Abstract
Pseudoxanthoma elasticum (PXE), a rare recessive genetic disease causing skin, eye and cardiovascular lesions is characterized by the calcification of elastic fibers. The disorder is due to loss-of-function mutations of the ABCC6 gene but the pathophysiology of the disease is still not understood. Here we investigated the transcriptional regulation of the gene, using DNase I hypersensitivity assay followed by luciferase reporter gene assay. We identified three DNase I hypersensitive sites (HS) specific to cell lines expressing ABCC6. These HS are located in the proximal promoter and in the first intron of the gene. We further characterized the role of the HS by luciferase assay and demonstrated the transcriptional activity of the intronic HS. We identified the CCAAT/Enhancer binding protein ß (C/EBPß) as a factor binding the second intronic HS by chromatin immunoprecipitation and corroborated this finding by luciferase assays. We also showed that C/EBPß interacts with the proximal promoter of the gene. We propose that C/EBPß forms a complex with other regulatory proteins including the previously identified regulatory factor HNF4α. This complex would account for the tissue-specific expression of the gene and might serve as a metabolic sensor. Our results point toward a better understanding of the physiological role of ABCC6.
INTRODUCTION
Pseudoxanthoma elasticum (PXE, OMIM 264800) is a rare Mendelian disorder characterized by calcification and fragmentation of the elastic fibers (Varadi et al. 2011; Li et al., 2009a; Uitto et al., 2010). Patients in their first decades of life develop yellowish papules at the neck and flector areas of the body. Plaques of papular lesions and redundant skin may appear during the slowly progressing disease in the axillae, the inguinal regions and the periumbilical area. The skin looses its elasticity and can become wrinkled. Ultrastructural analyses of the dermis show the fragmentation of the elastic fibers and the accumulation of calcium deposits, which can be visualized by von Kossa staining. Fragmentation of the elastic fibers of the Bruch’s membrane in the eye may lead to neovascularization and potentially severe loss of vision. In parallel, cardiovascular complications and coronary artery disease may complete the syndrome.
Recessive mutations in the ABCC6 ATP-binding cassette transporter gene lead to the development of the disease (Bergen et al., 2000; Le Saux et al., 2000; Ringpfeil et al., 2000). The mutation detection rate is over 90% (Vanakker et al., 2008) with more than 300 mutations reported and compiled in the ABCC6 mutation database. Stop codon mutation p.R1141X represents 25% of all observed mutations. It should be noted that PXE is also associated with a promoter polymorphism of the gene and other rare cases of promoter mutations exist as well (Hendig et al., 2008; Schulz et al., 2006). The biochemically well characterized ABCC6 gene product is a plasma membrane protein transporting a currently unknown molecule to the blood stream (Ilias et al., 2002). Surprisingly the gene has a very restricted tissue-specific expression pattern since it is expressed predominantly in the liver and it is absent from the tissues affected by the disease (Matsuzaki et al., 2005). Skin transplantation experiments between wild-type and knock-out mice and development of parabiotic animals confirmed the role of this expression pattern by showing that the disease should be considered metabolic (Jiang et al., 2009; Jiang et al.; Uitto et al., 2010). Recent data on acquired PXE after liver transplantation in human patients (Bercovitch et al., 2011) also corroborate the idea that PXE is a metabolic disorder caused either by the absence of a molecule from the serum secreted by the hepatocytes or the presence of a molecule, which is normally not present (Le Saux et al., 2006).
Several other pathologies are also associated to the PXE phenotype. While PXE is an autosomal recessive disorder and carriers of one loss-of-function mutant ABCC6 allele have no obvious clinical phenotype, they probably have an increased risk to develop coronary artery disease (Koblos et al., 2010; Trip et al., 2002). Furthermore, when they are carriers of other mutations as well, e.g. in the GGCX gene, PXE-like phenotype can develop (Li et al., 2009b). A significant portion (approximately 5%) of beta-thalassemic patients develops PXE-like phenotype (Aessopos et al., 2008; Hamlin et al., 2003) most probably due to the down-regulation of ABCC6 without any mutation in the gene (Martin et al., 2011).
Although several studies have been conducted to understand the transcriptional regulation of the gene (Aranyi et al., 2005; de Boussac et al., 2010; Jiang et al., 2006) and (Varadi et al., 2011 and references therein), to date only the essential role of hepatocyte nuclear factor 4 α (HNF4α) is known in the tissue-specific expression of the gene from ours and others work (Bolotin et al., 2010; de Boussac et al., 2010; Douet et al., 2006). We have also shown the regulatory role of the extracellular signal related kinase 1 and 2 (ERK1/2), which exerts its negative action on the expression of the gene via the inhibition of HNF4α (de Boussac et al., 2010). The role of DNA methylation and several other minor factors has been uncovered however, the mechanisms of relatively high expression levels of the gene in hepatocytes remained poorly understood (Varadi et al., 2011 and references therein). In light of future potential allele-specific therapy of PXE (Le Saux et al., 2011), improvement of PXE-related phenotypes and in order to decrease the ABCC6 haploinsufficiency associated cardiovascular risks, we aimed to identify crucial missing players in the transcriptional regulation of the human ABCC6 gene. We therefore searched for further regulatory sequences and identified a primate-specific activator element in the first intron of ABCC6 with strong activity in the human hepatoma HepG2 and the differentiated colon carcinoma Caco-2 cells. We also demonstrated that the activator interacts with the proximal promoter and binds C/EBPß and probably other presently unidentified factors.
RESULTS
Expression of the ABCC6 gene in model cell lines
In order to investigate the transcriptional regulation of the ABCC6 gene, we first chose model cell lines. We selected two cell lines characterized by high and two by low expression level of the gene. From our previous studies we already knew that HepG2 cells have a high expression level (Aranyi et al., 2005); HEK293 and HeLa have very low expression level or do not express the gene (Aranyi et al., 2005; de Boussac et al., 2010). Previous data showed that Abcc6 has a relatively high expression level in rodent small intestine (Maher et al., 2006; Maher et al., 2005). Indeed, we previously observed an intermediate expression level in Caco-2 cells (Aranyi et al., 2005). These cells are known to spontaneously differentiate to intestinal epithelial-like cells in post-confluent culture (Grasset et al., 1984). We now performed spontaneous differentiation experiments with Caco-2 cells. As a control we confirmed the increased expression of some differentiation markers (Gelebart et al., 2002; Ribiczey et al., 2007). The expression level changes of several ABC transporter genes were also analyzed. While ABCC1 levels progressively decreased, ABCC2, ABCC3 and ABCG5 expression level increased in parallel with that of ABCC6 (not shown). Indeed, after the cells reached 50% confluency, the expression level of ABCC6 consistently increased until post-confluent day 6 and became similar to that observed in HepG2 cells. These data confirmed that Caco-2 cells can be used as a model system in our study for high expression level of ABCC6.
DNase I assay revealed an active genomic region
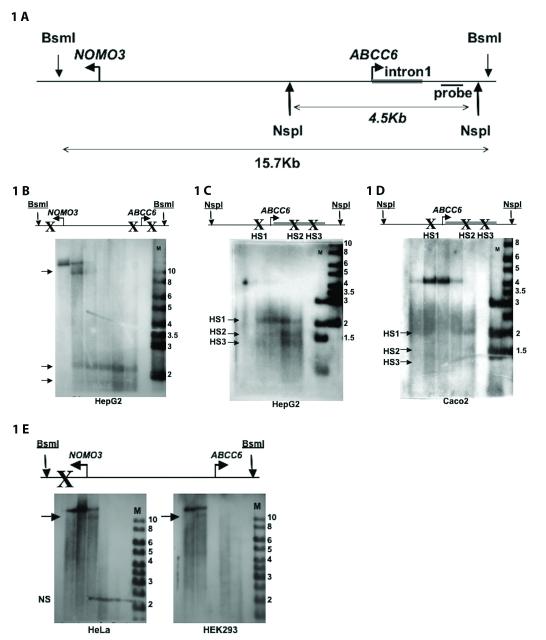
To identify regulatory sequences of the ABCC6 gene we performed DNase I hypersensitivity assay (DHA) (Lu and Richardson, 2004). The partial digestion of the genomic DNA in its nuclear context by DNase I reveals transcriptionally active regions due to their increased sensitivity to the enzyme. The great advantage of this technique is that it maps physiologically relevant regulatory sequences in a hypothesis free manner i.e. without any prior analysis of the region. We investigated the first intron of the ABCC6 gene and a 10kb upstream region between ABCC6 and NOMO3, the neighboring gene (Figure 1A). We mapped the hypersensitive sites (HS) of this region by Southern blot. We designed the ABCC6 specific probe to recognize the 3′ end of the first intron of the gene. This location allows the screening for potential HS in the ABCC6 regulatory sequences with high accuracy (Figure 1A).
Figure 1. DNase I hypersensitivity assay identifies cell-type specific hypersensitive sites.
A) Map of the NOMO3 – ABCC6 intergenic region. B) Detection of HS in HepG2 cells. C) Higher resolution analysis. D) Detection of HS in Caco-2 cells (95% confluency). E) Detection of HS in HeLa and HEK293 cells. Please note that the non-specific (“NS”) band on panel E) does not respect the borders of the loaded lanes. Arrows indicate HS, (M) is a 1kb marker (numbers indicate fragment sizes in kb). HS are indicated by “X” on the schematic genomic map. DNase I partial digestion was carried for 3 minutes with 0, (9), 11, 13, 15, 17 μg (HepG2), 0, 5, 10, 15, 20 μg (Caco-2) and 0, 15, 20, 25, 30 μg (HeLa and HEK293) enzyme.
After performing DHA in HepG2 cells, three HS were revealed in the studied region (Figure 1B). We identified the 11kb fragment as a product of enzymatic digestion at a HS in the first intron of NOMO3 gene. The two other fragments of 2.2kb and 1.8kb revealed HS close to the transcription start site (TSS) of the ABCC6 gene. We decided to further study only the two HS close to the TSS of the ABCC6 gene since no data indicate that the HS in the NOMO3 gene plays any role in the regulation of ABCC6.
The two closer HS were thus further investigated by the analysis of a 4.7kb DNA fragment containing the first intron and the proximal promoter of ABCC6. DHA analyzed by this higher resolution Southern blot showed that the two HS previously identified around the TSS of ABCC6 were in fact three different HS, represented in the Southern blot now by bands at 2.2kb, 1.7kb and 1.3kb. We named these HS sites from 5′ to 3′, HS1 to HS3 (Figure 1C).
Both the DNase I digestion and our Southern blot conditions have a resolution of approximately 150bp thus we located HS1 in the proximal promoter of the ABCC6 gene between −200bp and −50bp, while both HS2 and HS3 are situated in the first intron of ABCC6 between +310 and +460bp and between +600 and +750bp, respectively (Figure 1C).
Hypersensitive sites are cell-type specific
As ABCC6 has tissue-specific expression, we next asked whether the HS observed in HepG2 are also cell-type specific. To answer this question we performed DHA on Caco-2, HEK and Hela cells (Figure 1D and E). Caco-2 cells were approximately 95% confluent. We observed HS1 in the 4.7 kb genomic DNA fragment studied as earlier in the HepG2 cells. However, we obtained only very weak signals for HS2 and HS3, therefore we could not confirm undoubtedly the presence of these sites in Caco-2 cells.
In contrast, when we performed DHA in HEK and HeLa cells, we did not observe any HS in the same region. Indeed, HS1, 2 and 3 observed previously were absent from HEK and HeLa cells when we analyzed the larger 15.7 kb fragment. The entire region seemed to have a much more compact chromatin structure, as the HS in the first intron of NOMO3 appeared only upon using higher DNase I concentrations in the assay. The detection of this HS served also as a positive control for the experiment (Figure 1E) demonstrating the cell-type specificity of HS1-3.
Functional characterization of the HS in the proximal promoter and the first intron
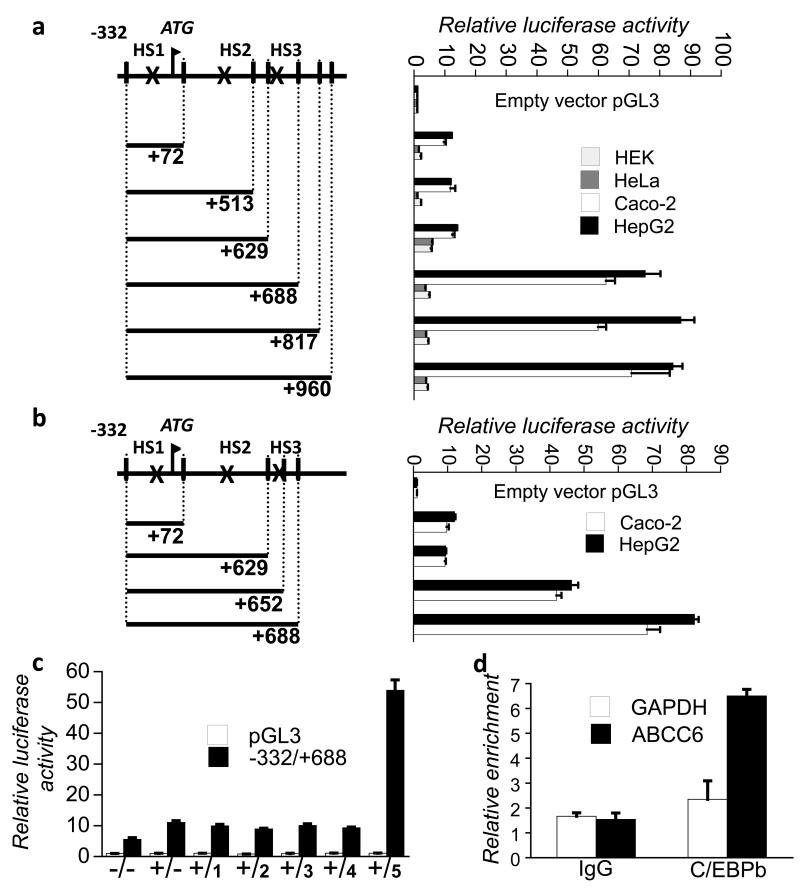
In the following experiments we investigated the functionality of the different HS and more specifically we focused on the two intronic HS by luciferase assay. We assumed from the localization of HS1 in the proximal promoter that it marks the binding of HNF4α (de Boussac et al., 2010) (and references therein). We mapped by luciferase assay the different HS with constructs starting at −332 (relative to the translation start site) and giving maximal promoter activation (Aranyi et al., 2005). The constructs were progressively truncated at the 3′ end from +960 (Figure 2A).
Figure 2. HS3 overlaps with a primate-specific intronic activator sequence, which binds C/EBPß.
Results of luciferase assays were standardized and expressed as fold induction of the empty pGL3-basic vector, mean±SEM (n = 5). A) Transcriptional activity of the first intron of ABCC6. The genomic map indicates the HS by “X”. B) Further mapping of the +629/+688 region. C) Putative transcription factors binding the 60bp sequence were tested in HeLa cells by luciferase assay in co-expression experiment with the −332/+688 construct and a vector containing HNF4α. Factors tested were as follows: 1: USF2; 2: RELA; 3: HNF3beta; 4: ELK1; 5: C/EBPbeta. HNF4alfa overexpression is indicated by + and empty vector is indicated by -. D) ChIP of C/EBPß in HepG2 cells. GAPDH and IgG were used as controls.
In HeLa and HEK cells we saw a low basal activity of the −332/+72 and −332/+513 constructs, which corresponds to the activation caused by the −145/+72 region observed in every cell type tested so far, as reported in our previous studies (Aranyi et al., 2005; de Boussac et al., 2010). The addition of the +514/+629 fragment to the −332/+513 construct led to the doubling of the reporter gene activity, which was not further increased by the addition of further intronic fragments (Figure 2A).
Surprisingly in HepG2 and Caco-2 cells, the activity of −332/+960 was approximately 6-8 times higher than the activity of the proximal promoter −332/+72. This activation remained at the same level when the construct was truncated at +688 but dropped to the level of the activity of the proximal promoter when the construct was truncated at +629 (Figure 2A). When we tried to further narrow down the response element, an intermediate activity was observed for the construct −332/+652 relative to the constructs −332/+629 and −332/+688 in both HepG2 and Caco-2 cells (Figure 2B). In our following experiments we have also shown that the 60bp intronic sequence has no enhancer activity in plasmids specifically designed for testing this (not shown). Altogether, our data suggest the presence of at least one strong cell-type specific activator element in the first intron of the ABCC6 gene between +629 and +688bp, which overlaps with HS3.
Identification of primate specific regulatory elements in the ABCC6 gene
Seeing the major role in the regulation of ABCC6 of the sequence overlapping with HS3, we next wanted to identify the regulatory proteins binding the HS3 region. Therefore we performed in silico analysis of the 60bp intronic segment. First, we investigated the evolutionary conservation of the region (Supplementary figure 1A). The +629/+652 fragment of the human gene was completely absent from the majority of investigated species except for primates and small conservation in the rabbit and the African elephant. The +653/+688 region remained completely absent from mouse, rat and chicken, while it was present in addition to primates in the African elephant and approximately 50% conserved in guinea pig, cow and rabbit. The ABCC6 proximal promoter was also investigated by the same bioinformatic approach, which demonstrated the presence of another primate specific sequence, while efficiently identified the HNF4α binding site in almost all analyzed species (Supplementary Figure 1B).
C/EBPß activates ABCC6 expression via HS3
Next, we investigated the human sequence for the presence of transcription factor binding sites in the 60bp long intronic region. The search engines indicated only few sites. Interestingly, some of the factors binding the putative sites are enriched in the liver (C/EBPß, HNF3).
We tested the potential role of the transcription factors identified by co-expressing them with our −332/+688 construct and HNF4α in HeLa cells. We co-expressed HNF4α since we hypothesized that its presence might be necessary for the activity of the factor(s) binding the putative intronic response element(s). The only transcription factor, which induced the expression of our construct after the co-expression with HNF4α was C/EBPß (Figure 2C).
Next, we wished to show the binding of C/EBPß to the ABCC6 intronic activator in the chromatin environment of HepG2 cells, where the gene is endogenously expressed. Therefore, we performed chromatin immunoprecipitation assay with an anti-C/EBPß antibody. We observed a more than 3-fold enrichment of the target region (+503/+700) in the immunoprecipitated fraction, demonstrating thereby the binding of C/EBPß to the first intron of the human ABCC6 gene in the chromatin context in HepG2 cells (Figure 2D). Altogether, these data prove a major role for C/EBPß in the transcriptional regulation of the human ABCC6 gene.
Interplay between the proximal promoter and the first intron
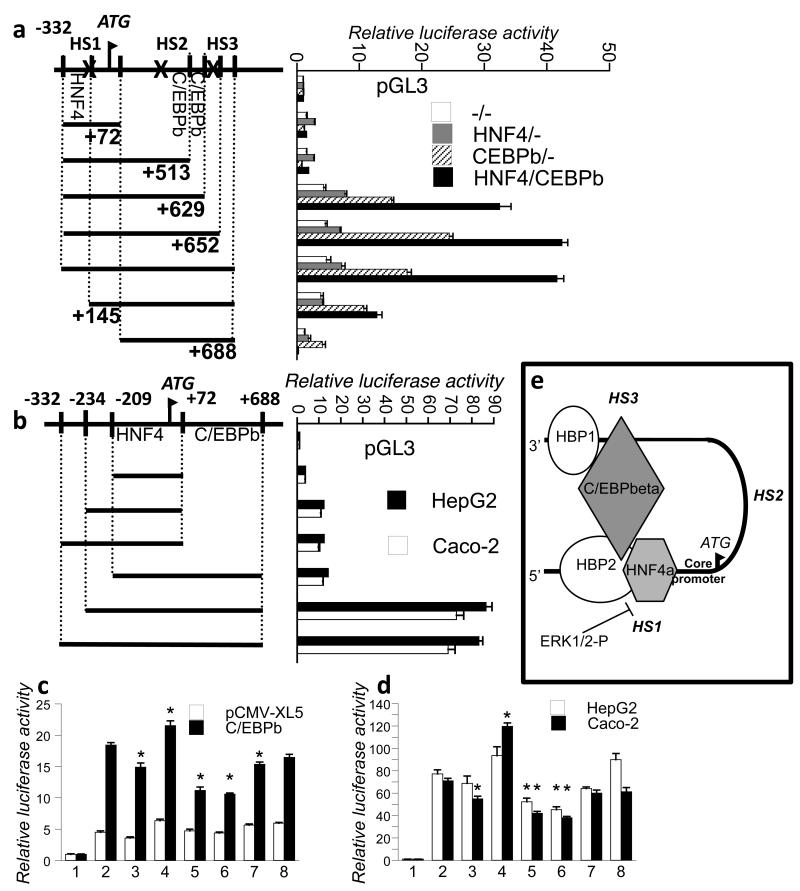
In our following experiment we mapped the region responsible for the binding of C/EBPß, as the resolution of the ChIP is not sufficient to locate exactly the response element. Different luciferase constructs were expressed alone or in the presence of C/EBPß or HNF4α or both in HeLa cells. We found in these experiments at least two C/EBPß binding sites between +513/+629 and +630/+652 (Figure 3A).
Figure 3. Characterization of C/EBPß binding to the intronic activator region.
Results of luciferase assays were standardized as in figure 2. A) Mapping of C/EBPß binding site. Luciferase constructs were co-expressed with HNF4α or C/EBPß or both or none in HeLa cells. B) Interaction of the C/EBPß binding region and the promoter in HepG2 and Caco-2 cells. ABCC6 luciferase constructs were tested in in HeLa cells overexpressing C/EBPß (C) and in HepG2 and Caco-2 cells (D). The following constructs were tested: 1: pGL3; 2: ABCC6(−332/+688); 3: ABCC6mut1(−332/+688); 4: ABCC6mut2(−332/+688); 5: ABCC6mut3(−332/+688); 6: ABCC6mut1,3(−332/+688); 7: ABCC6mut1,2,3(−332/+688); 8: ABCC6delHS2(−332/+72 +629/+688). Constructs are described in Materials and Methods. Asterisks indicate significant effect relative to wt. E) The scheme summarizes a working model for the transcriptional regulation of ABCC6.
In these experiments we have also observed that the overexpression of C/EBPß alone can reach a similar pattern of induction of the constructs than in the presence of HNF4α, which is therefore not an obligatory partner of C/EBPß in this experimental context (Figure 3A). Nevertheless, these experiments also suggested an interplay between the proximal promoter and the intronic region as concluded by the comparison of luciferase activities of constructs −145/+688 and −332/+688. We further explored the possible interactions between the proximal promoter of ABCC6 and the intronic hypersensitive sites in HepG2 and Caco-2 cells (Figure 3B) by testing new constructs. The new −234/+688 and −209/+688 constructs were compared with the previously described −332/+688 construct. The −332/+688 and the −234/+688 constructs showed similarly high luciferase activities (Figures 3B). According to our observations in HeLa cells, and in contrast to the longer constructs, the −209/+688 construct showed a low luciferase activity, confirming that the intronic activator sequence is interacting with the −234/−209 fragment and not with the −165/−145 sequence, which binds HNF4α (de Boussac et al., 2010). Interestingly, the −234/−209 fragment corresponds to the primate specific sequence identified in the proximal promoter (Supplementary Figure 1B).
In our final set of experiments we performed luciferase assays in HepG2, Caco-2 and HeLa cells with constructs harboring mutations at the predicted C/EBPß binding sites to determine the major site of action of the protein (Figure 3C and D). While the mutagenesis of site 1 (+539/+547) had only small effect, the mutagenesis of site 2 (+585/+594) increased the activity of the construct in HeLa cells when co-expressed with C/EBPß (Figure 3C). Therefore the role of the second site could not be evaluated. Interestingly, the mutagenesis of the third, most active site located between +632 and +640 abolished completely the activation of the site 3 by C/EBPß in HeLa cells (p<0.01). Indeed, the activity of the construct mut3(−332/+688) in the presence of C/EBPß was not different from that of the construct truncated at +629 (see Figure 3A). Double mutation of sites 1 and 3 had similar effect as the single mutation of site 3, while the triple mutation of the sites had smaller effect due to the adverse behavior of the mutation at site 2. Deletion of the HS2 region had no significant effect. Similar behavior of the mutant constructs was observed in HepG2 and Caco-2 cells (Figure 3D). However, it should be noted that constructs with mutations at site 3 expressed in HepG2 and Caco-2 still maintained a higher residual activity of the region than expected. This demonstrates that the +630/+652 sequence binds specifically C/EBPß and suggests that additional endogenous activator factor(s) bind(s) the +652/+688 fragment (Figure 3D and not shown).
DISCUSSION
ABCC6 is characterized by a strict tissue-specific expression overlapping with the expression pattern of the HNF4α transcription factor (Matsuzaki et al., 2005; Sladek FM, 1990). However, HNF4α and the other previously identified factors seemed to have only a small transcriptional activator potential in the regulation of ABCC6, suggesting that other elements are also required since the expression level of the gene is relatively high in hepatocytes. In the present study we have demonstrated that the human ABCC6 gene is under the control of a strong cell-type specific activator located in the first intron of the gene. We also showed that this sequence specifically binds C/EBPß and at least one further activator protein. Finally, we have shown that to develop its full activity, C/EBPß requires the interaction with the proximal promoter of the gene. Our results are summarized in Figure 3E.
We investigated model cell lines where ABCC6 is expressed (HepG2 and Caco-2) or is not expressed (HeLa and HEK) to identify further response elements and factors playing an important role in the regulation of the gene. We used DNase I hypersensitivity assay followed by luciferase reporter gene assay. It should be noted that the genome contains two human-specific ABCC6 pseudogenes, as a result of evolutionarily recent segmental duplications (Pulkkinen et al., 2001; Symmons et al., 2008). Due to the 99% sequence identity of the three loci it was impossible to perform an ABCC6 specific DHA, i.e. the observed HS may reflect any of the three genomic loci. Nevertheless, we considered that since both pseudogenes are expressed (Aranyi et al., 2005; Pulkkinen et al., 2001) and are located within large, almost identical duplicated genomic regions the same HS pattern might characterize all three loci. However, in the following luciferase reporter gene experiments, which were performed to study the functional role of the HS the plasmid constructs contained the ABCC6 specific sequence.
The approach proved to be efficient and cell-type specific activator elements (HS2 and 3) were found in the first intron of the gene similarly to CFTR, another member of the ABCC gene family (Smith et al., 1996). The physiologic role of HS3 was in the focus of this study. Interestingly this strong activator sequence turned out to be primate-specific as observed when aligned with other ABCC6 sequences from different species (Supplementary Figure 1A).
Our bioinformatic analysis predicted the binding of different transcription factors to the 60bp long intronic region of the human gene characterized by increased transcriptional activity. In our experiments only C/EBPß was found to specifically bind and transactivate the sequence. We showed the specific binding of the protein by several methods. ChIP assays demonstrated the binding of C/EBPß to at least one functional site between +200bp and +1kb considering the resolution of our technique (Figure 2D). We have extensively mapped the binding of C/EBPß in the first intron of ABCC6 by luciferase reporter gene assays as well (Figures 2C, and 3): i/ at least two binding sites exist between +513 and +688 conferring a 5-6-fold activation to the reporter constructs, as shown by co-expression of C/EBPß and HNF4α with wild-type constructs; ii/ as shown by the study of mutant constructs, the major site is located at +632/+640, which overlaps with the 5′ end of a 60bp region of HS3 (Figure 3C and D).
We have also demonstrated that the intronic activator region overlapping with HS3 interacts with HS1 located in the proximal promoter (Figures 3B). We have found that C/EBPß (HS3 region) to elicit its full activity has to interact with the proteins binding the −332/−145 (HS1) region. This latter sequence binds HNF4α and also harbors the −234/−209 sequence, the site for the second important cell-type specific activator of the proximal promoter needing HNF4α for its binding according to our previous data (Ratajewski et al., 2009).
In the present study we have shown that the activation of the intronic sequence by C/EBPß needs the presence of the −234/−209 sequence (Figures 3A and B). We hypothesize that in HeLa cells when C/EBPß was overexpressed a protein complex formed including C/EBPß and the protein binding the −234/−209 sequence even in the absence of HNF4α (Figure 3A, comparison of constructs −332/+688 and −145/+688 coexpressed with C/EBPß). We consider therefore that in these cells the primary interaction is between C/EBPß and the factor binding the −234/−209 sequence. This result also suggests that this hypothetical binding protein (HBP1), which is present in the cells, is unable to fully activate the construct in the absence of C/EBPß (Figure 3E). Although we considered previously that the activator that binds the −234/−209 sequence is a tissue-specific factor, this observation also suggests that HBP1 might be more widely expressed than previously thought (Ratajewski et al., 2009). Since our previous data indicated the dependence of HBP1 binding on HNF4α binding, we hypothesize that in HepG2 and Caco-2 cells, which reflect much better the physiological regulation of ABCC6 than HeLa cells, a complex composed of HNF4α, C/EBPß and HBP1 is playing the major regulatory role in the expression of the gene. Detailed luciferase analyses also showed that the −332/+652 construct had an intermediate activity compared to the −332/+688 and −332/+629 luciferase reporter gene constructs in both HepG2 and Caco-2 cells (Figure 2B). Our data clearly indicate that C/EBPß is the main activator of the +630/+652 region, while another hypothetical binding protein (HBP2) also binds the +652/+688 region. Most probably HBP2 is also included in the complex since in the absence of −332/−145 region the entire +630/+688 region looses its transcriptional activator potential (Figure 3B and E).
Interestingly and in agreement with our findings on the intronic segment, the −234/−209 sequence containing the binding site for HBP1 is not conserved evolutionarily according to our in silico analysis (Supplementary Figure 1), suggesting that the highlighted regulatory network is entirely primate specific. This strongly suggests that the rodent Abcc6 genes are not appropriate models of the transcriptional regulation of the human gene.
C/EBPß is a member of the CCAAT/enhancer binding protein family (Akira et al., 1990). These transcription factors have a basic leucin zipper domain and bind DNA as homodimers or heterodimerize with another member of the family and recognize very similar or identical sequences (Okazaki et al., 2002; Ramji and Foka, 2002). In hepatocytes two C/EBPs, α and ß are highly expressed. Our data therefore do not exclude the potential role of C/EBPα or other C/EBPs in the transcriptional regulation of ABCC6 in addition to that of C/EBPß. Interestingly, both C/EBPα and ß typically activate the transcription of genes playing a role in metabolism by binding their enhancer (Ramji and Foka, 2002). They often act in concert with HNF4α (Gautier-Stein et al., 2005; Schmidt et al.), with which they are major sensors of the metabolic state of hepatocytes.
Currently major objectives of the PXE research field are to understand the pathomechanism of the disease and to develop potential treatments of the various PXE symptoms and PXE associated phenotypes. Our results reported here and in our previous report (de Boussac et al., 2010) both point toward a potential unique role of ABCC6 among the other ABC transporters in the metabolic processes of the cell. This might indicate that the severity of PXE or various PXE-related phenotypes is directly linked to the metabolic status of the patients, potentially explaining the great variation of the clinical phenotype even within the same family (Varadi et al., 2011). This idea might serve as a basis of paradigm shift after the recently disproved hypothesis on the role of ABCC6 in vitamin K metabolism (Borst et al., 2008; Fulop et al., 2011) and help to decipher the pathomechanism of the disease.
Finally, our results contribute to the development of treatments of PXE and PXE related conditions as well. Indeed the better understanding of the transcriptional regulation of ABCC6 might contribute to the success of the recently proposed allele-specific therapy of PXE caused by mis-sense mutations, when the protein is mislocalized but is still active (Le Saux et al., 2011). In such a case inducing the expression of the gene and helping by chemical chaperones the proper targeting of the protein might cure the disease. In conclusion, our results may open new perspectives for targeting research and development for the treatment of PXE, PXE-like and ABCC6 mutation carrier-state associated phenotypes by focusing on the induction of ABCC6 expression as a new therapeutic target.
Materials and Methods
Cell culture
Cell lines were obtained from ATCC and cultured according to the manufacturer’s instructions. More specifically HepG2 human hepatoma cell line was cultured in advanced MEM supplemented with 10% FBS, 2mM glutamine, 100 U/ml penicillin and 100μg/ml streptomycin. HeLa and HEK293 cells were cultured in RPMI supplemented with 10% FBS, 2mM glutamine, 100 U/ml penicillin and 100μg/ml streptomycin. Caco-2 cells were cultured in advanced MEM supplemented with 20% FBS, glutamine and antibiotics like the other cell lines until reaching confluency (D0) and 14 more days as described previously (D14) (Ribiczey et al., 2007). Post-confluent Caco-2 cell cultures were re-fed by renewal of medium every second day.
DNase I hypersensitivity assay
6×106 Cells were collected and resuspended in 100 μl of buffer Ψ (11 mM K-phosphate pH 7.4; 108 mM KCl; 22 mM NaCl; 1 mM MgCl2; 1 mM DTT; 1 mM ATP) at 4°C, and were then treated with DNase I for 3 minutes on ice. The DNase I digestion was stopped by the addition of 2.5ml of lysis buffer (50 mM Tris-HCl pH 8; 20 mM EDTA; 1% SDS) followed by phenol extraction of the genomic DNA.
Southern blot
20 μg of DNase I-treated DNA was digested overnight with BsmI or NspI as indicated in the text. A probe located between +1435 and +1735bp (relative to the translation start site). Primers to amplify the probe were designed and tested for specificity (as all primers used in this study) by BiSearch (Aranyi et al., 2006). The probe was radiolabeled with the DecaLabel™ DNA Labeling Kit (Fermentas) and used for the Southern blot assay. The assay was performed according to standard protocols. Hybridization was carried out in QuickHyb (Stratagene) at 65°C for 3h. Membranes were exposed to hyperfilm (Amersham) for 24-36h at −80°C.
Promoter constructs transfections and Luciferase assay
Luciferase reporter gene assays were performed as described previously (de Boussac et al., 2010). C/EBPß and other transcription factor expression plasmids were purchased from Origene Technologies Inc. (Rockville, USA). New reporter plasmid vectors containing the ABCC6-PXE promoter sequences were constructed by PCR cloning. Human genomic DNA was used as template for amplification of the construct −332/+960, then this construct was used as template for the amplification of the others. The restriction sites (KpnI site for forward primers and HindIII site for reverse primers) were added to the 5′-end of primers, and promoter sequences were amplified using high fidelity thermostable DNA polymerase (TaqPfu). PCR products were cloned into the pGL3-Basic vector (Promega). The constructs were sequenced to avoid using constructs, which do not match the ABCC6-PXE gene sequence.
Site-directed mutagenesis
Mutations of the potential C/EBPß binding sites in the ABCC6 promoter were generated by a similar PCR-based method as in our previous study (Ratajewski et al., 2008). C/EBPbeta consensus binding site is TKNNGNAAK. Three putative sites are present in the 1st intron of the gene. The sites between +539/+547 (TAATACAAC mutated to TAATTCCCC – mutations in bold) and +585/+593 (GAAGGAAAC mutated to GAAGTACCC) are degenarate, while the third site between +632/+640 (TTGTGAAAC mutated to TTGTTACCC) is consensus. The introduced mutation of C/EBPß sites was based on a previous report of Hayashida et al. (Hayashida et al., 2009; Ratajewski et al., 2008).
The sequences of the mutated inserts were verified by automated sequencing. In double and triple mutant constructs the same mutations were included. To create a HS2 deletion construct we used the same method.
ChIP assay
Chromatin immunoprecipitation and quantification of the transcription factor-bound genomic DNA fragment were performed as described previously (de Boussac et al., 2010). The following antibodies were used: Normal Mouse IgG (part of the EZ-ChIP kit, Millipore) and anti-C/EBPß (E299, Abcam). Results were normalized to total extracted DNA amount in samples taken prior to immunoprecipitation by the deltaCt method.
Bioinformatics
In silico analysis of the genomic sequence were performed using the following web servers: P-Match http://gene-regulation.com/cgi-bin/pub/programs/pmatch/bin/p-match. cgi; Matinspector http://www.genomatix.de/cgi-bin/sessions/login.pl?s=ed1de7682d64bd03ebafefad4b12b450; TF-search http://www.cbrc.jp/research/db/TFSEARCH.html.
ABCC6 promoter regions from 13 different species were identified and downloaded using the UCSC genome browser (Kent et al., 2002) (http://genome.ucsc.edu/). Genomic sequences used are as follows: Homo sapiens (GRCh37/hg19 assembly), Pan troglodytes (CGSC 2.1/panTro2 assembly), Macaca mulatta (MGSC Merged 1.0/rheMac2 assembly), Callithrix jacchus (WUGSC 3.2/calJac3 assembly), Mus musculus (NCBI37/mm9 assembly), Rattus norvegicus (Baylor 3.4/rn4 assembly), Cavia porcellus (Broad/cavPor3 assembly), Oryctolagus cuniculus (Broad/oryCun2 assembly), Ailuropoda melanoleuca (BGI-Shenzhen 1.0/ailMel1 assembly), Loxodonta africana (Broad/loxAfr3 assembly), Pongo pygmaeus abelii (WUGSC 2.0.2/ponAbe2 assembly), Bos Taurus (Baylor 4.0/bosTau4 assembly) and Gallus gallus (WUGSC 2.1/galGal3 assembly). Multiple sequence alignment was performed using DIALIGN program with default parameters (Morgenstern, 2004).
Supplementary Material
ACKNOWLEDGMENTS
The authors are thankful to Drs. Károly Liliom, Béla Papp, Iannis Talianidis, Borbála Tihanyi and Krisztina Huszár for helpful discussions. This work was supported by the Hungarian research grants OTKA CK 80135 (A.V.), OTKA NK 81204 (A.V.), OTKA K 100638 (T.A.), OTKA T046814 (T.K.) and by NIH R01AR055225 (subaward) to A.V. T.A. is a Bolyai János fellow. This work was supported by a Polish-Hungarian academic co-operation grant. This work was also partially supported by the European Regional Development Fund under the Operational Programme Innovative Economy, grant POIG.01.01.02-10-107/09 and the statutory funds from the Institute of Medical Biology, Polish Academy of Sciences.
Abbreviations used
- PXE
pseudoxanthoma elasticum
- C/EBPß
CCAAT/Enhancer binding protein ß
- HNF4α
hepatocyte nuclear factor 4α
- DHA
DNase I hypersensitivity assay
- HS
hypersensitive site
- TSS
transcription start site
- HBP1, 2
hypothetical binding protein 1, 2
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- Aessopos A, Floudas CS, Kati M, Tsironi M, Giakoumi X, Livir-Rallatos C, et al. Loss of vision associated with angioid streaks in beta-thalassemia intermedia. Int J Hematol. 2008;87:35–38. doi: 10.1007/s12185-007-0014-y. [DOI] [PubMed] [Google Scholar]
- Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranyi T, Ratajewski M, Bardoczy V, Pulaski L, Bors A, Tordai A, et al. Identification of a DNA methylation-dependent activator sequence in the pseudoxanthoma elasticum gene, ABCC6. J Biol Chem. 2005;280:18643–18650. doi: 10.1074/jbc.M501139200. [DOI] [PubMed] [Google Scholar]
- Aranyi T, Varadi A, Simon I, Tusnady GE. The BiSearch web server. BMC Bioinformatics. 2006;7:431. doi: 10.1186/1471-2105-7-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovitch L, Martin L, Chassaing N, Hefferon TW, Bessis D, Vanakker O, et al. Acquired pseudoxanthoma elasticum presenting after liver transplantation. J Am Acad Dermatol. 2011;64:873–878. doi: 10.1016/j.jaad.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- Bolotin E, Liao H, Ta TC, Yang C, Hwang-Verslues W, Evans JR, et al. Integrated approach for the identification of human hepatocyte nuclear factor 4alpha target genes using protein binding microarrays. Hepatology. 2010;51:642–653. doi: 10.1002/hep.23357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–1579. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- de Boussac H, Ratajewski M, Sachrajda I, Koblos G, Tordai A, Pulaski L, et al. The ERK1/2-hepatocyte nuclear factor 4alpha axis regulates human ABCC6 gene expression in hepatocytes. J Biol Chem. 2010;285:22800–22808. doi: 10.1074/jbc.M110.105593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douet V, VanWart CM, Heller MB, Reinhard S, Le Saux O. HNF4alpha and NF-E2 are key transcriptional regulators of the murine Abcc6 gene expression. Biochim Biophys Acta. 2006;1759:426–436. doi: 10.1016/j.bbaexp.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop K, Jiang Q, Wetering KV, Pomozi V, Szabo PT, Aranyi T, et al. ABCC6 does not transport vitamin K3-glutathione conjugate from the liver: Relevance to pathomechanisms of pseudoxanthoma elasticum. Biochem Biophys Res Commun. 2011;415:468–471. doi: 10.1016/j.bbrc.2011.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier-Stein A, Mithieux G, Rajas F. A distal region involving hepatocyte nuclear factor 4alpha and CAAT/enhancer binding protein markedly potentiates the protein kinase A stimulation of the glucose-6-phosphatase promoter. Mol Endocrinol. 2005;19:163–174. doi: 10.1210/me.2004-0105. [DOI] [PubMed] [Google Scholar]
- Gelebart P, Kovacs T, Brouland JP, van Gorp R, Grossmann J, Rivard N, et al. Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. J Biol Chem. 2002;277:26310–26320. doi: 10.1074/jbc.M201747200. [DOI] [PubMed] [Google Scholar]
- Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247:C260–267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- Hamlin N, Beck K, Bacchelli B, Cianciulli P, Pasquali-Ronchetti I, Le Saux O. Acquired Pseudoxanthoma elasticum-like syndrome in beta-thalassaemia patients. Br J Haematol. 2003;122:852–854. doi: 10.1046/j.1365-2141.2003.04484.x. [DOI] [PubMed] [Google Scholar]
- Hayashida M, Okazaki K, Fukushi J, Sakamoto A, Iwamoto Y. CCAAT/enhancer binding protein beta mediates expression of matrix metalloproteinase 13 in human articular chondrocytes in inflammatory arthritis. Arthritis Rheum. 2009;60:708–716. doi: 10.1002/art.24332. [DOI] [PubMed] [Google Scholar]
- Hendig D, Langmann T, Kocken S, Zarbock R, Szliska C, Schmitz G, et al. Gene expression profiling of ABC transporters in dermal fibroblasts of pseudoxanthoma elasticum patients identifies new candidates involved in PXE pathogenesis. Lab Invest. 2008;88:1303–1315. doi: 10.1038/labinvest.2008.96. [DOI] [PubMed] [Google Scholar]
- Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Endo M, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol. 2009;129:348–354. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Matsuzaki Y, Li K, Uitto J. Transcriptional regulation and characterization of the promoter region of the human ABCC6 gene. J Invest Dermatol. 2006;126:325–335. doi: 10.1038/sj.jid.5700065. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Oldenburg R, Otsuru S, Grand-Pierre AE, Horwitz EM, Uitto J. Parabiotic heterogenetic pairing of Abcc6−/−/Rag1−/− mice and their wild-type counterparts halts ectopic mineralization in a murine model of pseudoxanthoma elasticum. Am J Pathol. 2010;176:1855–1862. doi: 10.2353/ajpath.2010.090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblos G, Andrikovics H, Prohaszka Z, Tordai A, Varadi A, Aranyi T. The R1141X loss-of-function mutation of the ABCC6 gene is a strong genetic risk factor for coronary artery disease. Genet Test Mol Biomarkers. 2010;14:75–78. doi: 10.1089/gtmb.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Bunda S, VanWart CM, Douet V, Got L, Martin L, et al. Serum factors from pseudoxanthoma elasticum patients alter elastic fiber formation in vitro. J Invest Dermatol. 2006;126:1497–1505. doi: 10.1038/sj.jid.5700201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Fulop K, Yamaguchi Y, Ilias A, Szabo Z, Brampton CN, et al. Expression and in vivo rescue of human ABCC6 disease-causing mutants in mouse liver. PLoS One. 2011;6:e24738. doi: 10.1371/journal.pone.0024738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25:223–227. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Pfendner E, Varadi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009a;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Grange DK, Armstrong NL, Whelan AJ, Hurley MY, Rishavy MA, et al. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol. 2009b;129:553–563. doi: 10.1038/jid.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Richardson B. DNaseI hypersensitivity analysis of chromatin structure. Methods Mol Biol. 2004;287:77–86. doi: 10.1385/1-59259-828-5:077. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cherrington NJ, Slitt AL, Klaassen CD. Tissue distribution and induction of the rat multidrug resistance-associated proteins 5 and 6. Life Sci. 2006;78:2219–2225. doi: 10.1016/j.lfs.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos. 2005;33:947–955. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- Martin L, Douet V, VanWart CM, Heller MB, Le Saux O. A Mouse Model of ß-thalassemia Shows a Liver-specific Downregulation of Abcc6 expression. Am J Pathol. 2011;178:774–783. doi: 10.1016/j.ajpath.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y, Nakano A, Jiang QJ, Pulkkinen L, Uitto J. Tissue-specific expression of the ABCC6 gene. J Invest Dermatol. 2005;125:900–905. doi: 10.1111/j.0022-202X.2005.23897.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern B. DIALIGN: multiple DNA and protein sequence alignment at BiBiServ. Nucleic Acids Res. 2004;32:W33–36. doi: 10.1093/nar/gkh373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, Li J, Yu H, Fukui N, Sandell LJ. CCAAT/enhancer-binding proteins beta and delta mediate the repression of gene transcription of cartilage-derived retinoic acid-sensitive protein induced by interleukin-1 beta. J Biol Chem. 2002;277:31526–31533. doi: 10.1074/jbc.M202815200. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L, Nakano A, Ringpfeil F, Uitto J. Identification of ABCC6 pseudogenes on human chromosome 16p: implications for mutation detection in pseudoxanthoma elasticum. Hum Genet. 2001;109:356–365. doi: 10.1007/s004390100582. [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajewski M, de Boussac H, Pulaski L. Liver-specific enhancer in ABCC6 promoter-Functional evidence from natural polymorphisms. Biochem Biophys Res Commun. 2009;383:73–77. doi: 10.1016/j.bbrc.2009.03.131. [DOI] [PubMed] [Google Scholar]
- Ratajewski M, Van de Ven WJ, Bartosz G, Pulaski L. The human pseudoxanthoma elasticum gene ABCC6 is transcriptionally regulated by PLAG family transcription factors. Hum Genet. 2008;124:451–463. doi: 10.1007/s00439-008-0570-0. [DOI] [PubMed] [Google Scholar]
- Ribiczey P, Tordai A, Andrikovics H, Filoteo AG, Penniston JT, Enouf J, et al. Isoform-specific up-regulation of plasma membrane Ca2+ATPase expression during colon and gastric cancer cell differentiation. Cell Calcium. 2007;42:590–605. doi: 10.1016/j.ceca.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2000;97:6001–6006. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Wilson MD, Ballester B, Schwalie PC, Brown GD, Marshall A, et al. Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science. 2010;328:1036–1040. doi: 10.1126/science.1186176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz V, Hendig D, Henjakovic M, Szliska C, Kleesiek K, Gotting C. Mutational analysis of the ABCC6 gene and the proximal ABCC6 gene promoter in German patients with pseudoxanthoma elasticum (PXE) Hum Mutat. 2006;27:831. doi: 10.1002/humu.9444. [DOI] [PubMed] [Google Scholar]
- Sladek FMZW, Lai E, Darnell JE., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990;4:2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Smith AN, Barth ML, McDowell TL, Moulin DS, Nuthall HN, Hollingsworth MA, et al. A regulatory element in intron 1 of the cystic fibrosis transmembrane conductance regulator gene. J Biol Chem. 1996;271:9947–9954. doi: 10.1074/jbc.271.17.9947. [DOI] [PubMed] [Google Scholar]
- Symmons O, Varadi A, Aranyi T. How segmental duplications shape our genome: recent evolution of ABCC6 and PKD1 Mendelian disease genes. Mol Biol Evol. 2008;25:2601–2613. doi: 10.1093/molbev/msn202. [DOI] [PubMed] [Google Scholar]
- Trip MD, Smulders YM, Wegman JJ, Hu X, Boer JM, ten Brink JB, et al. Frequent mutation in the ABCC6 gene (R1141X) is associated with a strong increase in the prevalence of coronary artery disease. Circulation. 2002;106:773–775. doi: 10.1161/01.cir.0000028420.27813.c0. [DOI] [PubMed] [Google Scholar]
- Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010;130:661–670. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanakker OM, Leroy BP, Coucke P, Bercovitch LG, Uitto J, Viljoen D, et al. Novel clinico-molecular insights in pseudoxanthoma elasticum provide an efficient molecular screening method and a comprehensive diagnostic flowchart. Hum Mutat. 2008;29:205. doi: 10.1002/humu.9514. [DOI] [PubMed] [Google Scholar]
- Varadi A, Szabo Z, Pomozi V, de Boussac H, Fulop K, Aranyi T. ABCC6 as a target in Pseudoxanthoma Elasticum. Curr Drug Targets. 2011;12:671–682. doi: 10.2174/138945011795378612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.