrial 60 kDa heat shock protein which is required for refolding and proper assembly of unfolded polypeptides generated under stress conditions in the mitochondrial matrix, Table 4). Although exercise caused a similar increase in aspartate aminotransferase in maternally separated and non-separated rats, the levels achieved in maternally separated rats remained 2.24 × lower than non-separated rats (NSR:SR = 2.24, p = 0.0013).
Table 4.
Combined effect of maternal separation and exercise on expression of hippocampal proteins in the 6-OHDA lesioned and non-lesioned hemispheres.
| Accession no. | %Cov(95) | Peptides | SR:SNR | p-value | |
|---|---|---|---|---|---|
| Lesion | |||||
| Energy metabolism | |||||
| Phosphoglycerate kinase 1 | sp|P16617| | 5.516 | 2 | 1.79 | 0.0247 |
| Neurotransmission / Signalling | |||||
| Aspartate aminotransferase, cytoplasmic | sp|P13221| | 11.62 | 4 | 1.73 | 0.0196 |
| Cytoskeletal /Structural | |||||
| Tenascin-R | sp|Q05546| | 5.236 | 5 | 1.47 | 0.0111* |
| Microtubule-associated protein 1a | sp|P34926| | 4.47 | 8 | 0.87 | 0.0059* |
| Glial fibrillary acidic protein | sp|P47819| | 12.56 | 7 | 1.51 | 0.0189 |
| Other | |||||
| Ribosomal protein S27a | tr|Q6PED0| | 23.08 | 6 | 0.75 | 0.0305 |
| 60 kDa heat shock protein, mitochondrial | sp|P63039| | 6.457 | 3 | 1.89 | 0.0007* |
| Non-lesion | |||||
| Energy metabolism | |||||
| Nucleoside diphosphate kinase B | sp|P19804| | 35.53 | 5 | 0.27 | 0.0207 |
| Malate dehydrogenase, mitochondrial | sp|P04636| | 19.53 | 7 | 0.51 | 0.0089* |
| Triosephosphate isomerase | sp|P48500| | 40.56 | 13 | 0.66 | 0.0313 |
| Enolase | tr|Q5EB49| | 20.05 | 8 | 0.85 | 0.0054* |
| Neurotransmission / Signalling | |||||
| 2′,3′-cyclic-nucleotide 3′-phosphodiesterase | sp|P13233| | 8.333 | 3 | 0.6 | 0.0432 |
| Alpha-synuclein | sp|P37377| | 37.86 | 5 | 0.34 | 0.0177 |
| 14-3-3 protein epsilon | sp|P62260| | 23.14 | 6 | 0.83 | 0.0032* |
| Cytoskeletal /Structural | |||||
| Non-erythroid spectrin beta | tr|Q6XD99| | 5.428 | 11 | 1.30 | 0.0398 |
| Receptor-type tyrosine-protein phosphatase zeta | sp|Q62656| | 1.425 | 2 | 1.41 | 0.0448 |
| Tenascin-R | sp|Q05546| | 5.236 | 5 | 0.46 | 0.0003* |
| Neurocan core protein | sp|P55067| | 1.671 | 3 | 0.66 | 0.0088* |
| Microtubule-associated protein | tr|Q64715| | 111.01 | 19 | 0.73 | 0.0028* |
| Brevican core protein | sp|P55068| | 3.058 | 4 | 0.78 | 0.0363 |
| Other | |||||
| Alpha-1-inhibitor 3 | sp|P14046| | 2.505 | 4 | 0.65 | 0.0111* |
| Ba1-667 | tr|Q7TP24| | 4.082 | 4 | 0.47 | 0.0002* |
| Histone H2B type 1 | sp|Q00715| | 36 | 7 | 0.6 | 0.0277 |
The % Cov (95) refers to the number of amino acids in the identified peptides that match the protein with a confidence of 95% or higher, divided by the total number of amino acids in the protein and expressed as a percentage. Peptides refer to the number of peptides in the protein that have been identified with a confidence of 95% or greater.
Significant with Bonferroni correction (p < 0.0166).
SR = maternally separated runners, SNR = maternally separated non-runners.
In the non-lesioned hemisphere of maternally separated rats, exercise (SR compared to SNR) decreased energy-related proteins (nucleoside diphosphate kinase, malate dehydrogenase, triosephosphate isomerase, enolase), neurotransmission (2′,3′-cyclic-nucleotide 3′-phosphodiesterase, alpha-synuclein, 14-3-3 protein epsilon) and structural proteins (tenascin-R, neurocan core protein, microtubule-associated protein, brevican core protein). Exercise increased other structural proteins (non-erythroid spectrin beta, receptor-type tyrosine-protein phosphatase zeta) and decreased the antioxidant (Ba1-667) and the protease inhibitor (alpha-1-inhibitor 3).
Discussion
Animals subjected to early life maternal separation and subsequently challenged with 6-OHDA infused into the medial forebrain bundle, displayed long-term changes in energy metabolism and synaptic plasticity in the hippocampus. Maternal separation up-regulated hippocampal proteins involved in energy metabolism (nucleoside diphosphate kinase B, triosephosphate isomerase), synaptic vesicle fusion (alpha-synuclein), extracellular matrix (tenascin-R), cytoskeleton (neurocan and brevican core protein), and iron metabolism (Ba1-667), in the non-lesioned hemisphere of the 6-OHDA lesioned rat brain. Exercise reversed the effects of maternal separation on these proteins in the hippocampus in the non-lesioned hemisphere.
Parkinson's disease has been described as a metabolic syndrome or a mitochondrial complex I (NADH CoQ dehydrogenase) disorder (Schapira et al. 1990), so it is not surprising that the 6-OHDA lesion in the present study resulted in increased mitochondrial proteins, specifically energy related proteins, with decreased RNA binding motif protein (protein synthesis) and increased aspartate aminotransferase (the enzyme that converts aspartate into the major excitatory neurotransmitter, glutamate). Evidence has repeatedly implicated mitochondrial dysfunction in Parkinson's disease (Schapira et al. 1990; Navarro and Boveris, 2009). Complex I inhibitors were shown to reduce the rate of respiration / ATP production in cultured cells and to produce Parkinsonian symptomology in animals, suggesting a pivotal role for mitochondrial dysfunction in the degeneration of dopamine neurons (Langston et al. 1983; Gomez et al. 2007; Höllerhage et al. 2009) and hence the effect on dopamine axon terminals in the hippocampus.
Maternal separation caused an increase in glycolytic and citric acid cycle enzymes (triosephosphate isomerase, nucleoside diphosphate kinase B and aconitate hydratase), vesicle associated proteins (alpha-synuclein, adaptin ear-binding coat-associated protein, protein kinase C and casein kinase substrate), cytoskeletal proteins (spna2 protein, brevican core protein, neurocan core protein, tenascin-R) and antioxidants (ferric iron binding protein Ba1-667). The finding that alpha-synuclein is increased by maternal separation may provide an explanation for the increased vulnerability of dopamine neurons to 6-OHDA in rats subjected to perinatal stress (Pienaar et al., 2008). High levels of mitochondrial alpha-synuclein have been found in the hippocampus, striatum and substantia nigra of non-lesioned rats (Zhang et al. 2008). Alpha-synuclein associates with complex I in the mitochondrial electron transport chain (Devi et al. 2008) and may contribute to dopamine neuron pathology as in the case of Parkinson's disease and cocaine abuse by impairing mitochondrial energy metabolism (Mash et al. 2003; Büeler, 2009). Increased levels of alpha-synuclein in maternally separated rats may interfere with complex I activity and hence increase susceptibility of dopamine neurons to 6-OHDA lesion (Büeler, 2009). This may explain the increased severity of locomotor deficits produced by 6-OHDA in maternally separated rats (Pienaar et al. 2008; Mabandla and Russell, 2010).
Maternal separation also altered the effect of the 6-OHDA lesion on hippocampal proteins. Mitochondrial enzymes that were increased by 6-OHDA in non-separated rats, were decreased in maternally separated rats. This seemed to suggest that maternal separation and 6-OHDA acted on similar energy-related metabolic pathways. Maternal separation also blocked the 6-OHDA-induced increase in glutamate transmission (cytoplasmic aspartate aminotransferase) which prevented the beneficial effect of exercise on this protein from achieving the high levels observed in non-separated rats.
Early life stress and exercise resulted in opposing changes in hippocampal metabolic protein levels in the non-lesioned hemisphere. Maternally separated rats that were allowed to exercise in freely moving running wheels displayed an opposing down-regulation of hippocampal enzymes involved in energy metabolism (nucleoside diphosphate kinase B, malate dehydrogenase, triosephosphate isomerase, enolase,), neurotransmission (alpha-synuclein), structure (tenascin-R, neurocan and brevican core protein) and iron metabolism (Ba1-667) in the non-lesioned side of the brain. In agreement with previous findings (Ding et al. 2006; Kirchner et al. 2008), exercise, a stressor itself, was found to up-regulate proteins involved in energy metabolism (triosephosphate isomerase) and synaptic plasticity (aspartate aminotransferase, dynactin, spectrin, RNA binding motif protein, tyrosine-protein phosphatase zeta) in the hippocampus of non-separated rats. These findings suggest that maternal separation and exercise target similar metabolic processes in the hippocampus and that prior exposure to maternal separation may have interfered with the effects of exercise by reducing energy-related proteins and neuroplasticity. Interestingly, exercise reversed the effects of maternal separation on energy metabolism, cytoskeletal proteins, iron metabolism and alpha-synuclein by interacting with these same systems. This may provide a mechanism by which exercise reduces the adverse effects of maternal separation on motor function (Marais et al. 2009; Mabandla and Russell, 2010). Once again, these findings highlight the compensatory role of exercise observed in previously stressed rats in the present study, by decreasing maternal separation-induced protein levels in the non-lesioned hemisphere of the 6-OHDA treated rat brain. In agreement with the suggestion that exercise and maternal separation activate similar stress-related pathways, Daniels et al. (2011) found that maternal separation and exercise affected the same functional classes of proteins. Our results show that prior exposure to maternal separation alters these pathways so that they do not respond to exercise in the same way as in non-separated rats
The beneficial effects of exercise have been attributed to neurotrophin-mediated hippocampal plasticity which has been shown to be dependent on the regulation of metabolic and oxidative proteins (Vaynman et al. 2006; Maniam and Morris, 2010; Tajira et al. 2010). Similar to the present study, exercise has been shown to normalize proteins affected by early life maternal separation, including hippocampal glucocorticoid receptors, 5HT1A receptors and BDNF mRNA (Maniam and Morris, 2010). Tajiri et al. (2010) also showed that exercise caused a compensatory increase in hippocampal BDNF and glial cell line-derived neurotrophic factor (GDNF), in the non-lesioned hemisphere of exercised rats which could explain some of the effects of exercise observed in the present study.
Tenascins are glycoproteins that form part of the extracellular matrix (ECM). They function as structural support proteins and hence orchestrate various cell-cell interactions and also influence cell interactions with the protein components of the ECM (Jones and Jones, 2000). Tenascin-R in particular is widely distributed throughout the central nervous system (Joester and Faissner, 2001). It plays an important role in determining the postsynaptic threshold for induction of long-term potentiation (LTP), the neural correlate of learning and memory (Montag-Sallaz and Montag, 2003). Maternal separation resulted in increased hippocampal tenascin-R levels in the non-lesioned hemisphere, while exercise reduced the levels of tenascin-R in the non-lesioned hemisphere and increased tenascin-R levels in the lesioned hemisphere of rats previously subjected to maternal separation. The increase in tenascin-R by exercise, might relate to exercise improving learning and memory and hence synaptic plasticity mediated by BDNF (Ding et al. 2006; Vaynman et al. 2006; Maniam and Morris, 2010).
Brevican and neurocan core proteins are lecticans which form part of the multidomain hyaluronan-binding chondroitin sulfate proteoglycans and bind to both cell surface molecules (e.g. neural cell adhesion molecule (NCAM)) and structural ECM proteins (e.g. tenascin-C and –R, hyaluronan, heparin). They are potent inhibitors of neuronal adhesion and neurite outgrowth and thus play a modulatory role in signal transduction (Friedlander et al. 1994; Sango et al. 2003). Although neurocan and brevican are dispensable for brain development, mice deficient in either brevican or neurocan protein display abnormalities in hippocampal LTP and hence altered synaptic plasticity (Zhou et al. 2001; Brakebusch et al. 2002). In the present study, maternal separation increased neurocan in the lesioned and non-lesioned hemisphere and brevican in the non-lesioned side, while exercise decreased both neurocan and brevican core protein in the non-lesioned hemisphere. If the inhibitory action on synaptic plasticity of neurocan and brevican core proteins are taken into account it is not surprising that maternal separation resulted in increased expression of these proteins, as previous studies have shown that maternal separation impairs synaptic plasticity and hence learning and memory (Aisa et al. 2009), while exercise has opposing beneficial effects. Exercise appeared to attempt to restore altered levels of proteins, a finding that has been confirmed as exercise counteracts stress effects and improves cognitive function (Cotman and Berchtold, 2002) which has been linked to increasing growth factor expression, especially BDNF (Zheng et al. 2006), being a predictor of learning efficacy.
Conclusion
Proteomic analysis of the hippocampus revealed that exercise partially reversed changes in hippocampal proteins caused by maternal separation in the non-lesioned hemisphere which may contribute to the beneficial effects of exercise. Maternal separation altered the expression of metabolic proteins which possibly reduced the beneficial effect of exercise. These findings provide insight into the mechanism by which maternal separation stress prevents the beneficial effects of exercise on CNS function by affecting proteins involved in energy metabolism and neuroplasticity.
Figure 1.
Exercise reversed changes induced by maternal separation (MS) in hippocampal proteins. A. Effect of MS in non-exercised rats. Depiction of data in Table 2. B. Effect of exercise in MS rats. Diagram of data in Table 4. SNR = maternally separated non-runners, NSNR = non-separated non-runners, SR = maternally separated runners, SNR = maternally separated non-runners.
Acknowledgments
This material is based upon work supported financially by the National Research Foundation (NRF), the National Institutes of Health (NIH) Fogarty International Center grant R01TW008040 to Michael J. Zigmond, and the University of Cape Town. Any opinion, findings and conclusions, or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereto. The authors declare no conflict of interest.
References
- Aisa B, Elizalde N, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: implications for spatial memory. Hippocampus. 2009;19:1222–1231. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- Büeler H. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease. Exp Neurol. 2009;218:235–246. doi: 10.1016/j.expneurol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H, Krug M, Böckers TM, Zhou X, Kreutz MR, Montag D, Gundelfinger ED, Fässler R. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22:7417–7427. doi: 10.1128/MCB.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Daniels WMU, Marais L, Stein DJ, Russell VA. Exercise normalizes altered expression of proteins in the ventral hippocampus of rats subjected to maternal separation. Exp Physiol. 2011 doi: 10.1113/expphysiol.2011.061176. [DOI] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Pradhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Faure J, Stein DJ, Daniels W. Maternal separation fails to render animals more susceptible to methamphetamine-induced conditioned place preference. Metab Brain Dis. 2009;24:541–559. doi: 10.1007/s11011-009-9158-1. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chrondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Bandez MJ, Navarro A. Pesticides and impairment of mitochondrial function in relation with the parkinsonian syndrome. Front Biosci. 2007;12:1079–1093. doi: 10.2741/2128. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Höllerhage M, Matusch A, Champy P, Lombès A, Ruberg M, Oertel WH, Höglinger GU. Natural lipophilic inhibitors of mitochondrial complex I are candidate toxins for sporadic neurodegenerative tau pathologies. Exp Neurol. 2009;220:133–142. doi: 10.1016/j.expneurol.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Howells FM, Russell VA, Mabandla MV, Kellaway LA. Stress reduces the neuroprotective effects of exercise in a rat model for Parkinson's disease. Behav Brain Res. 2005;165:210–220. doi: 10.1016/j.bbr.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Joester A, Faissner A. The structure and function of tenascins in the nervous system. Matrix Biol. 2001;20:13–22. doi: 10.1016/s0945-053x(00)00136-0. [DOI] [PubMed] [Google Scholar]
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kirchner L, Chen W-Q, Afjehi-Sadat L, Viidik A, Skalicky M, Höger H, Lubec G. Hippocampal metabolic proteins are modulated in voluntary and treadmill exercise rats. Exp Neurol. 2008;212:145–151. doi: 10.1016/j.expneurol.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Mabandla MV, Russell VA. Voluntary exercise reduces the neurotoxic effects of 6-hydroxydopamine in maternally separated rats. Behav Brain Res. 2010;211:16–22. doi: 10.1016/j.bbr.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabandla MV, Kellaway L, St Clair GA, Russell VA. Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metab Brain Dis. 2004;19:43–50. doi: 10.1023/b:mebr.0000027416.13070.c3. [DOI] [PubMed] [Google Scholar]
- Mabandla MV, Kellaway L, Daniels WM, Russell VA. Effect of exercise on dopamine neuron survival in prenatally stressed rats. Metab Brain Dis. 2009;24:525–539. doi: 10.1007/s11011-009-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: role of hippocampus. Psychoneuroendocrinology. 2010;35:1553–1564. doi: 10.1016/j.psyneuen.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Marais L, Stein DJ, Daniels WMU. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab Brain Dis. 2009;24:587–597. doi: 10.1007/s11011-009-9157-2. [DOI] [PubMed] [Google Scholar]
- Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WMU. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res. 2008;61:106–112. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Mash DC, Ouyang Q, Pablo J, Basile M, Izenwasser S, Lieberman A, Perrin RJ. Cocaine abusers have an overexpression of α-synuclein in dopamine neurons. J Neurosci. 2003;23:2564–2571. doi: 10.1523/JNEUROSCI.23-07-02564.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag-Sallaz M, Montag D. Severe cognitive and motor coordination deficits in tenascin-R-deficient mice. Genes Brain Behav. 2003;2:20–31. doi: 10.1034/j.1601-183x.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Brain mitochondrial dysfunction and oxidative damage in Parkinson's disease. J Bioenerg Biomembr. 2009;41:517–521. doi: 10.1007/s10863-009-9250-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- Pienaar IS, Kellaway LA, Russell VA, Smith AD, Stein DJ, Zigmond MJ, Daniels WM. Maternal separation exaggerates the toxic effects of 6-hydroxydopamine in rats: implications for neurodegenerative disorders. Stress. 2008;11:448–456. doi: 10.1080/10253890801890721. [DOI] [PubMed] [Google Scholar]
- Ryu V, Yoo SB, Kang DW, Lee JH, Jahng JW. Post-weaning isolation promotes food intake and body weight gain in rats that experienced neonatal maternal separation. Brain Res. 2009;1295:127–134. doi: 10.1016/j.brainres.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Sango K, Oohira A, Ajiki K, Tokashiki A, Horie M, Kawano H. Phosphacan and neurocan are repulsive substrata for adhesion and neurite extension of adult rat dorsal root ganglion neurons in vitro. Exp Neurol. 2003;182:1–11. doi: 10.1016/s0014-4886(03)00090-6. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I. Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res. 2010;1310:200–207. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- Van Heerden JH, Russell V, Korff A, Stein DJ, Illing N. Evaluating the behavioural consequences of early maternal separation in adult C57BL/6 mice; the importance of time. Behav Brain Res. 2010;207:332–342. doi: 10.1016/j.bbr.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Wu A, Gómez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Ueda K, Yu S, Yang H. Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody. Brain Res. 2008;1244:40–52. doi: 10.1016/j.brainres.2008.08.067. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X-H, Brakebusch C, Matthies H, Oohashi T, Hirsch E, Moser M, Krug M, Seidenbecher CI, Boekers TM, Rauch U, Buettner R, Gundelfinger ED, Fässler R. Neurocan is dispensible for brain development. Mol Cell Biol. 2001;21:5970–5978. doi: 10.1128/MCB.21.17.5970-5978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]