Abstract
Apolipoprotein E (APOE) genotype affects outcomes of Alzheimer’s Disease and other conditions of brain damage. Using APOE knock-in mice, we have previously shown that APOE- ε4 Targeted Replacement (TR) mice have fewer dendritic spines and reduced branching in cortical neurons. Since dendritic spines are postsynaptic sites of excitatory neurotransmission, we used APOE TR mice to examine whether APOE genotype affected the various elements of the glutamate-glutamine cycle. We found that levels of glutamine synthetase and glutamate uptake transporters were unchanged among the APOE genotypes. However, compared to APOE- ε3 TR mice, APOE-ε4 TR mice had decreased glutaminase levels (18%, p<0.05), suggesting decreased conversion of glutamine to glutamate. APOE-ε4 TR mice also had increased levels of the vesicular glutamate transporter VGLUT1 (20%, p<0.05), suggesting that APOE genotype affects presynaptic terminal composition. To address whether these changes affected normal neurotransmission, we examined the production and metabolism of glutamate and glutamine at 4–5 months and 1 year. Using high frequency 13C/1H nuclear magnetic resonance (NMR) spectroscopy, we found that APOE-ε4 TR mice have decreased production of glutamate and increased levels of glutamine. These factors may contribute to the increased risk of neurodegeneration associated with APOE-ε4, and also act as surrogate markers for AD risk.
Keywords: APOE, Glutaminase, Glutamine, VGLUT1, pre-synaptic, 13C/1H NMR
Introduction
Apolipoprotein E (APOE) is the strongest genetic risk factor for late-onset Alzheimer’s Disease (AD), and there are several hypotheses about how APOE affects AD pathogenesis (for review see (Bales et al. 2002)). APOE also affects normal brain function independent of AD pathology, as evidenced by studies of young, non-demented humans (Scarmeas et al. 2005, Filippini et al. 2009, Reiman et al. 2004). The ε4 allele of APOE is associated with deficits in glucose metabolism in cognitively unimpaired individuals in their 50s–60s (Small et al. 1995, Reiman et al. 1996), and with alterations in cerebral activation (measured by PET or fMRI) in young adults (Scarmeas et al. 2005, Filippini et al. 2009, Reiman et al. 2004). These findings suggest that APOE’s impact on brain function earlier in life could contribute to the susceptibility to damages later in life. Consistent with this idea, APOE genotype affects outcomes of other conditions of brain damage, including traumatic brain injury (Zhou et al. 2008), HIV dementia (Burt et al. 2008), and stroke (Savva & Stephan 2010). These observations support the development of a new class of therapeutics to compensate for APOE-ε4 related changes prior to overt signs of disease. However, before one could evaluate such therapies, it would be necessary to identify biomarkers that can be used in assessing therapeutic outcome.
To address this need, we and others have utilized APOE Targeted Replacement (TR)mice. These animals express the human APOE alleles (APOE-ε2, APOE-ε3, or APOE-ε4) under the mouse APOE promoter, and do not develop the plaques and tangles diagnostic of AD (Sullivan et al. 1997). Despite the lack of neuropathological changes, APOE-ε4 TR mice have simpler neuronal structures in the amygdala (Wang et al. 2005) and cortex (Dumanis et al. 2009), abnormalities in hippocampal LTP, (Korwek et al. 2009, Trommer et al. 2005), and alterations in hippocampal structure (Andrews-Zwilling et al. 2010). The APOE-ε4 animals have behavioral deficits in some Morris Water Maze tasks such as the probe test but not the learning rates while training (Grootendorst et al. 2005, Bour et al. 2008, Andrews-Zwilling et al. 2010). Moreover, aged female APOE-ε4 TR mice display increased errors in avoidance conditioning tasks that are not observed in younger cohorts (Bour et al. 2008, Grootendorst et al. 2005). Interestingly, a recent study has reported an emergence of a seizure phenotype in aged APOE-ε4 TR mice and abnormal cortical EEG activity (Hunter et al. 2012). These studies support the human studies described above, which suggest that APOE genotype affects brain structure and activity in the absence of overt signs of AD.
Based on these findings, we asked whether APOE genotype affected normal excitatory neurotransmission at the synapse by examining the elements of the glutamate-glutamine cycle in the brain. APOE-ε4 TR mice had lower levels of glutaminase and higher levels of the VGLUT1 transporter. Moreover, we examined the production and metabolism of glutamate (GLU) and glutamine (GLN). Using high frequency 13C/1H nuclear magnetic resonance (NMR), we found that incorporation of 13C label from glucose into C4 and/or C3 isotopomers of glutamate was decreased in APOE-ε4 TR mice. However, APOE-ε4 TR mice had higher levels of brain glutamine. Taken together, these data suggest that APOE genotype affects presynaptic terminal composition and impacts the normal GLU-GLN cycle.
Materials and Methods
Mice
Human APOE-ε2, APOE-ε3 and APOE-ε4 TR mice (on a C57Bl6/J background), which express each of the human APOE isoforms regulated by the endogenous murine APOE promoter (Sullivan et al. 1997) were bred in house. C57BL/6J wild type mice were obtained from Jackson Laboratories (Bar Harbor, Maine, USA). Experiments were performed on age-matched male mice at 4–5 months, 6 months or 1 year of age. All animal experiments were conducted in compliance with the rules and regulations of the Institutional Animal Care and Use Committee at Georgetown University.
Preparation of Brain Homogenates
Whole brains were homogenized with a dounce homogenizer in ice-cold TBS buffer (50 mM Tris-HCl, 150 mM NaCl, 1x protease inhibitor and phosphate inhibitor cocktails, pH 7.4). The homogenates were centrifuged at 47,000 RPM for 45 min at 4°C and the supernatants were labeled as the TBS (soluble) fraction. The remaining pellet was sonicated in ice-cold TBS-X buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1x protease inhibitor and phosphate inhibitor cocktails, pH 7.4). The resuspended pellet was centrifuged at 47,000 RPM for 45 min at 4°C and the supernatants were labeled as the TBS-X (membrane) fraction. Total protein concentration was determined by BCA protein assay kit (Pierce, Rockford, IL).
Immunoblot Analysis
Protein samples were boiled in Laemmli buffer (4% SDS) and electrophoresed on 10% gels with equal amounts of total protein loaded per lane. Separated proteins were transferred onto nitrocellulose membranes and analyzed by Western blotting. The following primary antibodies from Abcam were used: rabbit anti-glutaminase, rabbit anti-glutamine synthetase, rabbit anti-EAAT1 and rabbit anti-EAAT3. The following primary antibodies from Millipore were used: mouse anti-VGLUT1 and rabbit anti-EAAT2. The following primary antibodies from Sigma were used: rabbit anti-tubulin and mouse anti-β actin. After incubation with the appropriate HRP-conjugated secondary antibody, membranes were developed using SuperSignal West PICO or DURA luminol/enhancer solution (Pierce). For negative controls, blots were analyzed in the absence of primary antibodies, to define endogenous mouse antibody bands by secondary antibodies. The X-ray film was scanned and the density of bands was quantified using Quantity One software (Biorad).
Immunohistochemistry
50 micrometer free-floating coronal sections were processed for peroxidase immunohistochemistry using rabbit anti-glutaminase (Abcam, 1:400). Tissue sections were incubated in biotinylated secondary antibodies, and detection of the peroxidase reaction product with diaminobenzidine was performed with the ABC Elite kit (Vector Laboratories, Burlingame, CA). Cressyl Violet was used as a counterstain for nuclei.
High-frequency [1H/13C] Magnetic Resonance Spectroscopy
Animals were fasted overnight with free access to tap water and were intraperitoneally (i.p.) injected with [1-13C] glucose solution (0.5 mol/l) over 10 s (0.3 ml/25–30 g body weight; 200 mg/kg). 45 min post-injection with [1-13C] glucose, animals were sacrificed by cervical dislocation and brains immediately immersed in 6% ice-cold perchloric acid, 50 mM NaH2PO4. This time point was chosen from previous studies where we had tested that 45 min was good for 13C labeling of a range of metabolites in the mouse brain (Khandelwal et al. 2011). After homogenization and lyophilization, extracts were re-suspended in 0.65 ml D2O containing 2 mM sodium [13C] formate as an internal intensity and chemical shift reference (δ 171.8). Metabolite pool size was identified on 1H [13C-decoupled] NMR spectra. Peak areas were adjusted for nuclear Overhauser effect, saturation and natural abundance effects and quantified by reference to [13C] formate. Metabolite pool sizes were determined by integration of resonances in fully relaxed 400 MHz [13C-decoupled] 1H spectra using N-acetylaspartate as internal intensity reference. Incorporation of 13C into isotopomers was measured in reference to [13C] formate. All data were collected on a 9.7 Tesla Varian Spectrometer with dual 13C/1H probe. [13C-decoupled]-1H spectra were acquired with 3000 scans, pulse width 45°, relaxation delay 1 s, line broadening 0.5 Hz, acquired data points 13.132 and transformation size 32 K at room temperature. [1H-decoupled]-13C spectra were acquired with 30,000 scans and 31,875 data points. Spectra were integrated and quantified using MestReNova (Master Lab Research).
Statistical Analysis
Data are expressed as mean ± SEM unless otherwise specified. Statistical analysis was performed using the SPSS 10.0 software package (Graphpad, San Diego, CA). One-way analyses of variance (ANOVA) were used to analyze the effect of APOE genotype on various biomarkers of interest. Tukey’s post-hoc analyses were used to detect statistical differences among the groups using *, P < 0.05, **, p<0.01, ***, p<0.001 compared to APOE-ε3 TR mice unless otherwise noted.
Results
Brain glutaminase levels are lower in APOE-ε4 TR mice
The APOE TR mice show differential effects on spine density and neuronal morphology, with APOE-ε4 TR mice having reduced dendritic complexity and spine density compared to APOE-ε3 and APOE-ε2 TR mice (Dumanis et al. 2009, Wang et al. 2005). Dendritic spines are postsynaptic sites of excitatory neurotransmission, and glutamate (GLU) is the primary excitatory neurotransmitter in the brain. Glutamine (GLN) derived GLU from the GLU-GLN cycle is necessary for the maintenance of GLU at the nerve terminals (Laake et al. 1995, Lebon et al. 2002). Therefore, we asked whether APOE genotype impacted the GLU-GLN cycle.
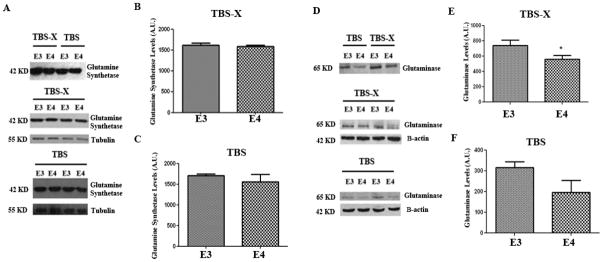
We examined the brains of APOE TR mice at 4–5 months of age for levels of glutamine synthetase, the enzyme responsible for converting GLU to GLN. Brains were extracted in buffers to isolate soluble and membrane bound proteins, and immunoblotted for glutamine synthetase. Glutamine synthetase was present in both the cytoplasm and membrane bound fractions (Fig 1A, upper panel), but there were no significant differences in the levels between the APOE-ε3 and APOE-ε4 TR mouse brains in either fraction (Fig 1A–C).
Figure 1. Glutaminase is reduced in APOE-ε4 TR mice.
4–5 month old APOE TR mouse brains were homogenized by TBS and then TBS-X by sequential fractionation. Homogenates were probed for Glutamine Synthetase (A–C) and Glutaminase (D–F). (A) A representative immunoblot showing that Glutamine Synthetase is present in both the TBS and TBSX fraction of these mice (upper panel). A representative blot showing the levels of Glutamine Synthetase in the TBSX Fraction (middle panel) and the TBS fraction (lower panel). Tubulin was used as a loading control. (B) Quantification of Glutamine Synthetase levels in the TBS-X and (C) TBS fractions. (D) Representative immunoblot showing that glutaminase is present in the TBSX and TBS fractions. (E) Quantification of the Glutaminase levels in the TBSX and (F) TBS fractions. n=5 per condition. *, p<0.05 compared to APOE-ε3 TR mice. Error bars expressed as S.E.M.
We also tested the levels of phosphate-activated glutaminase (PAG), the enzyme responsible for converting GLN to GLU. PAG was more prevalent in the membrane bound fraction of brain extracts (Fig 1D, upper panel), consistent with its partial location at the outer face of the inner membrane wall of mitochondria (Kvamme et al. 2001). In brains of 4–5 month old mice, the membrane bound levels of PAG were significantly lower in the APOE-ε4 TR mice than the APOE-ε3 TR mice (23%, p<0.05) (Fig 1E). We tested these results in a second cohort of 4–5 month old mice. Consistent with Fig 1, we found that PAG was significantly lower in the membrane fraction of APOE-ε4 TR brains compared to APOE-ε3 TR brains (18%, p<0.05; data not shown). The cytoplasmic levels of PAG in APOE TR were not significantly different between the genotypes (Fig. 1F).
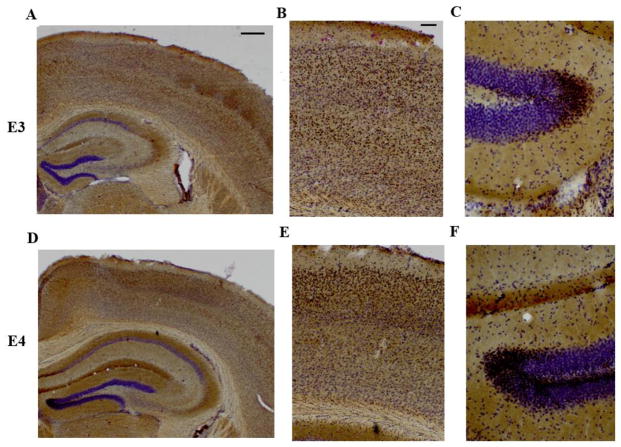
To determine whether APOE-ε4 TR mice had differences in the brain distribution of PAG compared to APOE-ε3 TR mice, we performed an immunostain for PAG in coronal brain sections. PAG was observed predominantly in the soma of neuronal cells in layers II/III and in layer V of the cortex. There was also intense staining for glutaminase in the dentate gyrus, the stratum moleculare and the stratum oriens of the hippocampus consistent with previous reports (Altschuler et al. 1985) (Fig 2). No differences in the distribution of PAG were observed between the APOE genotypes (Fig 2).
Figure 2. Glutaminase regional distribution is similar in the APOE-ε4 and APOE-ε3 TR mice.
6 month old APOE TR mouse brain sections (50 microns thick) were stained and processed via peroxidase immunohistochemistry for glutaminase staining and cresyl violet counter-staining. (A–C) APOE3 TR representative image and (D–F) APOE4 TR representative image (A, D). Image at 2X magnification, scale bar to 500 microns. (B, E) Cortical image and (C, F) Hippocampal image of the dentate gyrus at 20x magnification. Scale bar set to 100 microns.
Brain VGLUT1 levels are significantly higher in APOE-ε4 TR mice
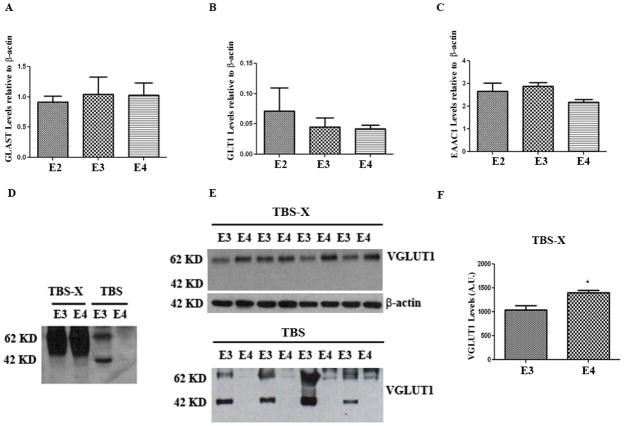
Glutamate re-uptake transporters in human brain are the Excitatory Amino Acid Transporters (EAATs) and they play an important role of regulating concentrations of glutamate in the synaptic cleft (for review see (Kanai & Hediger 1992)). In other mammalian systems, the nomenclature for the EAATs is different. EAAT1 (GLAST) is located on glial cells, while EAAT3 (EAAC1 carrier) is predominantly neuronal (Kanai & Hediger 1992); EAAT2 (GLT1) can be found on both the glial and neuronal plasma membranes (Shashidharan et al. 1994, Desilva et al. 2012). Although these transporters are ubiquitously expressed throughout the brain, GLT1 is the major glutamate transporter in the forebrain and GLAST is predominantly expressed in the cerebellum (Takamori 2006, Rothstein et al. 1994). Consistent with previous reports (Maragakis et al. 2004), we observed GLAST, EAAC1, and GLT1 mostly in the membrane bound fraction but not in the cytosolic fraction of mouse brain extracts (data not shown). None of these transporters (GLAST, EAAC1, and GLT1) showed significantly different levels between APOE-ε3 and APOE–ε4 TR brains (Fig 3A–C).
Figure 3. VGLUT1 levels are higher in the TBSX fraction of APOE-ε4 TR mice.
4–5 month old APOE TR mouse brains were homogenized by RIPA and the levels of (A) GLAST, (B) GLT1, and (C) EAAC1 were analyzed by western blot and quantified. These levels were unchanged among the genotypes. (D–F) 4–5 month old APOE TR mouse brains were homogenized by TBS followed by TBS-X via sequential fractionation. (D) VGLUT1 is predominantly in the TBS-X fraction as seen in the representative immunoblot. (E) The immunboblot for VGLUT1 in the TBS-X fraction (top) and TBS fraction (bottom). (F) Quantification for the TBS-X fraction in (E). *, p<0.05. n=4 animals/condition. Error bars expressed as S.E.M.
We also examined the levels of VGLUT1, a transporter responsible for packaging GLU into presynaptic vesicles. As expected, VGLUT1 was predominantly localized to the membrane bound fraction (Fig 3D). Interestingly, the levels of VGLUT1 were significantly higher in APOE-ε4 TR mice compared to APOE-ε3 TR mice (20%, p<0.05) (Fig 3E, F). We also observed low levels of VGLUT1-positive fragments in the cytosolic brain fraction; VGLUT1 fragments were observed in APOE-ε3 TR mice, but not in the APOE-ε4 TR mice (Fig 3D, E). The data on glutaminase and VGLUT1 are summarized in Table 1. Together they suggest that APOE genotype may affect vesicular glutamate within the presynaptic compartments of the glutamatergic synapses.
Table 1. Glutaminase and VGLUT1 Summary for APOE TR mice.
A table summarizing the protein level findings from Figure 1 and Figure 3 between APOE-ε3 TR mice and APOE-ε4 TR mice TR mice.
| Levels in APOE-ε4 TR mice compared to APOE3-ε3 TR mice | ||
|---|---|---|
| Glutaminase | ||
| Soluble Fraction | Unchanged | p>0.10 |
| Membrane Bound Fraction | Decreased by 23% | p<0.05 |
| VGLUT1 | ||
| Soluble Fraction | Decreased by 90%* | p<0.01 |
| Membrane Bound Fraction | Increased by 20% | p<0.05 |
APOE-ε4 TR mice have significantly higher brain levels of glutamine
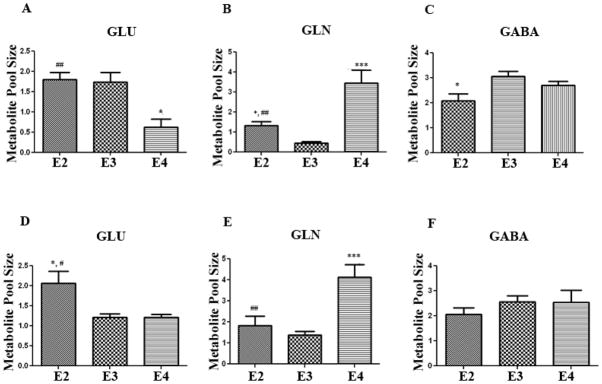
The differences in PAG levels (Fig 1) and VGLUT1 levels (Fig 3), suggest that APOE-ε4 TR mice may have altered glutamate metabolism. Therefore, we measured whole brain levels of GLU and two of its downstream products, glutamine (GLN) and gamma-aminobutyric acid (GABA), using high-frequency 1H NMR spectroscopy in mice at 4–5 months of age (n= 4 per genotype). We chose the 4–5 month age because it was consistent with the timepoint chosen for our immunoblotting analysis. We found that GLU levels were significantly lower in APOE-ε4 TR mice by over 50% compared to APOE-ε3 and APOE-ε2 TR mice (Fig 4A) (p<0.05). In contrast, the total levels of GLN were significantly higher by 3 fold (p<0.01) in the APOE-ε4 TR mice compared to the APOE-ε3 and APOE-ε2 TR mice (Fig 4B). Total levels of GABA were not different among the APOE genotypes (Fig 4C).
Figure 4. Glutamine pool sizes are higher in APOE-ε4 TR mice.
4–5 month old APOE TR brains were lyopholized and resuspended in deuterium. 1H-NMR was used to measure total metabolite pool sizes for GLU (A), GLN (B), and GABA (C). 1 year old APOE TR brains were lyopholized and resuspended in deuterium. 1H-NMR was used to measure total metabolite pool sizes for GLU (D), GLN (E), and GABA (F). At both time points, GLN levels were significantly increased by over two fold in the APOE-ε4 TR mice compared to the other genotypes. *, p <0.05, **, p<0.01, ***, p<0.001 compared to APOE-ε3 TR mice.#, p<−.05, ##, p<0.01 compared to APOE-ε4 TR mice. Error bars expressed as S.E.M. (n=4 animals/genotype/time-point).
We repeated the 1H NMR analysis in a second cohort of APOE TR mice at 1 year of age. Brain levels of GLU were not significantly different between the APOE-ε4 and APOE-ε3 TR mice, but the APOE-ε2 TR mice still had significantly higher levels of GLU (Fig 4D) (p<0.05). Again, we did not observe significant differences in levels of GABA among the genotypes (Fig 4F). Consistent with the 4–5 month results, the levels of GLN were significantly higher (by 123%, p<0.001) in the APOE-ε4 TR mice compared to APOE-ε3 and APOE-ε2 TR mice (Fig 4E). These consistent data on higher GLN levels in APOE-ε4 TR mice, taken together with the reduction in PAG levels (Fig 1), suggest that there is decreased metabolism of GLN in APOE-ε4 TR mice compared to controls. This observation does not exclude the possibility that there is also increased GLN synthesis in APOE-ε4 TR mice compared to controls.
APOE-ε4 TR mice have significantly lower brain 13C incorporation of glutamate
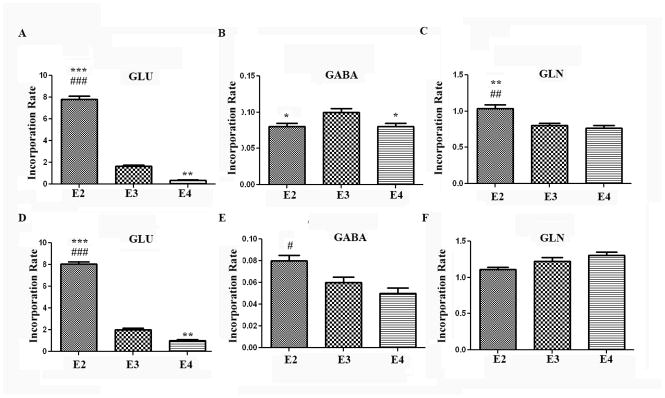
To address the mechanism by which GLN levels are higher in the APOE-ε4 TR mice, we used 13C NMR spectroscopy. Animals were intraperitoneally injected with [1-13C] glucose, and brain tissue was collected 45 minutes later (see Methods for more detail). This method enables the measurement of 13C label incorporation from glucose into various metabolites. The data at 1 year and 4.5 months were consistent with each other. APOE-ε2 TR mice showed significantly higher levels of newly-synthesized GLU compared to the other genotypes at both 4–5 months (Fig 5A), and at 1 year of age (Fig 5D). These data are consistent with the higher total levels of GLU in APOE-ε2 TR mice observed in Figure 4. GLU production at 4.5 months and 1 year (Fig 5A, D) was significantly lower in the APOE-ε4 TR mice compared to the other genotypes (90% compared to APOE-ε2 TR, and 60% compared to APOE-ε3 TR, p<0.001 at 1 year), suggesting that 13C incorporation of GLU was lower or GLU breakdown was higher in these animals (Fig 5D).
Figure 5. Glutamine incorporation is unchanged among the genotypes.
4–5 month old APOE TR mice were injected i.p. with [1-13C] glucose solution, and brains were extracted 45 minutes later. 13C NMR analysis was used to determine the incorporation of 13C for GLU C4 (A), and GABA C2 (B), and GLN C4 (C). 1 year old APOE TR mice were injected i.p. with [1-13C] glucose solution, and brains were extracted 45 minutes later. 13C NMR analysis was used to determine the incorporation of 13C for GLU C4 (D), and GABA C2 (E), and GLN C4 (F). ###, p<0.001, ##, p<0.01, #, p<0.05 compared to APOE-ε4 TR mice. ***, p<0.001, **, p<0.01, *, p<0.05 compared to APOE-ε3 TR mice. The GLU turnover rate was increased in the APOE-ε2 TR mice and decreased in the APOE-ε4 TR mice when compared to APOE-ε3. (n=4 animals/genotype). Error bars expressed as S.E.M.
We also examined turnover rates for GABA, which can be synthesized from GLU. Incorporation of 13C into GABA was slightly, but significantly, higher in the younger APOE-ε3 animals (Fig 5B). In the 1 year old cohort, the APOE-ε2 animals had the higher incorporation of label, and there were no differences between the APOE-ε3 and APOE-ε4 genotypes (Fig 5E). The overall incorporation rate of 13C into GABA was much lower than the 13C incorporation rate into GLU or GLN (Fig 5) suggesting that the 13C incorporation rate into GABA is less efficient perhaps because it is in a different compartment (GABAergic interneurons compared to the GLU-GLN cycle). While there were no consistent differences among APOE genotypes in incorporation of 13C into GABA, other NMR conditions may need to be used to increase GABA enrichment to test for a clear APOE isoform dependent effect.
Interestingly, GLN production was not significantly different among the APOE genotypes at 4.5 months (5C) and at 1 year of age (Fig. 5F), suggesting that the higher GLN levels in APOE-ε4 TR mice (Fig 4B, D) were not due to increased GLN synthesis derived from the GLU-GLN cycle. Succinate (a product of the TCA cycle) turnover rates were also not different among the genotypes (Fig. 6) suggesting that the lower level of glutamate production was not due to a deficit in entry of glucose-derived 13C label into the TCA cycle.
Figure 6. Succinate incorporation is unchanged among the genotypes.
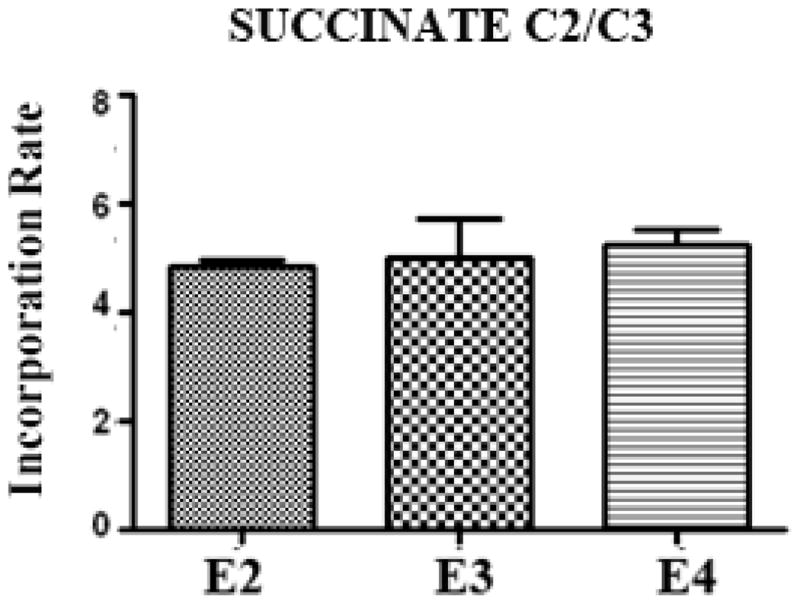
1 year old mice were injected i.p. with [1-13C] glucose solution, and brains were extracted 45 minutes later. 13C NMR analysis was used to determine the incorporation of 13C for Succinate C2/C3.
Discussion
Various findings in humans and APOE knock-in mice indicate that APOE genotype affects normal brain function independent of the accumulation of AD-associated neuropathological changes. We have been able to address this hypothesis by analyzing whether there are effects of APOE genotype in knock-in mice on the metabolism of GLU, the major excitatory amino acid in the brain. We used immunoblots to measure proteins important to GLU metabolic pathways and 1H-decoupled 13C NMR to measure various GLU metabolites. We found several indications that GLU metabolism was altered in APOE-ε4 TR mouse brains, including decreased levels of glutaminase and increased levels of VGLUT1. We also found that 13C incorporation into GLU is lower in APOE-ε4 TR mice compared to APOE-ε3 and APOE-ε2 TR mice at 4–5 months and at 1 year of age. Human APOE-ε4 carriers have reduced glucose utilization (Scarmeas et al. 2005, Reiman et al. 2001, Small et al. 1995) and the APOE-ε4 knock-in mice have a more permeable blood brain barrier (Nishitsuji et al. 2011, Bell et al. 2012), suggesting that reduced GLU levels observed via 13C NMR may be related to reduced uptake of glucose by astrocytic endfeet. However, APOE-ε4 TR mice did not have a lower 13C incorporation into succinate C2/C3 or GLN C4 (Fig. 6, Fig. 5C, F). If decreased glucose uptake in APOE-ε4 TR mice were responsible for the reduced 13C incorporation into GLU C4 observed, then all measured glucose metabolites should have a reduced rate of production.
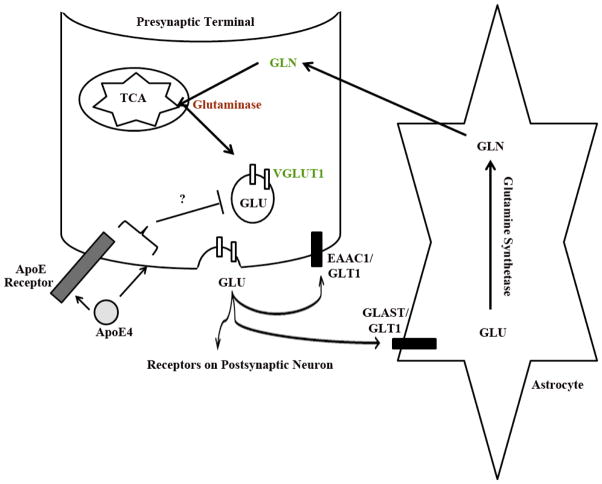
We also found that total GLN levels were over two fold higher in the APOE-ε4 TR mice compared to APOE-ε3 and APOE-ε2 TR mice; this result was consistent in two independent cohorts of animals (Fig 4). We examined the various transporters and enzymes involved in the GLU-GLN cycle. The lower glutaminase levels in APOE-ε4 TR brains (Fig 1) would result in reduced recycling of GLN back into GLU and thus explain the higher levels of total available GLN. The GLU-GLN cycle is a major pathway for GLU repletion in the brain (Laake et al. 1995). These findings have suggested that presynaptic mechanisms among the APOE genotypes may account for the observed differences in GLN levels (See model, Fig. 7). We hypothesize that apoE maybe inhibiting vesicle release either by altering presynaptic membrane lipid composition or altering presynaptic ApoE receptor signaling. Future studies will begin to address this hypothesis.
Figure 7. The effects of APOE4 on presynaptic markers.
A model depicting the alterations in APOE-ε4 TR mice compared to APOE-ε3 TR mice. APOE-ε4 TR mice have increased total GLN (in green) and increased VGLUT1 (in green) but decreased glutaminase (red) compared to APOE-ε3 TR mice. All other aspects of the GLU-GLN cycle are unaffected (such as Glutamine Synthetase and the other Glutamate transporters). We hypothesize that apoE maybe inhibiting vesicle release either by altering synaptic lipid membrane composition or affecting signaling via a pre-synaptic ApoE receptor.
Although the total levels of GLU in APOE-ε4 TR mice were unchanged at 1 year compared to the APOE-ε3 TR mice (Fig 4E), the metabolic flux of 13C into GLU C4 was lower (Fig 5), suggesting that APOE-ε4 TR mice may compensate by having a larger pool of readily available GLU. VGLUTs are transporters which use a chloride and proton gradient to shuttle cytoplasmic GLU into pre-synaptic vesicles. The total level of VGLUTs correlates with the amount of GLU stored and released (Daniels et al. 2006, Takamori et al. 2000, Wojcik et al. 2004, Wilson et al. 2005) at the presynaptic terminal. A recent report showed that APOE-ε4 TR mice on a control diet have higher total VGLUT1 levels compared to APOE-ε3 TR mice (Kariv-Inbal et al. 2012), consistent with our findings (Fig 3), suggesting increased vesicular GLU in APOE-ε4 TR mice. The higher VGLUT1 in APOE-ε4 TR mice would thus compensate for the reduced GLU turnover by increasing the quantel content of GLU in synaptic vesicles (See model, Fig 6). Although VGLUT1 is predominately expressed on synaptic vesicles, when the vesicles fuse to the plasma membrane and release GLU, VGLUT1 is also present on the plasma membrane. Plasma membrane VGLUT1 is involved in phosphate transport (Takamori 2006). High concentrations of phosphate are needed to activate glutaminase, and VGLUT1 on the plasma membrane correlates with an increase in glutaminase activity (Takamori 2006). Thus, in APOE-ε4 TR mice, higher VGLUT1 levels at the plasma membrane may be a compensatory mechanism for the observed reduction in glutaminase levels.
In contrast to the membrane fractions, we observed decreases in VGLUT1 levels in the cytoplasmic fractions of APOE-ε4 TR mice (Fig 3E). The presence of full-length VGLUT1 and VGLUT1 fragments in cytoplasmic extracts may reflect turnover of membrane-bound VGLUT1, although little is known about VGLUT1 metabolism (Lobo et al. 2011). Recently, it has been reported that VGLUT1 levels on synaptic vesicles can be impacted by the diurnal cycle (Yelamanchili et al. 2006, Darna et al. 2009), however, the precise mechanism regulating VGLUT1 levels are unknown. The observation of lower levels of VGLUT1 immunoreactive bands in APOE-ε4 mice may indicate that they have reduced rates of VGLUT1 turnover. The apoE4 protein has been observed to reduce recycling of endocytic vesicles (Chen et al. 2010, Heeren et al. 2004), and it may similarly impact VGLUT1 recycling in synaptic vesicles.
Here, we focused on elements of the GLU-GLN cycle, but GLU can also be derived from other sources in the brain. For example, leucine, which is transported across the blood brain barrier, can be transaminated into GLU (Kanamori et al. 1998). It is possible that the leaky blood brain barrier of APOE-ε4 knock-in mice may impact leucine trafficking into the brain, which subsequently could impact GLU metabolism.
Our observed alterations in GLU metabolism support earlier studies showing synaptic alterations in APOE-ε4 TR mice (Trommer et al. 2004, Korwek et al. 2009, Wang et al. 2005, Dumanis et al. 2009). They may also relate to a recent study that found seizure activity as well as abnormal EEG cortical activity in APOE-ε4 TR mice (Hunter et al. 2012). Alterations in the precise balance of excitatory and inhibitory neurotransmission could underlie the susceptibility of APOE-e4 TR mice to seizures. This recent finding in mice correlates with another recent study, which has found that healthy human APOE-ε4 carriers have abnormal EEG activity following hyperventilation (Ponomareva et al. 2008).
Understanding the biochemical variations that correlate with APOE genotype in these mouse models could allow advancement for clinical approaches in APOE-ε4 carriers. There are reports of aberrant expression of glutaminase as well as glutamine synthesis and glutamate dehydrogenase (which converts α-ketoglutorate to glutamate) in AD brains compared to controls (Robinson 2001, Burbaeva et al. 2005). Unfortunately, in these studies, it is impossible to delineate whether these changes were present prior to onset of AD. These APOE TR mice allow us to examine such changes independent of the AD pathology.
Due to the limitations of NMR sensitivity, we were using whole brain extracts enriched with 13C label to achieve our signal, therefore the effects that we observe have been averaged across brain regions. Future studies will begin to elucidate the brain specific region effects of APOE-ε4 TR compared to the other genotypes. Such studies would be interesting, especially with the conflicting reports of LTP abnormalities in APOE-ε4 TR mice: LTP is enhanced in the CA1 region of the hippocampus (Korwek et al. 2009) but reduced in the dentate gyrus (Trommer et al. 2004).
Here, we have defined several presynaptic effects of APOE genotypes. However, we and others have found that APOE genotype also affects post-synaptic measures. APOE-ε4 correlates with decreased dendritic spines in both mice (Wang et al. 2005, Dumanis et al. 2009) and humans (Ji et al. 2003). Primary neurons treated with recombinant apoE4 recombinant protein have reduced surface level expression of glutamate receptors (AMPA and NMDA subunits) compared to control and apoE3 treated neurons (Chen et al. 2010). Future in vitro studies will address whether apoE receptors are involved in modulating the presynaptic (glutamate synthesis, VGLUT1 levels, glutaminase levels) and postsynaptic (GLU trafficking, dendritic spines) effects of APOE4, to begin elucidating the mechanism of these isoform specific effects. The various in vivo measures have thus allowed us to focus future in vitro analyses on specific neurotransmission pathways and test whether the pre- and post-synaptic changes in APOE-ε4 TR mice are independent or related events.
A potential approach to manipulating apoE isoform effects is through diet. APOE-ε4 TR mice fed on a diet high in DHA content resulted in lower levels of VGLUT1, abolishing the effects of APOE-ε4 (Kariv-Inbal et al. 2012). These results indicate that not only does diet interact with APOE genotype to affect the lifecycle of GLU, but also that preventative measures may allow reduction of the differences in APOE-ε4 and APOE-ε3 TR genotypes. The various measures of proteins and metabolites from this study could act as biomarkers of AD risk. ApoE is a cholesterol transporter and essential to maintaining lipid and cholesterol homeostasis in the membrane. Although total membrane composition of cholesterol and lipids is not strikingly different in these animal models (Kariv-Inbal et al. 2012), it is quite possible that the membrane composition at the synaptic vesicle is altered. Thus, AD preventative treatments of normal mice could be monitored using these new in vivo markers of APOE genotype.
Interestingly, APOE-ε2 TR mice are markedly different from the APOE-ε3 and APOE-ε4 TR mice, potentially relating to the decreased risk of AD associated with APOE-ε2 (Sullivan et al. 1998). We observed some opposite effects of APOE-ε2 compared to APOE-ε4 in these mice, such as higher 13C label incorporation into GLU in the APOE-ε2 TR animals (Fig 5A, Table 1), as well as higher levels of brain GLU (Figure 4). As in humans, APOE-ε2 is associated with type III hyperlipoproteinemia in these mice (Sullivan et al. 1998), resulting in atherosclerotic plaques and over double the cholesterol and triglyceride levels of the other APOE genotypes. The observed differences in brain GLU flux or level of 13C incorporation (E2>E3>E4) could be related to differences in lipid metabolism or could reflect differences in risk of neurodegeneration by APOE genotype.
In this work, using a variety of measures, we have shown that APOE genotype modulates glutamate metabolism in a mouse model displaying normal expression of human APOE alleles. In the APOE-ε4 TR mice, glutamate level was low while glutamine levels were high; in addition, glutaminase levels were low and VGLUT1 levels were high. These various factors may contribute to the increased risk of neurodegeneration associated with APOE-ε4, and could thus act as surrogate markers for AD risk.
Acknowledgments
This work was supported by NIH R01 AG035379 (GWR), P01 AG030128 (GWR), AG30378 (CEM), Georgetown University fund (CEM) and the National Science Foundation Graduate Fellowship DGE-0903443 (SBD). We thank Dr. Susan McKenna and Dr. Susanna Scafidi for their helpful discussions.
Abbreivations
- APOE
ApolipoproteinE
- AD
Alzheimer’s Disease
- GLU
Glutamate
- GLN
Glutamine
- NMR
Nuclear magnetic resonance
- TR
Targeted Replacement
- PAG
Phosphate-Activated Glutaminase
- EAAT
Excitatory Amino Acid Transporter
- GABA
gamma-aminobutyric acid
Footnotes
None of the authors have a conflict of interest to declare with this work.
References
- Altschuler RA, Monaghan DT, Haser WG, Wenthold RJ, Curthoys NP, Cotman CW. Immunocytochemical localization of glutaminase-like and aspartate aminotransferase-like immunoreactivities in the rat and guinea pig hippocampus. Brain Res. 1985;330:225–233. doi: 10.1016/0006-8993(85)90681-x. [DOI] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, et al. Apolipoprotein E4 causes age-and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Dodart JC, DeMattos RB, Holtzman DM, Paul SM. Apolipoprotein E, amyloid, and Alzheimer disease. Mol Interv. 2002;2:363–375. 339. doi: 10.1124/mi.2.6.363. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Burbaeva G, Boksha IS, Tereshkina EB, Savushkina OK, Starodubtseva LI, Turishcheva MS. Glutamate metabolizing enzymes in prefrontal cortex of Alzheimer’s disease patients. Neurochem Res. 2005;30:1443–1451. doi: 10.1007/s11064-005-8654-x. [DOI] [PubMed] [Google Scholar]
- Burt TD, Agan BK, Marconi VC, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A. 2010;107:12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, DiAntonio A. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 2006;49:11–16. doi: 10.1016/j.neuron.2005.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darna M, Schmutz I, Richter K, Yelamanchili SV, Pendyala G, Holtje M, Albrecht U, Ahnert-Hilger G. Time of day-dependent sorting of the vesicular glutamate transporter to the plasma membrane. J Biol Chem. 2009;284:4300–4307. doi: 10.1074/jbc.M805480200. [DOI] [PubMed] [Google Scholar]
- Desilva TM, Borenstein NS, Volpe JJ, Kinney HC, Rosenberg PA. Expression of EAAT2 in neurons and protoplasmic astrocytes during human cortical development. J Comp Neurol. 2012 doi: 10.1002/cne.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, et al. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res. 2005;159:1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Heeren J, Grewal T, Laatsch A, Becker N, Rinninger F, Rye KA, Beisiegel U. Impaired recycling of apolipoprotein E4 is associated with intracellular cholesterol accumulation. J Biol Chem. 2004;279:55483–55492. doi: 10.1074/jbc.M409324200. [DOI] [PubMed] [Google Scholar]
- Hunter JM, Cirrito JR, Restivo JL, et al. Emergence of a seizure phenotype in aged apolipoprotein epsilon 4 targeted replacement mice. Brain Res. 2012 doi: 10.1016/j.brainres.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kanamori K, Ross BD, Kondrat RW. Rate of glutamate synthesis from leucine in rat brain measured in vivo by 15N NMR. J Neurochem. 1998;70:1304–1315. doi: 10.1046/j.1471-4159.1998.70031304.x. [DOI] [PubMed] [Google Scholar]
- Kariv-Inbal Z, Yacobson S, Berkecz R, Peter M, Janaky T, Lutjohann D, Broersen LM, Hartmann T, Michaelson DM. The isoform-specific pathological effects of apoE4 in vivo are prevented by a fish oil (DHA) diet and are modified by cholesterol. J Alzheimers Dis. 2012;28:667–683. doi: 10.3233/JAD-2011-111265. [DOI] [PubMed] [Google Scholar]
- Khandelwal PJ, Herman AM, Hoe HS, Rebeck GW, Moussa CE. Parkin mediates beclin-dependent autophagic clearance of defective mitochondria and ubiquitinated Abeta in AD models. Hum Mol Genet. 2011;20:2091–2102. doi: 10.1093/hmg/ddr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korwek KM, Trotter JH, Ladu MJ, Sullivan PM, Weeber EJ. ApoE isoform-dependent changes in hippocampal synaptic function. Mol Neurodegener. 2009;4:21. doi: 10.1186/1750-1326-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvamme E, Torgner IA, Roberg B. Kinetics and localization of brain phosphate activated glutaminase. J Neurosci Res. 2001;66:951–958. doi: 10.1002/jnr.10041. [DOI] [PubMed] [Google Scholar]
- Laake JH, Slyngstad TA, Haug FM, Ottersen OP. Glutamine from glial cells is essential for the maintenance of the nerve terminal pool of glutamate: immunogold evidence from hippocampal slice cultures. J Neurochem. 1995;65:871–881. doi: 10.1046/j.1471-4159.1995.65020871.x. [DOI] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo AC, Gomes JR, Catarino T, et al. Cleavage of the vesicular glutamate transporters under excitotoxic conditions. Neurobiol Dis. 2011;44:292–303. doi: 10.1016/j.nbd.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Dietrich J, Wong V, Xue H, Mayer-Proschel M, Rao MS, Rothstein JD. Glutamate transporter expression and function in human glial progenitors. Glia. 2004;45:133–143. doi: 10.1002/glia.10310. [DOI] [PubMed] [Google Scholar]
- Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomareva NV, Korovaitseva GI, Rogaev EI. EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol Aging. 2008;29:819–827. doi: 10.1016/j.neurobiolaging.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR. Changes in the cellular distribution of glutamine synthetase in Alzheimer’s disease. J Neurosci Res. 2001;66:972–980. doi: 10.1002/jnr.10057. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Savva GM, Stephan BC. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, Stern Y. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005;76:1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharan P, Huntley GW, Meyer T, Morrison JH, Plaitakis A. Neuron-specific human glutamate transporter: molecular cloning, characterization and expression in human brain. Brain Res. 1994;662:245–250. doi: 10.1016/0006-8993(94)90819-2. [DOI] [PubMed] [Google Scholar]
- Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J Clin Invest. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–351. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Shah C, Yun SH, et al. ApoE isoform-specific effects on LTP: blockade by oligomeric amyloid-beta 1–42. Neurobiol Dis. 2005;18:75–82. doi: 10.1016/j.nbd.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Shah C, Yun SH, et al. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004;15:2655–2658. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- Wang C, Wilson WA, Moore SD, Mace BE, Maeda N, Schmechel DE, Sullivan PM. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol Dis. 2005;18:390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelamanchili SV, Pendyala G, Brunk I, Darna M, Albrecht U, Ahnert-Hilger G. Differential sorting of the vesicular glutamate transporter 1 into a defined vesicular pool is regulated by light signaling involving the clock gene Period2. J Biol Chem. 2006;281:15671–15679. doi: 10.1074/jbc.M600378200. [DOI] [PubMed] [Google Scholar]
- Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008;25:279–290. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]