Abstract
Patients and their families have, for many decades, detected subtle changes in cognition subsequent to surgery, and only recently has this been subjected to scientific scrutiny. Through a combination of retrospective human studies, small prospective biomarker studies, and experiments in animals, it is now clear that durable consequences of both anesthesia and surgery occur, and that these intersect with the normal processes of aging, and the abnormal processes of chronic neurodegeneration. It is highly likely that inflammatory cascades are at the heart of this intersection, and if confirmed, this suggests a therapeutic strategy to mitigate enhanced neuropathology in vulnerable surgical patients.
Keywords: neuroinflammation, peripheral inflammation, cytokines, Alzheimer transgenic mice, POCD, biomarkers
1. Introduction
Sporadic, or late onset, Alzheimer disease, is the most common form of dementia, and is thought to be due to an interaction between specific susceptibility genes and environmental factors. But the environmental factors are poorly understood, exemplified by the recent National Institutes of Health consensus statement that “…firm conclusions cannot be drawn about the association of any modifiable risk factor with cognitive decline or Alzheimer’s disease” (Daviglus et al., 2010). Nevertheless, due to the very common complaint of cognitive decline from patients and families following major illness or surgery, there has been recent focus on these environmental factors as enhancing the neuropathology when in the setting of genetic vulnerabilities. The purpose of this brief review is to review the literature to date on surgery and major illness (primarily sepsis) as risk factors for both the timing and prevalence of Alzheimer’s dementia.
2. Surgery
2.1 Human Studies
Anecdotes describing post-operative cognitive decline (POCD) have been around for many decades. It was first characterized by Bedford in 1955 (Bedford, 1955), and then more carefully by Moller, Johnson, Monk and others in the last decade (Johnson et al., 2002;Moller et al., 1998;Monk et al., 2008). Although strict definitions and diagnostic criteria have not been agreed on (Crosby & Culley, 2011;Evered et al., 2011), POCD seems to be a largely time-limited cognitive syndrome in the days to weeks following surgery. It is most common in the elderly, and after prolonged surgical procedures, but few other risk factors have been identified. No evidence yet exists to implicate any particular feature of the perioperative experience. For example, it appears to be just as common after regional as after general anesthesia (Williams-Russo et al., 1995). It occurs with all forms of surgery (Moller et al., 1998). But it appears to resolve; few studies show persistence of the cognitive effects longer than 3 months after surgery. But is this really resolution or merely deployment of cognitive reserve? Longitudinal studies after heart surgery suggest that despite apparent resolution at 6 months, there exists a later decline at about 5 years after surgery (Newman et al., 2001). More recently, however, this has been questioned (Selnes et al., 2012) through studies that included medically treated control patients. In fact, because of a lack of pre-operative cognitive trajectory information, even the existence of POCD as a real clinical entity has been questioned (Avidan et al., 2009).
Whether or not POCD really exists, or what the diagnostic criteria are, the question of whether surgery is associated with either an earlier onset of dementia, or a higher risk of dementia, remains. This issue was initially tackled by Bohnen et al, using the same cohort of 252 patients in two different study designs (case-control and correlative analysis). These authors were able to demonstrate a significant inverse relationship between the cumulative history of prior surgeries and the age of dementia diagnosis (Bohnen et al., 1994a). The somewhat different question of whether surgery was a risk factor for Alzheimer’s diagnosis at any time could not be answered with confidence (Bohnen et al., 1994b). They estimated that being diagnosed with Alzheimer’s after having surgery had an odds ratio of 1.5, although clinically an important effect size, was not statistically different from an odds ratio of 1 because of limited power.
Subsequent studies have been largely consistent with this finding (Gasparini et al., 2002), except that Lee et al found a significant increase in the odds ratio (1.7) for being diagnosed with Alzheimer’s disease after coronary artery bypass surgery (CABG) (Lee et al., 2005). Looking at this from a different perspective, Knopman asked whether already diagnosed Alzheimer disease patients have a higher risk of having had CABG surgery in the past. Using this somewhat different approach, no association was detected (Knopman et al., 2005).
Alzheimer disease has a pre-clinical course measured in decades, thus, biochemical and imaging biomarkers that predict the disease have become popular goals. Because the cognitive effects of an intervention might be similarly delayed, perioperative investigators have begun to turn to such biomarkers in an attempt to more quickly ascertain whether surgery causes an increment that might predict an acceleration in dementia diagnosis. For example, Palotas et al, found that cerebrospinal fluid (CSF) tau and amyloid β changed from entirely normal levels to a pattern consistent with mild cognitive impairment (MCI) 6 months after CABG surgery (Palotas et al., 2010). Tang et al also found that even minor surgery causes the CSF tau/amyloid β ratio to change towards an Alzheimer pattern only 48 hours after surgery (Tang et al., 2011a). Not surprisingly, this was also associated with elevations in pro-inflammatory cytokines in the CSF, such as Interleukin-6 (IL-6). Finally, a recent study has shown that even structural changes in the brain may follow surgery. Kline et al showed that cortical and hippocampal gray matter volume was about 10% lower than preoperative values an average of 6 months after surgery in patients entered into an Alzheimer Disease Neuro Imaging (ADNI) center database (Kline et al., 2012). These effects were no longer detectable at the next imaging visit, suggesting reversal or plasticity is retained even at this age. Thus, independent of cognitive changes, there is slowly growing evidence that suggests neuropathology known to underlie Alzheimer’s disease is enhanced by a surgical procedure, but the context (i.e., condition of the brain) may be critical as to the eventual outcome.
Detailed anesthetic histories on any of the patients in the above cited studies were not available, thus it is not clear whether the actual surgical procedure, or any other aspect of perioperative care might have been causally related to cognitive dysfunction. Understanding causation will be enormously difficult in any human study because anesthesia without surgery and surgery without anesthesia will never be done, and anesthesia choice and depth will always be a surrogate for the magnitude and duration of the surgical procedure. Searching for rank order effects with a range of different anesthetic types and drugs may provide some insight if differences exist, but it is likely that animal studies will be necessary to gain mechanistic information.
2.2 Animal Studies
A large number of in vitro studies have provided a mechanistic rationale for anesthetic-induced acceleration in neurodegenerative diseases, which will be reviewed in other articles within this issue (see review in this issue, Xie). Fewer studies using animals have appeared, but in general have also provided evidence for delayed cognitive effects of anesthesia, especially using the anesthetic, isoflurane, in aged wild type (Bianchi et al., 2008, Culley et al., 2004a, Culley et al., 2004b, Green et al., 2006, Lu et al., 2010, Planel et al., 2008, Whittington et al., 2011, Zhang et al., 2011, Zhang et al., 2012) and transgenic animals (see review in this issue, Tang and Eckenhoff). Most of these animal studies have only looked at cognitive events in the days to weeks following exposure, but a few have documented cognitive effects out as long as two months afterward. In general the long term effects of anesthetic exposure per se were small or absent, although anesthetic-induced improvements have been noted (Culley et al., 2003, Tang et al., 2011b). Surgery, on the other hand, appears to produce more robust detrimental effects in the small number of studies that have been reported. For example, Wan et al found that orthopedic surgery, in young wild type mice under an intravenous anesthetic, produced significant post-operative cognitive effects, while the anesthetic alone did not. The effects of surgery lasted only a week, however (Wan et al., 2007). These acute effects of surgery have been attributed to the effects of the peripheral inflammatory response, such as Tumor Necrosis Factor-α (TNFα) (Terrando et al., 2010, Terrando et al., 2011), IL-6 and Interleukin 1- beta (IL-1β) (Cao et al., 2010, Rosczyk et al., 2008). A more recent study in our lab examined the effects of abdominal surgery both in wild type and in the triple transgenic mouse model of Alzheimer disease (3xTgAD). This surgical procedure, cecal resection, approximated an uncomplicated appendectomy or colectomy in humans, very common procedures. Similar to the Wan et al, and the Terrando et al studies, we also found that the anesthetic alone did not produce a statistically significant effect on cognition in the wild type (WT) animal. Even the transgenic animal did not show statistically significant declines, although they seemed to fare a bit worse that the WT. We were able to show that there existed a striking cognitive decline due to the addition of surgery, but only in the transgenic animal. Most importantly, we were able to detect this cognitive impairment out until at least 3 months following the operation (Tang et al., 2012). This strongly suggests that the effect of anesthesia and surgery might only accrue in a brain with ongoing pathology. In the case of our studies, this smoldering pathology was created by the transgenes, but other genetics and environmental factors likely exist that produce similar vulnerabilities.
In summary, although the largely retrospective human studies have suggested a linkage between surgery and cognitive outcome, there is much that remains unanswered. For example, is POCD different from dementia? Is it predictive of dementia? Does surgery accelerate neurodegeneration independently of POCD? The answers to these questions should not only help us define POCD, but also help to understand the cognitive risks of surgery in the elderly. Animal studies have provided evidence that both the anesthetic (largely isoflurane) and the operation can independently influence cognition, but that the effect due to surgery appears to be dominant.
3. Major illness
It has long been assumed that major illness has a deleterious and perhaps prolonged effect on cognition, and perhaps may be a risk factor for dementia. A recent review of the subject concluded that “…neurocognitive sequelae following critical illness are common, may be permanent, and are associated with impairments in daily function” (Hopkins & Jackson, 2006). Two recent human studies have looked more closely at the issue, using similar designs but different patient datasets. Avidan et al, using an Alzheimer Disease Research Center (ADRC) cohort, were unable to detect an effect of either surgery or major illness on the trajectory of cognitive decline in patients in the eighth decade of life (Avidan et al., 2009). On the other hand, Ehlenbach et al, using a larger prospective cohort of similarly aged subjects, and with similar numbers of cognitive evaluations, found that cognitive decline was significantly greater in patients after hospitalizations for even non-critical illness (Ehlenbach et al., 2010). They also looked at the correlation of illness with dementia. The adjusted risk of being diagnosed with dementia appeared to display a “dose-response” effect, in that it was 1.4 (1.1-1.7) for non-critical illness hospitalization and 2.3 (0.9-5.7) for critical illness hospitalization. It is intriguing that these risk ratios for incident dementia after hospitalization are remarkably similar to those presented by Bohnen over 15 years ago for surgery and anesthesia (Bohnen et al., 1994b). The lack of power in these pioneering studies make distinction between various types of critical illness difficult, although there appear to be more studies on the survivors of the adult respiratory distress syndrome (ARDS) and sepsis, the two biggest causes of intensive care unit admission.
In ARDS, up to 75% of survivors had deficits in several neurocognitive domains at 6 months after discharge, dropping to 45% at 1 year. Thereafter, no significant improvement was detected out to 5 years (Rothenhausler et al., 2001). Sepsis is similar, and is particularly interesting because the effects on the immune system may reveal clues for the mechanism of cognitive dysfunction – if they were better understood. It is well recognized that coincident with sepsis, a delirium-like state, termed sepsis associated encephalopathy (SAE), is common. The mechanisms underlying SAE are complex and involve both inflammatory and non-inflammatory pathways (Iacobone et al., 2009). But like ARDS, the incidence of cognitive dysfunction in sepsis survivors is estimated to be as high as 78% (Iwashyna et al., 2010, Yende & Angus, 2007). Long term cognitive outcome studies have not been reported, but will be confounded by very high mortality. This high mortality produces what is known as the “survivor effect”, which simply states that factors responsible for facilitating survival may extend to other systems or measures under study, such as cognition. A well known example would be ischemic pre-conditioning. The high mortality of sepsis, then, may skew results to appear that it actually caused improvement. Studying non-fatal infective episodes, Dunn et al found a significant association between incident dementia and more than two such episodes (Dunn et al., 2005). In another recent example, serum levels of the proinflammatory cytokines TNFα and IL-6, measured during a variety of infectious episodes, correlated with both the rate and extent of cognitive decline and in older patients over a 6 month period (Holmes et al., 2009, Holmes et al., 2011).
Thus, like surgery, there appears to be evidence for an acceleration in cognitive decline, and increase in incident dementia subsequent to major illness. In fact, surgery is indicated for some forms of major illness (e.g., inflammatory bowel disease), and thus dissection of the various contributors to post-operative dementia is likely to be difficult in patients. Animal models of either ARDS, infection or sepsis have not yet been evaluated for long term cognitive effects, although studies on the impact of inflammation have appeared (Godbout et al., 2005, Jurgens et al., 2012, for review Corona et al., 2012).
4. Neuroinflammation
There have been many proposed mechanisms for the linkage between cognition and the above discussed medical and surgical conditions, but the most obvious, and also most studied, is the inflammatory response, both systemic and focal. The involvement of neuroinflammation in the smoldering pathogenesis of neurodegenerative disorders, now established, was initially suspected because of the salutary effects of chronic non-steroidal anti-inflammatory drugs (NSAID) usage, but more recently through careful study of Alzheimer pathology in patient’s brains, blood and CSF. The literature on the role of inflammation in Alzheimer disease has exploded, and because many good reviews are now available (Perry et al., 2007), we will only touch on a few relevant points.
The accumulation of pathologic protein aggregates, whether amyloid beta, prion protein or hyperphosphorylated tau, small oligomers or mature senile plaques, are thought to activate the immune cells in the brain, such as astrocytes or microglia. Additional inflammatory cells can be recruited from peripheral monocytes, which gain access to the central nervous system via either a compromised blood brain barrier or the circumventricular organs (Saijo & Glass, 2011). Microglia are responsible for immune surveillance in the brain, and are now thought to become activated by some forms of amyloid beta aggregate, cytokines, chemokines, and other cellular material (Graeber, 2010). Their activation causes a phenotypic shift with a change in the surface expression of specific antigens. They also produce reactive oxygen and nitrogen radicals and various other mediators in order to neutralize a perceived threat. Simultaneously, microglia produce cytokines that further activate the immune system. Taken together, these processes are aimed at scavenging and removal of misfolded protein and cellular debris, but collateral damage to adjacent structures occurs as well. A secondary anti-inflammatory component mitigates the positive feedback, and returns these cells to a surveillance mode, although intermediate states of activation probably exist. It is now thought that microglia are “primed” by the chronic, smoldering, subclinical neurodegeneration (Cunningham et al., 2005, Perry et al., 2007), and that this same chronic process may compromise the integrity of the blood brain barrier. In essence, this is like a mine, that has been fused and is lying in wait. The trigger in this case is the occurrence of an acute peripheral inflammatory event, such as surgery, infection or major illness, with its large pro-inflammatory response. The signals either travel through vagal afferents, or a cytokine burst (typically, IL-6, IL-1β and TNF-α) that simply traverse the now leaky blood brain barrier, ultimately activating already primed microglia. Should the blood brain barrier actually be compromised by AD, or by surgery and anesthesia, it would also facilitate entry of peripheral inflammatory cells, further augmenting the damage. The result is predicted to be an exaggerated and detrimental neuroinflammatory response, which may go on to damage synapses and neurons. There also exists evidence that normally opposing compensatory anti-inflammatory response to neuroinflammation, such as Interleukin-4 (IL-4), Interleukin-10 (IL-10), or transforming growth factor-β (TGFβ) release, is muted by the chronic neurodegeneration, contributing further to the pro-inflammatory damage (Swardfager et al., 2010).
These observations lead to an obvious therapeutic approach to mute the inflammatory response to predictable, transient events, like surgery. While very specific anti-cytokine therapies are, or may become available, the complexity and redundancy of the response suggests a less specific approach. A variety of broad spectrum anti-inflammatory drugs are available which have been shown to dampen the systemic inflammatory response to surgery and infection. Two major classes of drug are available, the non-steroidal anti-inflammatory drugs, as well as the large class of steroids; both have been used to reduce the systemic inflammatory response in various forms of surgery, but not yet as a matter of routine to reduce the neuroinflammatory response in the elderly. There are important side effects of these drugs for surgery, such as bleeding for the NSAIDS, and wound healing for the steroids, but in general, these effects are minimal with the transient usage of these drugs proposed for the effect here (Cardwell et al., 2005). The commonly used local anesthetic lidocaine has also been shown to reduce cognitive consequences of isoflurane exposure in aged rats, presumably due to its well-known anti-inflammatory effects (Lin et al., 2012).
The efficacy of anti-inflammatory drugs in Alzheimer disease prospective trials has been disappointing, but this might be due to delivery well after the majority of pathology and damage had occurred, and the inherent variability of primary endpoint commonly used: neurocognitive testing (Martin et al., 2008). Biomarker studies, especially imaging, have made more progress. For example, recent work has shown that elderly NSAID users have lower age-related loss of gray matter volume than age matched controls (Bendlin et al., 2010, Walther et al., 2011). These results emphasize the dissociated (temporally, at least) nature of cognitive and pathologic changes in the chronic neurodegenerative diseases, and give hope that some of these therapeutic approaches may be efficacious if instituted early in life. Also, these data do not directly address the more limited application to prevent the transient, incremental triggering inflammatory response to surgical trauma. Both in animals and in people, NSAIDS and steroids have been shown to diminish the inflammatory response to surgery (Fillinger et al., 2002, Kamer et al., 2012, Nakamura et al., 2006, Srinivasa et al., 2011, Zargar-Shoshtari et al., 2009). Definition of the optimal timing and duration of such treatments requires further work.
Finally, it should be mentioned that even the animal studies cannot fully separate anesthesia from surgery, because the surgical procedure is always performed with an anesthetic, and it is becoming clear that certain anesthetics can modulate the inflammatory or immune response. Isoflurane and sevoflurane, for example, have been shown to inhibit and bind with high affinity to the leukocyte functional antigen-1α (LFA-1α) inserted (I) domain of integrin (Yuki et al., 2008, Yuki et al., 2010). Propofol and ketamine are widely recognized as having anti-inflammatory and antioxidant properties, and have been shown to mute the systemic inflammatory response (Corcoran et al., 2006, Kalimeris et al., 2011, Tang et al., 2011a). A large number of cytokines have a four helix bundle fold which has been identified as a favored anesthetic-binding motif (Johansson et al., 2000). Thus, the potential for the anesthetic to modulate the effect of surgery on innate immunity is likely to be real (Chiang et al., 2008), although much understudied at this point. Studies in animals should include side-by-side comparisons of the many different anesthetics available in order to inform clinicians of the best potential choices.
5. Conclusion
Major illness and surgery have both been associated with an enhancement in the pathogenesis of chronic neurodegenerative diseases, such as Alzheimer’s disease, and in some cases, worsening of the cognitive outcome of this pathogenesis. Anesthesia, or anesthetics, although implicated mechanistically in both in vitro and animal studies, appear to play a smaller role than surgery, although their potential to modulate the inflammatory response remains understudied. The surgical effect, like that of major illness, is likely to be mediated through inflammatory cascades that ultimately trigger neuroinflammation. The outcome of neuroinflammation will depend on the brain context; in some cases producing no functional outcome because of intact compensatory anti-inflammatory plastic responses. However, some brains are made vulnerable by smoldering pathology, inflammation and dysfunctional anti-inflammatory pathways. In such cases, super-imposed peripheral inflammation may exaggerate neuroinflammation and cause neuronal and synaptic destruction, with the inevitable decline in cognitive reserve. This potential mechanism immediately suggests a relatively low risk approach to reduce incremental neuropathology – dampen the perioperative inflammatory response to surgery.
Fig. 1.
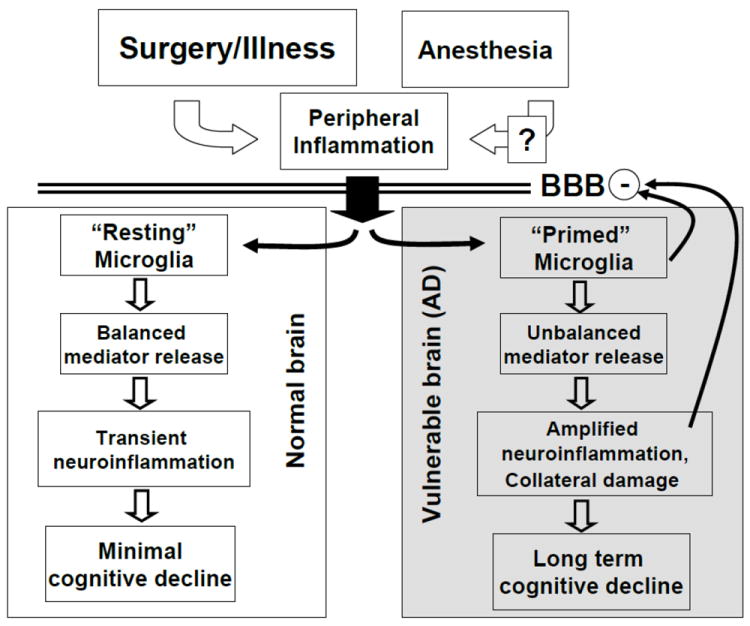
A simplified conceptual model for what might be happening subsequent to anesthesia, surgery and/or illness in both the normal and the vulnerable brain. The presumed immediate effect of surgery is peripheral inflammation, the magnitude of which might be modulated by the anesthetic choice (indicated by the “?”). The peripheral inflammatory mediators (or neural afferents), cross the blood brain barrier (BBB), and activate microglia. Depending on the prior state of these immune cells, the response could be appropriate and balanced, as indicated for the normal brain, or amplified and unbalanced in the vulnerable Alzheimer (AD) brain. Presumably, the microglia in the vulnerable brain have already been activated, or “primed”, by disease-related proteins, like amyloid-β. Structural injury in the vulnerable brain may include the BBB, which further enhances inflammatory mediator entry. The degree and duration of cognitive loss will depend on the degree of inflammation-induced structural (synaptic) injury.
Acknowledgments
This work was supported in part by NIH AG031742 and the Austin Lamont Endowment.
Abbreviations
- POCD
post-operative cognitive decline
- CABG
coronary artery bypass surgery
- MCI
mild cognitive impairment
- CSF
cerebrospinal fluid
- ADNI
Alzheimer Disease Neuro Imaging
- TNFα
Tumor Necrosis Factor-α
- 3xTgAD
triple transgenic mouse model of Alzheimer disease
- ADRC
Alzheimer Disease Research Center
- ARDS
adult respiratory distress syndrome
- SAE
sepsis associated encephalopathy
- IL-6
Interleukin-6
- IL-4
Interleukin-4
- IL-10
Interleukin-10
- TGFβ
transforming growth factor-β
- NSAID
non-steroidal anti-inflammatory drugs
- LFA-1α
leukocyte functional antigen-1α
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avidan MS, Searleman AC, Storandt M, Barnett K, Vannucci A, Saager L, et al. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111:964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269:259–263. doi: 10.1016/s0140-6736(55)92689-1. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Newman LM, Ries ML, Puglielli L, Carlsson CM, Sager MA, et al. NSAIDs may protect against age-related brain atrophy. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, et al. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen N, Warner MA, Kokmen E, Kurland LT. Early and midlife exposure to anesthesia and age of onset of Alzheimer’s disease. Int J Neurosci. 1994a;77:181–185. doi: 10.3109/00207459408986029. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Warner MA, Kokmen E, Beard CM, Kurland LT. Alzheimer’s disease and cumulative exposure to anesthesia: a case-control study. J Am Geriatr Soc. 1994b;42:198–201. doi: 10.1111/j.1532-5415.1994.tb04952.x. [DOI] [PubMed] [Google Scholar]
- Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian AY, et al. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1426–1432. doi: 10.1016/j.pnpbp.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Cardwell M, Siviter G, Smith A. Non-steroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD003591.pub2. CD003591. [DOI] [PubMed] [Google Scholar]
- Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS One. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran TB, Engel A, Sakamoto H, O’Shea A, O’Callaghan-Enright S, Shorten GD. The effects of propofol on neutrophil function, lipid peroxidation and inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function. Br J Anaesth. 2006;97:825–831. doi: 10.1093/bja/ael270. [DOI] [PubMed] [Google Scholar]
- Corona AW, Fenn AM, Godbout JP. Cognitive and behavioral consequences of impaired immunoregulation in aging. J Neuroimmune Pharmacol. 2012;7:7–23. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- Crosby G, Culley DJ. Surgery and anesthesia: healing the body but harming the brain? Anesth Analg. 2011;112:999–1001. doi: 10.1213/ANE.0b013e3182160431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–1009. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004a;99:1393–1397. doi: 10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004b;100:309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Jr, Cox NJ, et al. National Institutes of Health State-of-the-Science Conference statement: preventing alzheimer disease and cognitive decline. Ann Intern Med. 2010;153:176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis Assoc Disord. 2005;19:91–94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–1185. doi: 10.1213/ANE.0b013e318215217e. [DOI] [PubMed] [Google Scholar]
- Fillinger MP, Rassias AJ, Guyre PM, Sanders JH, Beach M, Pahl J, et al. Glucocorticoid effects on the inflammatory and clinical responses to cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:163–169. doi: 10.1053/jcan.2002.31057. [DOI] [PubMed] [Google Scholar]
- Gasparini M, Vanacore N, Schiaffini C, Brusa L, Panella M, Talarico G, et al. A case-control study on Alzheimer’s disease and exposure to anesthesia. Neurol Sci. 2002;23:11–14. doi: 10.1007/s100720200017. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Culliford D, Perry VH. Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology. 2011;77:212–218. doi: 10.1212/WNL.0b013e318225ae07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–878. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T. Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med. 2009;37:S331–S336. doi: 10.1097/CCM.0b013e3181b6ed58. [DOI] [PubMed] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JS, Scharf D, Davies LA, Reddy KS, Eckenhoff RG. A designed four-alpha-helix bundle that binds the volatile general anesthetic halothane with high affinity. Biophys J. 2000;78:982–993. doi: 10.1016/S0006-3495(00)76656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T, Monk T, Rasmussen LS, Abildstrom H, Houx P, Korttila K, et al. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96:1351–1357. doi: 10.1097/00000542-200206000-00014. [DOI] [PubMed] [Google Scholar]
- Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimeris K, Christodoulaki K, Karakitsos P, Batistatou A, Lekka M, Bai M, et al. Influence of propofol and volatile anaesthetics on the inflammatory response in the ventilated lung. Acta Anaesthesiol Scand. 2011;55:740–748. doi: 10.1111/j.1399-6576.2011.02461.x. [DOI] [PubMed] [Google Scholar]
- Kamer AR, Galoyan SM, Haile M, Kline R, Boutajangout A, Li YS, et al. Meloxicam improves object recognition memory and modulates glial activation after splenectomy in mice. Eur J Anaesthesiol. 2012 doi: 10.1097/EJA.0b013e3283534f56. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kline RP, Pirraglia E, Cheng H, De SS, Li Y, Haile M, et al. Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment. Anesthesiology. 2012;116:03–612. doi: 10.1097/ALN.0b013e318246ec0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology. 2005;65:986–990. doi: 10.1212/01.wnl.0000171954.92119.c7. [DOI] [PubMed] [Google Scholar]
- Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005;7:319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- Lin D, Cao L, Wang Z, Li J, Washington JM, Zuo Z. Lidocaine attenuates cognitive impairment after isoflurane anesthesia in old rats. Behav Brain Res. 2012;228:319–327. doi: 10.1016/j.bbr.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z. Anesthetic sevoflurane causes neurotoxicity differently in neonatal naive and Alzheimer disease transgenic mice. Anesthesiology. 2010;112:1404–1416. doi: 10.1097/ALN.0b013e3181d94de1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Kitagawa Y, Ozawa S, Suda K, Ando N, Ueda M, et al. Role of steroid administration to reduce inflammation after thoracotomy in a rat surgical stress model. J Surg Res. 2006;135:364–369. doi: 10.1016/j.jss.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- Palotas A, Reis HJ, Bogats G, Babik B, Racsmany M, Engvau L, et al. Coronary artery bypass surgery provokes Alzheimer’s disease-like changes in the cerebrospinal fluid. J Alzheimers Dis. 2010;21:1153–1164. doi: 10.3233/jad-2010-100702. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Planel E, Krishnamurthy P, Miyasaka T, Liu L, Herman M, Kumar A, et al. Anesthesia-induced hyperphosphorylation detaches 3-repeat tau from microtubules without affecting their stability in vivo. J Neurosci. 2008;28:12798–12807. doi: 10.1523/JNEUROSCI.4101-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenhausler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23:90–96. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366:250–257. doi: 10.1056/NEJMra1100109. [DOI] [PubMed] [Google Scholar]
- Srinivasa S, Kahokehr AA, Yu TC, Hill AG. Preoperative glucocorticoid use in major abdominal surgery: systematic review and meta-analysis of randomized trials. Ann Surg. 2011;254:183–191. doi: 10.1097/SLA.0b013e3182261118. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011a;115:727–732. doi: 10.1097/ALN.0b013e31822e9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JX, Mardini F, Caltagarone BM, Garrity ST, Li RQ, Bianchi SL, et al. Anesthesia in presymptomatic Alzheimer Disease: A 3xTgAD study. Alzheimers Dement. 2011b;7:521–531. doi: 10.1016/j.jalz.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JX, Mardini F, Janik LS, Garrity ST, Li RQ, Bachlani G, et al. Modulation of murine Alzheimer pathogenesis and behavior by surgery. Ann Surg. 2012 doi: 10.1097/SLA.0b013e318269d623. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther K, Bendlin BB, Glisky EL, Trouard TP, Lisse JR, Posever JO, Ryan L. Anti-inflammatory drugs reduce age-related decreases in brain volume in cognitively normal older adults. Neurobiol Aging. 2011;32:497–505. doi: 10.1016/j.neurobiolaging.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- Whittington RA, Virag L, Marcouiller F, Papon MA, Khoury NB, et al. Propofol directly increases tau phosphorylation. PLoS One. 2011;6:e16648. doi: 10.1371/journal.pone.0016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Russo P, Sharrock NE, Mattis S, Szatrowski TP, Charlson ME. Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial. JAMA. 1995;274:44–50. [PubMed] [Google Scholar]
- Yende S, Angus DC. Long-term outcomes from sepsis. Curr Infect Dis Rep. 2007;9:382–386. doi: 10.1007/s11908-007-0059-3. [DOI] [PubMed] [Google Scholar]
- Yuki K, Astrof NS, Bracken C, Soriano SG, Shimaoka M. Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology. 2010;113:600–609. doi: 10.1097/ALN.0b013e3181e89a77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, Soriano SG, Shimaoka M. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 2008;22:4109–4116. doi: 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargar-Shoshtari K, Sammour T, Kahokehr A, Connolly AB, Hill AG. Randomized clinical trial of the effect of glucocorticoids on peritoneal inflammation and postoperative recovery after colectomy. Br J Surg. 2009;96:1253–1261. doi: 10.1002/bjs.6744. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhen Y, Dong Y, Xu Z, Yue Y, Golde TE, et al. Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and Abeta oligomerization. Plos One. 2011;6:e27019. doi: 10.1371/journal.pone.0027019. [DOI] [PMC free article] [PubMed] [Google Scholar]


