Abstract
As a public health problem, prostate cancer engenders huge economic and life-quality burden. Developing effective chemopreventive regimens to alleviate the burden remains a major challenge. Androgen signaling is vital to the development and progression of prostate cancer. Targeting androgen signaling via blocking the production of the potent ligand dihydrotestosterone has been shown to decrease prostate cancer incidence. However, the potential of increasing the incidence of high-grade prostate cancers has been a concern. Mechanisms of disease progression after the intervention may include increased expression of androgen receptor (AR) in prostate tissue and expression of the constitutively-active AR splice variants (AR-Vs) lacking the ligand-binding domain. Thus, novel agents targeting the receptor, preferentially both the full-length and AR-Vs, are urgently needed. In the present study, we show that ginsenoside 20(S)-protopanaxadiol-aglycone (PPD) effectively downregulates the expression and activity of both the full-length AR and AR-Vs. The effects of PPD on AR and AR-Vs are manifested by an immediate drop in proteins followed by a reduction in transcripts, attributed to PPD induction of proteasome-mediated degradation and inhibition of the transcription of the AR gene. We further show that although PPD inhibits the growth as well as AR expression and activity in LNCaP xenograft tumors, the morphology and AR expression in normal prostates are not affected. This study is the first to show that PPD suppresses androgen signaling through downregulating both the full-length AR and AR-Vs, and provides strong rationale for further developing PPD as a promising agent for the prevention and/or treatment of prostate cancer.
Keywords: 20(S)-protopanaxadiol-aglycone, androgen receptor, prostate cancer
INTRODUCTION
Prostate carcinogenesis is characterized by a latency of 20 to 40 years.1 Most prostate cancers remain asymptomatic for years, even though they are malignant histologically.1 The long latency of prostate carcinogenesis presents an ideal opportunity for chemoprevention to inhibit or delay the clinical symptoms or to reverse the progression of the disease. Although the etiology of prostate cancer remains poorly understood, the vital role of androgen signaling in the development and progression of prostate cancer has been documented extensively.2 Finasteride and dutasteride are inhibitors of 5α-reductases, which are responsible for the conversion of testosterone to the more potent androgen dihydrotestosterone (DHT). So far, they are the only chemopreventive agents that have been shown definitively in large-scale randomized trials to decrease prostate cancer incidence.3,4 However, for both agents, the decrease appears to be limited to low-grade tumors with a Gleason score of 6 or lower, and significant increase in the incidence of high-grade prostate cancers has been observed in the treatment groups.3–5 As a consequence, 5α-reductase inhibitors are not approved by FDA for the prevention of prostate cancer.5
The mechanism underlying the increased incidence of high-grade prostate cancers after finasteride and dutasteride treatments is still unknown. A recent study reported significant upregulation of androgen receptor (AR) in prostate tissue by finasteride treatment in patients with benign prostatic hyperplasia.6 Increase in AR expression has been shown to be sufficient to convert prostate tumor growth from an androgen-sensitive to castration-resistant stage,7 and therefore may represent a mechanism underlying disease progression after finasteride treatment. Furthermore, the levels of constitutively-active, ligand-independent AR splice variants (AR-Vs) have been reported to be upregulated during prostate cancer progression.8–11 These AR-Vs lack the ligand-binding domain (LBD), and thus may not be responsive to treatment with finasteride or dutasteride. Therefore, it becomes imperative to develop new chemopreventive agents that could disrupt AR signaling through both the full-length and splice variants of AR.
The ginseng root is one of the most commonly used medicinal herbs in the United States.12 It is used as a tonic for enhancing well-being and promoting longevity, and also as a complementary therapy particularly for cancer intervention.12 It has been used for centuries by humans and reported to have minimal side effects. Ginsenosides are considered the main active ingredients responsible for the pharmaceutical functions of the ginseng root.13 There are two major groups of ginsenosides, the protopanaxadiol and the protopanaxatriol group. Orally ingested ginsenosides are mainly metabolized by intestinal bacteria through deglycosylation.13 The sugar moieties of the ginsenosides in the protopanaxadiol and the protopanaxatriol group are cleaved to give 20(S)-protopanaxadiol-aglycone (PPD) and 20(S)-protopanaxatriol-aglycone, respectively.13 PPD has been reported to inhibit the growth of prostate cancer cells both in vitro and in vivo.14,15 The growth inhibition has been attributed to PPD suppression of cell proliferation and induction of apoptosis, which are accompanied by downregulation of cyclins and cyclin-dependent kinases as well as induction of p21 expression and PARP cleavage.14,15 Beyond these, very little is known about the mechanism underlying the action of PPD in prostate cancer.
While taking a close look at the structure of PPD, we found that it shares the four trans-ring rigid steroid skeleton with steroid hormones, including androgens (Figure 1A). Accordingly, it is reasonable to believe that PPD might be able to modulate AR signaling. In fact, PPD inhibition of the growth of LNCaP prostate cancer cells has been reported to lead to reduced secretion of prostate-specific antigen (PSA), a well-known target of AR, although it is unclear as to whether the reduction in PSA secretion is a direct consequence of PPD inhibition of AR signaling.15 In this report, we describe a series of experiments that were designed to characterize the effect of PPD on AR signaling pathway.
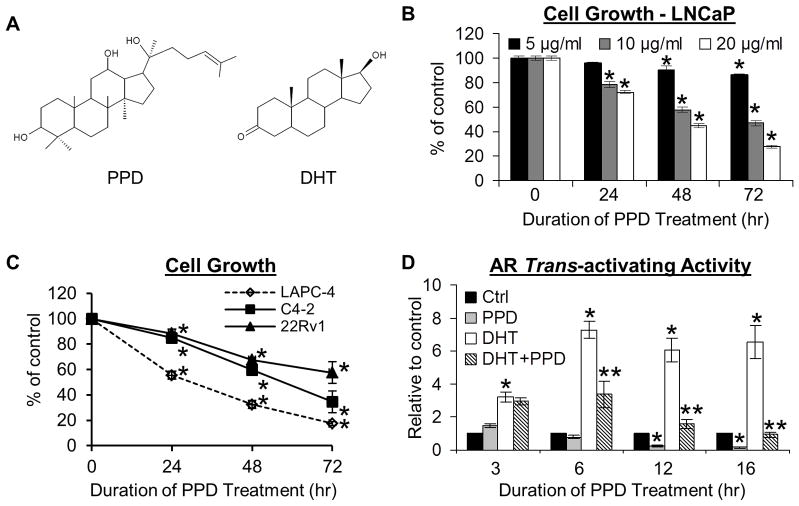
Fig. 1.
A. Structures of PPD and DHT. B & C. PPD inhibition of the growth of LNCaP cells (B), as well as LAPC-4, C4-2 and 22Rv1 cells (C). Cells were treated with different doses of PPD as indicated (B) or 20 μg/ml PPD (C) for 24, 48, or 72 hr, and growth response determined by the SRB assay. *, P < 0.01 from vehicle control. D. PPD suppression of AR transactivation. LNCaP cells transfected with the ARE-luciferase construct were treated with 20 μg/ml PPD for indicated time in the presence or absence of 1 nM DHT, and luciferase activity analyzed. * and **, P < 0.01 from control or DHT-treated sample, respectively.
MATERIAL AND METHODS
Cell Lines and Reagents
LNCaP, 22Rv1, and PC-3 cells were obtained from American Type Culture Collection at Passage 4. LAPC-416 and C4-217 were provided by Drs. Charles Sawyers and Shahriar Koochekpour, respectively, and cultured as described.17,18 Cells used in this study were within 20 passages (~3 months of non-continuous culturing). Cell growth was determined by using the Sulforhodamine B (SRB) assay as described.19 PPD was obtained from the Organic Chemistry Laboratory at Jilin University, Changchun, China. Compound of purity of >98%, as determined by high-performance liquid chromatograph, was used in cell culture studies, and that of >95% was used in animal studies.
Construction of AR-promoter-luciferase Reporter Gene Plasmids
An 8 kb (−6885 to +1115) and a 1.7 kb (−600 to +1115) fragment of the 5′-flanking region of the human AR gene were PCR amplified from an AR-containing BAC clone, RP11-807F19 (Roswell Park Cancer Institute). These two fragments were then separately cloned into the pCR™2.1-TOPO® vector (Invitrogen), and subcloned into the pGL4.19[luc2CP/Neo] rapid response luciferase expression vector (Promega). The authenticity of the constructs, pGL4-ARpro8 and pGL4-ARpro1.7, was confirmed by DNA sequencing.
Reporter Gene Assay
The androgen-responsive element (ARE)-luciferase reporter plasmid, containing three repeats of the ARE region ligated in tandem to the luciferase reporter,20 or the pGL4-ARpro8 or pGL4-ARpro1.7 AR-promoter-luciferase reporter construct, was transiently transfected into cells as described.21 To control for uniform transfection efficiency, transfection was conducted in a 10-cm dish, and the transfected cells were subsequently replated in triplicate onto 24-well plates and allowed to recover overnight before treatments. Luciferase activity was normalized by the protein concentration of the sample.
Quantitative Reverse Transcription-PCR (qRT-PCR)
RNA was isolated with the PerfectPure RNA Cultured Cell Kit (Fisher Scientific). The TaqMan® PCR primers and probes for β-actin, AR, and PSA were from Applied Biosystems, and the qRT-PCR analysis was performed as described.22 The primer sequences for AR isoforms and the qRT-PCR analysis of AR isoforms were as described in.9
Western Blotting
The procedure was described previously.23 Immunoreactive bands were quantitated by densitometry and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following antibodies were used: anti-PSA (Neomarkers), anti-GAPDH (Millipore), anti-AR (Millipore), anti-AR3 (Precision Antibody), anti-peroxisome proliferator-activated receptor γ (PPARγ) (Santa Cruz Biotechnology), anti-p53 (Cell Signaling), and anti-histone H3 (Cell Signaling).
Cell-Free AR Ligand-Binding Assay
The assay was performed by using the PolarScreen™ AR Competitor Assay Kit (Invitrogen) as per manufacturer’s protocol. In essence, the relative affinity of the test compound to AR was analyzed by competing with a fluorescent androgen ligand (Fluormone™ AL Green) for binding to the His- and GST-tagged AR-LBD. The Fluormone™ AL Green or different concentrations of the test compound (PPD, DHT, or bicalutamide) were incubated with AR-LBD (His-GST) in duplicate in dark for 4 hr, and the polarization values were then measured. The fluorescence polarization values of 1 x AR-LBD (His-GST)/Fluormone™ AL Green Complex and 1 x AR-LBD (His-GST) were considered as 0% or 100% AR bound by the test compound, respectively.
Whole-Cell AR Ligand-Binding Assay
The assay was performed by using the test compound (PPD, DHT, or bicalutamide) to compete with 0.1 nM [H3]-R1881 (a potent synthetic androgen) for AR binding. Cells were incubated with different concentrations of PPD, DHT, or bicalutamide in the presence of 0.1 nM [H3]-R1881 (PerkinElmer) for 1.5 hr. At the end of the incubation, the cells were washed three times with ice-cold PBS to remove excess [H3]-R1881. The AR-bound R1881 was extracted with ethanol, and radioactivity measured by scintillation counting.
Immunoprecipitation Assay
Cells were lysed with a lysis buffer containing 20 mM Tris-HCl (pH7.5), 50 mM NaCl, 20 mM Na2MoO4, 0.5% NP-40, 1 mM EDTA, 1mM EGTA, and 2 mM DTT. Cell lysate (1.2 mg) was incubated with 4 μg anti-AR antibody (Millipore) or mouse IgG (as a negative control) overnight at 4°C. Dynabeads® Protein G (Invitrogen) was then added. After an additional incubation for 1 h at 4°C on a platform rocker, the beads were captured by using a magnetic separation rack, washed four times with the lysis buffer, boiled in SDS-loading buffer for 10 min, and subjected to Western blot analysis with an antibody against Hsp90 (Santa Cruz Biotechnology) or AR (Millipore).
AR N-C InteractionAssay
The intensity of AR N-C interaction was assessed by using a mammalian two-hybrid system.24 This system includes two fusion protein constructs, VP-A1 and GALD-H, as well as one reporter gene plasmid, G5E1bLuc. VP-A1 is the fusion construct of the N-terminal residues 1–503 of AR and the activation domain of the herpes simplex virus VP16 protein. GALD-H is the fusion construct of the C-terminal ligand-binding domain of AR (624–919) and the GAL4 DNA-binding domain. The G5E1bLuc construct contains the luciferase reporter gene downstream of five consensus GAL4 binding sites and the minimal promoter of the adenovirus E1b gene. To control for uniform transfection efficiency, the three constructs were co-transfected into AR-null PC-3 cells at 1:1:5 ratio in a 15-cm dish as described.21 Cells were subsequently replated in triplicate onto 6-well plates and allowed to recover overnight before treatments. Luciferase activity was normalized by the protein concentration of the sample. The use of AR-null cells in this assay was to avoid interference of the interaction between transfected AR N- and C-terminal fragments from the endogenous AR.25
LNCaP Tumor Xenograft Model
Male nude mice were obtained from the NCI Animal Production Center at 5–6 weeks of age. After one week of adaptation, mice were inoculated subcutaneously with 4×106 LNCaP cells suspended in 50% Matrigel on both dorsal flanks. The day following inoculation, mice were randomly assigned to two groups and received 40 mg/kg PPD in olive oil or olive oil as control through oral gavage 6 days weekly. Body weights and tumor dimensions were monitored weekly. Tumor volume was calculated as 0.524 × width2 × length.26 At the termination of the experiment, mice were anesthetized, and blood collected for serum PSA determination using quantitative ELISA (United Biotech). Tumors were removed, weighed, and fixed in 10% formalin for paraffin embedding and histological analyses. All animal procedures were approved by the Tulane University Institutional Animal Care and Use Committee.
AR Immunohistochemical Analyses
Sections of paraffin-embedded blocks were deparaffinized, rehydrated, and immersed in 3% H2O2 to quench endogenous peroxidase activity. Antigen retrieval was achieved by heating samples in 10 mM citrate buffer (pH 6.0) to near boiling for 20 min. The slides were cooled to room temperature, and preincubated with blocking serum to prevent non-specific binding. Primary antibody incubation, with a monoclonal AR antibody (BioGenex), was conducted at room temperature for 1 hr. The reaction was visualized using the ABC Staining System (Santa Cruz Biotechnology), with diaminobenzidine as the chromogen. Sections were counterstained with hematoxylin. As the negative control, the primary antibody was replaced with a non-immune IgG at the same concentration, and no reactivity was detected.
Images were sampled sequentially throughout each section using a 20× objective, with areas of necrosis, preparation artifacts, or edges avoided. Each field of view was digitized at 200× magnification. All digitized images were analyzed using the ImageJ software (NIH). The diaminobenzidine color was extracted from each digitized image using the color deconvolution function in ImageJ, and the extracted color image was transformed into an 8-bit grayscale image. A histogram was subsequently derived for each image using the Auto Threshold tool in ImageJ. The two peaks on each histogram depict the distribution of pixels in areas stained positively (left peak) or negatively (right peak) for AR. The floor between the two peaks defined background intensity. The relative density of each immuno-positive pixel was calculated as: relative density = log (I0) − log (Ii), where I0 is the intensity of the background in each field, and Ii the intensity of individual pixel.27
Statistical Analysis
The Student’s two-tailed t test was used to determine the mean differences between treatment and control. Data are presented as mean ± SEM.
RESULTS
PPD inhibition of the growth of prostate cancer cells
We first assessed the effect of PPD on the growth of androgen-dependent LNCaP cells by the SRB assay. As presented in Figure 1B, PPD inhibited the growth of LNCaP cells in a dose- and time-dependent manner. LNCaP cells express a mutant but functional AR.28 We also examined the effect of PPD on the growth of androgen-dependent, wild-type-AR-expressing LAPC-4, the C4-2 castration-resistant derivative of LNCaP, and castration-resistant 22Rv1 cells. 22Rv1 expresses both the full-length AR and the constitutively-active LBD-truncated AR-Vs, which have been correlated with prostate cancer progression and recurrence.8–11 As shown in Figure 1C, PPD also caused a progressive inhibition of the growth of LAPC-4, C4-2, and 22Rv1 cells as a function of time. The data thus confirm previous reports showing the ability of PPD to inhibit the growth of prostate cancer cells.14,15
PPD Suppression of AR Signaling
We then investigated the effect of PPD on AR transactivation by the reporter gene assay. LNCaP cells transfected with the ARE-luciferase reporter construct20 were exposed to PPD for 3, 6, 12, or 16 hr. As shown in Figure 1D, PPD inhibited not only DHT-induced but also basal AR trans-activating activity. Reduction of DHT-induced AR activity appeared earlier, at 6 hr, whereas decrease of basal AR activity was not observed until 12 hr post-treatment. We proceeded to examine the effect of PPD on an endogenous AR-target gene, PSA, by qRT-PCR and Western blotting. As shown in Figures 2A and 2B, the PSA data were consistent with the reporter gene result, displaying a time-dependent downregulation. A similar decrease of PSA level was also detected in LAPC-4, C4-2, and 22Rv1 cells (Figure 2C).
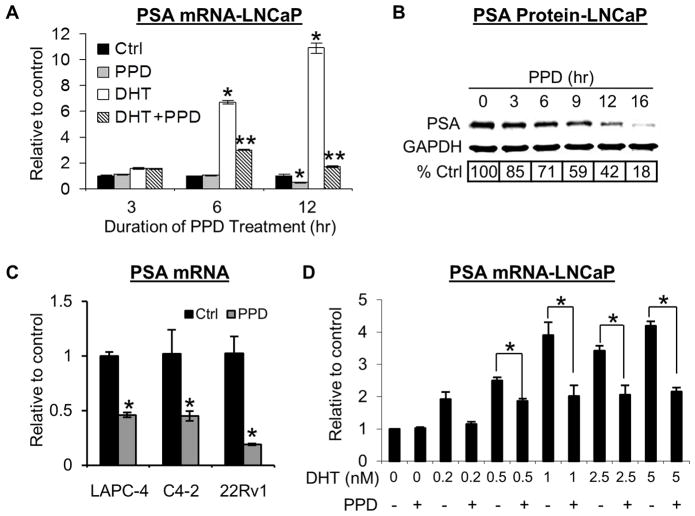
Fig. 2.
A. PPD downregulation of PSA mRNA in LNCaP cells. Cells were treated with 20 μg/ml PPD for indicated time in the presence or absence of 1 nM DHT, and PSA mRNA determined by qRT-PCR. * and **, P < 0.01 from control or DHT-treated sample, respectively. B. PPD downregulation of PSA protein in LNCaP cells. Cells were treated with 20 μg/ml PPD for indicated time, and PSA protein detected by Western blotting. C. PPD downregulation of PSA mRNA in LAPC-4, C4-2, and 22Rv1 cells. Cells were treated with 20 μg/ml PPD for 12 hr, and PSA mRNA determined. *, P < 0.01 from vehicle control. D. No weakening of PPD effect on PSA expression by increasing concentrations of DHT. LNCaP cells were treated with 20 μg/ml PPD and indicated concentration of DHT for 6 hr, and PSA mRNA determined by qRT-PCR. *, P < 0.01 from respective DHT-treated sample.
Limited Ability of PPD to Compete with Androgens for Binding to AR
Since PPD bears structural similarity to androgens, it is conceivable that PPD may inhibit AR transactivation by functioning as an AR antagonist. We carried out a whole-cell AR ligand-binding assay in LNCaP cells and a cell-free AR ligand-binding assay to characterize the interaction between PPD and AR. Both assays showed that the binding affinity of PPD to AR is ~10,000–40,000-fold less than that of DHT and ~80-fold less than that of the anti-androgen bicalutamide. We then studied whether the suppressive activity of PPD on AR signaling differs in the presence of different concentrations of DHT by using PSA mRNA expression as the read-out. As presented in Figure 2D, the ability of PPD to downregulate PSA expression was not weakened with increasing concentrations of DHT. Taken together, the data indicate that PPD suppression of AR transactivation is unlikely to be mediated by competing with androgens for binding to AR. It is of note that PPD does not have agonistic activity against AR either. This is evidenced by the AR activity data presented in Figure 1D under androgen-depleted condition.
PPD Downregulation of AR Protein Levels
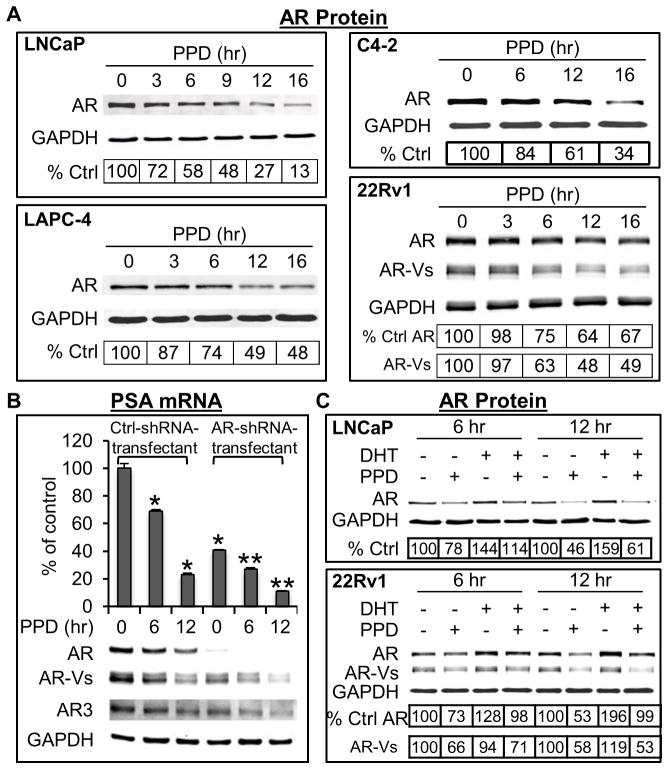
To understand the mechanism by which PPD inhibits AR transactivation, we assessed PPD modulation of AR protein. As shown in Figure 3A, in all the four cell lines examined, a decrease of AR protein was already evident at 6 hr after PPD treatment, and the decrease became more significant with time. In contrast, no decrease of another nuclear receptor, PPARγ, was observed (Supplementary Figure), indicating the specificity of the effect. It is important to note that PPD downregulated not only the full-length AR but also the constitutively-active truncated AR-Vs (the 22Rv1 panel of Figure 3A). The magnitude of downregulation of the AR-Vs was similar to that of the full-length AR. The downregulation of AR-Vs also resulted in a decrease in the activity of AR-Vs. This is indicated by the ability of PPD to inhibit PSA expression even after specific knockdown of the full-length AR (Figure 3B).
Fig. 3.
A. PPD downregulation of AR protein. Western blot analysis of AR protein in cells treated with 20 μg/ml PPD. B. Specific knockdown of the full-length AR does not affect PPD downregulation of PSA mRNA. 22Rv1 cells infected with lentivirus encoding the control shRNA or a full-length-AR-specific shRNA9 were treated with 20 μg/ml PPD in androgen-depleted condition, and the cell lysates subjected to qRT-PCR analysis of PSA mRNA or Western blot analysis of AR proteins. * and **, P < 0.01 from untreated control-shRNA-transfected or AR-shRNA-transfected cells, respectively. C. PPD downregulation of AR protein in the presence or absence of DHT. Western blot analysis of AR protein in LNCaP or 22Rv1 cells treated with 20 μg/ml PPD in the presence or absence of 1 nM DHT for 6 or 12 hr. The numbers in the tables denote relative normalized intensities of the AR protein bands compared to the untreated control value of 100.
To further determine whether PPD downregulation of AR requires the presence of androgen, we cultured the LNCaP and 22Rv1 cells in androgen-deprived condition, and treated the cells with PPD in the presence or absence of DHT. The data are presented in Figure 3C. In accordance with the ability of androgen to stabilize the full-length AR protein and the lack of the LBD of the AR-Vs, an increase of the full-length AR protein, but not the AR-Vs, was evident after exposure of cells to DHT. PPD downregulated basal and androgen-stabilized AR protein to a similar extent, indicating that PPD downregulation of AR does not require the presence of androgen. The data thus lend further support to our conclusion that PPD suppression of AR signaling is unlikely to be mediated by competing with androgens for binding to AR.
PPD Suppression of AR Transcription
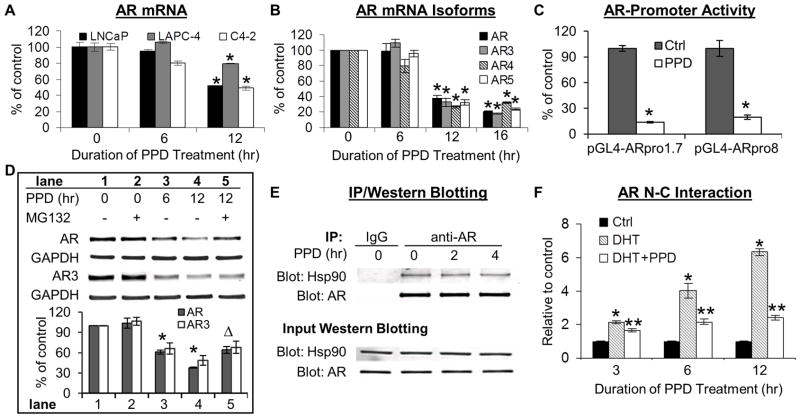
We then examined the changes of AR transcripts by qRT-PCR. As shown in Figures 4A and 4B, PPD treatment also downregulated the mRNA levels of the full-length AR and the AR-Vs, namely AR3, AR4, and AR5. In order to determine whether the reduced transcript levels were contributed by PPD inhibition of AR promoter activity, we constructed two reporter gene plasmids of the human AR promoter, pGL4-ARpro8 and pGL4-ARpro1.7. The 8 kb fragment (−6885 to +1115) in pGL4-ARpro8 contains ~6.9 kb of the 5′-flanking region upstream of the entire 5′-untranslated region, and the 1.7 kb fragment (−600 to +1115) in pGL4-ARpro1.7 contains 600 bp of the 5′-flanking region upstream of the entire 5′-untranslated region. LNCaP cells transfected with pGL4-ARpro8 or pGL4-ARpro1.7 were exposed to PPD for 12 hr. As shown in Figure 4C, PPD treatment led to a ~80% reduction of the activity of both promoters. The data therefore demonstrate the ability of PPD to suppress the transcription of the AR gene. However, suppression of transcription may not contribute to the initial decline of AR protein. This is because PPD downregulation of AR transcripts was not observed until 12 hr after treatment (Figures 4A and 4B), lagging behind the drop in protein (evident already at 6 hr, Figure 3A). The initial rapid decline of AR protein, instead, may be the result of PPD modulation of AR protein degradation.
Fig. 4.
A & B. PPD downregulation of AR transcripts. LNCaP, LAPC-4, C4-2 cells (A) or 22Rv1 cells (B) were treated with 20 μg/ml PPD, and subjected to qRT-PCR analysis of the full-length (AR) and truncated isoforms (AR3, AR4, and AR5) of AR mRNA. *, P < 0.01 from control. C. PPD suppression of AR transcription. LNCaP cells transfected with the AR-promoter-luciferase construct, pGL4-ARpro8 or pGL4-ARpro1.7, were treated with 20 μg/ml PPD for 12 hr, and luciferase activity analyzed. *, P < 0.01 from untreated control. D. PPD induction of proteasome-mediated AR degradation. Western blot analysis of AR or AR3 protein in LNCaP or 22Rv1 cells, respectively. Cells were pretreated with 20 μg/ml PPD for 6 hr prior to the combined treatment with PPD and 10 μM MG132 for an additional 6 hr. The bar graph represents densitometry analysis of the Western blot data from three independent experiments. * and Δ, P < 0.01 from control or cells treated with PPD alone for 12 hr, respectively. E. No effect of PPD on Hsp90-AR association. LNCaP cells cultured in hormone-deprived condition were treated with 20 μg/ml PPD for 2 or 4 hr, and immunoprecipitation was conducted with anti-AR antibody or mouse IgG. Two top panels are Hsp90 or AR Western analysis of the immunoprecipitates. Two bottom panels are Hsp90 or AR Western analysis of the input samples. F. PPD disruption of AR N-C interaction. AR N-C interaction assay in PC-3 cells transfected with a mammalian two-hybrid system. Cells were treated with 1 nM DHT ± 20 μg/ml PPD. * and **, P < 0.01 from control or DHT-treated sample, respectively.
PPD Induction of Proteasome Degradation of AR
Proteasome-mediated pathway has been described as the main machinery regulating AR protein degradation.29–31 To determine the involvement of proteasome in PPD-mediated decrease of AR protein, we assessed the effect of the MG132 proteasome inhibitor on PPD downregulation of AR in LNCaP cells and AR3 (the only AR-V for which a specific antibody is available) in 22Rv1 cells. Cells were pretreated with PPD for 6 hr prior to the addition of MG132, and the treatment continued for an additional 6 hr. The reason that MG132 was only present during the last 6 hr of treatment was because of the cytotoxicity associated with longer treatment with PPD and MG132. As shown in Figure 4D, treatment with MG132 alone did not cause a significant change in AR or AR3 protein level (comparing lanes 1 & 2). This is consistent with previous reports.32,33 However, MG132 treatment was able to reverse the PPD effect, bringing PPD-suppressed AR or AR3 level respectively from 38% or 48% of control (lane 4) back to 64% or 68% of control (lane 5), the same as the pre-MG132 level (lane 3). The data thus indicated that promoting AR degradation through the proteasome pathway contributes significantly to PPD downregulation of both the full-length and truncated AR. To demonstrate the specificity of PPD-induced AR degradation, we assessed the effect of PPD on the level of the p53 protein, which is known to be regulated by the proteasome-mediated pathway.34 No change in p53 protein level was detected (Supplementary Figure 1).
PPD Disruption of AR N-C Interaction
The stability of the AR protein has been reported to be regulated by Hsp90 and the interaction between the N- and C-termini of AR. Inhibiting Hsp90 activity or disrupting Hsp90-AR association leads to reduced stability and increased proteasomal degradation of AR.32,35 AR N-C interaction stabilizes AR by preventing ligand dissociation and AR degradation.36 We therefore characterized the effect of PPD on Hsp90-AR association and AR N-C interaction. The association of Hsp90 and AR was evaluated by immunoprecipitation. No change in Hsp90-AR association was observed (Figure 4E). We then assessed AR N-C interaction using a mammalian two-hybrid system24 in AR-null PC-3 cells to avoid interference from the endogenous AR.25 Cells transfected with the mammalian two-hybrid system were exposed to DHT with or without PPD for 3, 6, or 12 hr. As shown in Figure 4F, exposure to DHT resulted in AR N-C interaction, which was markedly inhibited by PPD.
PPD Suppression of Tumor Growth and AR Expression in vivo
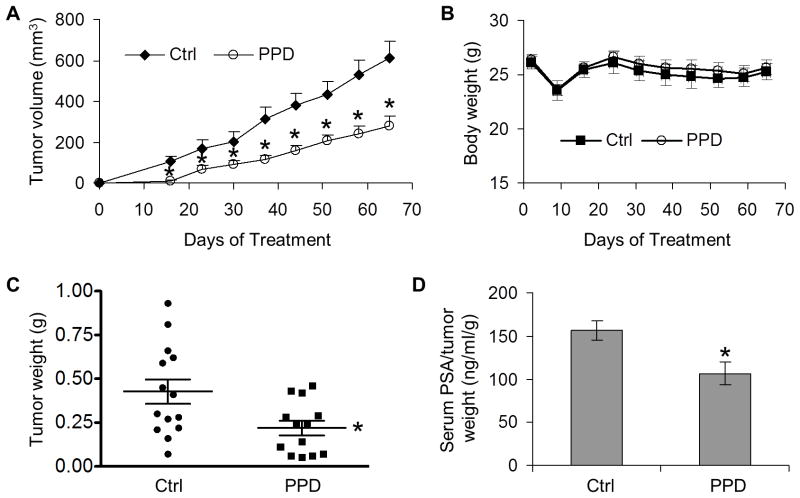
Male nude mice implanted with LNCaP cells on both dorsal flanks were divided into two groups receiving either olive oil as control (n = 8 mice) or 40 mg/kg PPD (n = 11 mice) through oral gavage 6 days per week. As shown in Figure 5A, palpable tumors appeared in both groups on day 16. However, the average tumor size in the PPD group was significantly smaller than that in the control group. At the conclusion of the study (day 65), the tumor incidence was 87.5% (14/16) in the control group, but 59% (13/22) in the PPD group, representing a 32.6% decrease of tumor take rate by PPD. Additionally, the average weight of the tumors at the end of the study was 0.43±0.07 g in the control group, but 0.22±0.04 g in the PPD group, indicating a ~49% inhibition of tumor growth by PPD (Figure 5C). Each individual tumor was considered as independent in the above data analysis. We also calculated tumor volumes and weights considering each individual mouse as independent, and tumor volumes and weights remain statistically different between control and PPD treatment groups (Supplementary Figure 2). PPD supplementation did not appear to cause severe toxicity since mice in the treatment group had similar body weights as the control mice (Figure 5B).
Fig. 5. PPD inhibition of the growth of LNCaP xenograft tumors and PSA secretion.
A. Mean tumor volumes in each group (n=14 for the control group; n=13 for the PPD group). *, P < 0.05 from the control group. B. Mean body weights of the mice in each group. C. Weights of individual tumors in each group at the conclusion of the experiment. *, P < 0.01 from the control group. D. Mean serum PSA levels, normalized by tumor weights, in each group at the conclusion of the study. *,P < 0.05 from the control group.
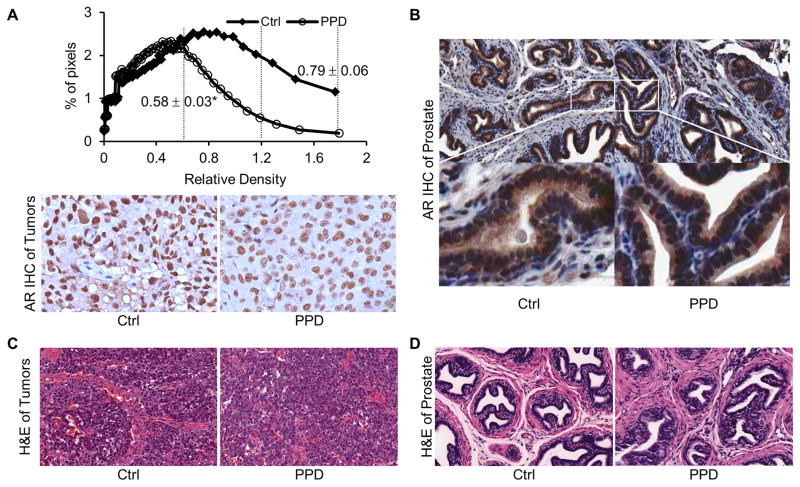
We then measured PSA levels by ELISA in mouse serum and AR protein expression by immunohistochemistry in formalin-fixed tissues. Mean serum PSA concentration after normalization by tumor weight was reduced by one third by PPD (Figure 5D). The result of quantitative analysis of AR immunostaining of the xenograft tumors is shown in the top panel of Figure 6A. The quantitation was performed according to the method developed by Kim et al. for optimized immunohistochemical quantitation of AR.27 Images were sampled sequentially throughout sections from four tumors in the control group and five tumors in the PPD group using a 20× objective, with areas of necrosis, preparation artifacts, or edges avoided. Depending on the size of the tumors, 10–25 image fields were captured for each tumor section, and digitized and quantitated as described in Material and Methods. The data are presented as percentage of pixels with varying AR immunostaining intensity. PPD treatment resulted in a shift in the distribution of AR intensity, from the higher two tertiles to the lowest tertile. The mean pixel intensity in the PPD group was decreased to 73% of the control. The data therefore corroborated our in vitro results, showing the effectiveness of PPD in downregulating AR level and activity in cancer cells in vivo. On the other hand, no change in AR expression was observed in the normal prostate glands of mice (Figure 6B). Hematoxylin and eosin (H & E) staining of the tissues showed no apparent histopathological change of the tumors or the prostate glands after PPD treatment (Figures 6C and 6D).
Fig. 6. PPD downregulation of AR in LNCaP xenograft tumors.
A. AR immunohistochemical staining of sections of LNCaP xenograft tumors. Upper panel: Quantitation of the results, which is presented as % of pixels in each image field with different AR intensity. The numbers next to the distribution curves represent the mean pixel intensity of the respective group ± SEM. *, P < 0.01 from the control group. The vertical dashed lines segment the three tertiles of immunostaining intensity. Lower panels: Representative images from control and PPD groups (magnification, 200×). B. AR staining of sections of mouse dorsolateral prostates. C & D. H&E staining of sections of LNCaP xenograft tumors or mouse dorsolateral prostates, respectively.
DISCUSSION
The present study represents the first to demonstrate PPD as an effective agent to downregulate the expression and activity of both the full-length AR and the AR-Vs in prostate cancer. This report is also the first to show the in vivo efficacy of PPD against AR-expressing prostate cancer cells, which constitute the majority of clinical prostate carcinomas. We further show that PPD suppression of tumor growth is accompanied by a considerable decline in AR expression in prostate tumor xenografts and PSA levels in the serum. The decline in serum PSA levels is not simply a consequence of reduction of tumor sizes by PPD, as the effect remains pronounced after normalization by tumor weight. The data instead indicate the ability of PPD to suppress AR activity in the tumors.
Despite its structural similarity with androgens, PPD has limited ability to compete with androgens for binding to AR. Instead, the effect of PPD on AR is manifested by an immediate drop in protein, followed by a reduction in mRNA. AR is known to negatively auto-regulate its transcription.37 A decline of AR protein would be expected to lead to an increase in AR transcription. The fact that we observe a decrease in AR transcription following a drop of protein indicates that PPD could turn on a mechanism counteracting AR negative auto-regulation. This is significant because it could lead to a sustained downregulation of AR.
We further demonstrate that the initial decrease in AR protein could be attributed to induction of proteasome-mediated AR degradation, possibly resulting from PPD disruption of AR N-C interaction. The intermolecular interaction between the AR N-terminal and C-terminal regions is known to stabilize the full-length AR by preventing ligand dissociation and AR degradation.36 Since AR3 and all the other AR-Vs identified to date retain an intact N-terminal domain,8–11,38,39 it is reasonable to believe that the full-length AR can also form N-C interaction with the AR-Vs. In fact, ARv567es, another major AR-V, has been shown by coimmunoprecipitation assay to interact with the full-length AR.11 On the other hand, AR/AR3 complex has not been detected by coimmunoprecipitation in cell lines expressing a relatively high level of endogenous AR3, including 22Rv1,9,38 although the activity of AR3 has been indicated to rely on the presence of the full-length AR.39 We have also used an antibody recognizing both AR and AR-Vs or specific for AR3 or an anti-FLAG antibody to precipitate the complex in an AR-null cell line with exogenously introduced AR3 together with non-tagged full-length AR or AR3 together with FLAG-tagged full-length AR. However, no interaction between these two forms of AR was observed even when both were highly expressed in the cells (data not shown). Thus, whether PPD induction of AR3 protein degradation results from PPD disruption of AR N-C interaction awaits to be determined.
While PPD suppresses AR expression in the human prostate tumor xenografts, no change in AR expression is detected in normal mouse prostates. Would the differential response be due to differences in regulation of mouse versus human AR expression or the existence of a cancer-specific factor that is amenable to PPD modulation? In aligning the sequences of the human and mouse AR proteins, we found that the sequences are highly homologous, with 100% homology at the DNA-binding domain, LBD, dimer interface, and co-activator recognition sites and 90% homology at the N-termini. The nucleotide sequences of the 5′-flanking region of the human and mouse AR gene are also highly conserved, and the transcription factors that have been shown to regulate the transcription of the mouse AR gene have also been demonstrated to control the expression of the human AR gene.37,40–42 Mechanism underlying degradation of the mouse AR protein is largely unknown. It is possible that differences in AR protein degradation pathways exist between the two species, and could contribute to the differential response of the human tumor xenografts and normal mouse prostates to PPD. On the other hand, a cancer-associated factor(s) could also be involved in the differential response. Further research is needed to address these possibilities.
Androgens play a key role in the development of prostate cancer. The ability of finasteride and dutasteride to reduce prostate cancer incidence attests to the viability of targeting androgen signaling axis for prostate cancer chemoprevention. PPD suppresses androgen signaling through reducing receptor availability, and thus is at a different level from finasteride and dutasteride, which block the formation of DHT. Thus, combining these two types of drugs would be expected to produce a more pronounced suppression of AR transactivation and thereby an increased chemopreventive efficacy. Additionally, finasteride treatment has been shown to lead to AR upregulation in prostate tissue.6 The fact that PPD downregulates not only the full-length AR but also the AR-Vs that lack the LBD indicates that it could be helpful for targeting the androgen signaling that is resistant to finasteride and dutasteride.
The benefit of PPD intervention might also be extended to the prevention of relapse after androgen deprivation therapy. Although recurrent castration-resistant prostate cancer is no longer responsive to androgen deprivation, the expression and signaling of AR are maintained.43 Agents such as abiraterone are effective in lowering PSA and prolonging survival in patients with castration-resistant prostate cancer, but even this irreversible CYP17 inhibitor has a relatively short time to disease progression in these patients.44 One mechanism of resistance to abiraterone has been ascribed to increased expression of the full-length AR and AR-Vs.45 Elevated expression of the full-length AR has been reported to be sufficient to convert prostate cancer growth from an androgen-dependent to castration-resistant stage.7 Prevalent upregulation of AR-Vs in castration-resistant prostate cancer has also been shown to contribute to castration resistance.8–11 Thus, the fact that PPD can effectively reduce the abundance of both the full-length AR and the AR-Vs also indicates its great potential as an adjuvant to androgen deprivation therapy. In addition to PPD, several other natural or synthetic compounds have been shown to inhibit AR-Vs.46–50 The discovery of these compounds should generate excitement because there is no drug currently in clinical trials to target castration-resistant progression driven by the AR-Vs. Taken together, the findings from the current study provide strong rationale for further developing PPD or its analogue as a promising agent for the prevention and/or treatment of prostate cancer.
Supplementary Material
Acknowledgments
We would like to thank Dr. Charles Sawyers, now at Memorial Sloan Kettering Cancer Center, for LAPC-4 cells, Dr. Shahriar Koochekpour now at Roswell Park Cancer Institute for C4-2 cells, and Dr. Elizabeth Wilson at the University of North Carolina for the AR N-C interaction mammalian two-hybrid system. We are very grateful to Mary Price (The Louisiana Cancer Research Consortium FACS Core) and Dina Gaupp (Tulane Center for Gene Therapy Histology Core Facility) for their excellent technical assistance. This work was supported by National Cancer Institute grant K01CA114252 (YD), American Cancer Society grant RSG-07-218-01-TBE (YD), Chinese Scholarship Council Fellowship (BC), Louisiana Cancer Research Consortium Start-up Fund (YD and HZ), Tulane Cancer Center Developmental Fund (YD), Tulane University School of Medicine Pilot Fund (YD), and National Major Special Project for Science and Technology Development of Ministry of Science and Technology of China 2009ZX09304-003 (JG).
Abbreviations
- AR
androgen receptor
- AR-Vs
androgen receptor splice variants
- PPD
20(S)-protopanaxadiol-aglycone
- ARE
androgen-responsive element
- DHT
dihydrotestosterone
- PSA
prostate-specific antigen
- SRB
Sulforhodamine B
- qRT-PCR
quantitative reverse transcription-PCR
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PPARγ
peroxisome proliferator-activated receptor γ
- LBD
ligand-binding domain
- H & E
hematoxylin and eosin
Footnotes
Novelty and Impact of the Work: This is the first report on 20(S)-protopanaxadiol-aglycone as an effective agent to downregulate the expression and activity of the full-length androgen receptor and its constitutively-active splice variants in prostate cancer. It is also the first to show the in vivo efficacy of 20(S)-protopanaxadiol-aglycone against androgen-receptor-expressing prostate tumors. Considering the critical roles of androgen receptor and its splice variants in disease progression, our findings suggest that 20(S)-protopanaxadiol-aglycone is a promising agent for prostate cancer intervention.
References
- 1.Waterbor JW, Bueschen AJ. Prostate cancer screening (United States) Cancer Causes Control. 1995;6:267–74. doi: 10.1007/BF00051798. [DOI] [PubMed] [Google Scholar]
- 2.Montie JE, Pienta KJ. Review of the role of androgenic hormones in the epidemiology of benign prostatic hyperplasia and prostate cancer. Urology. 1994;43:892–9. doi: 10.1016/0090-4295(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, et al. Effect of Dutasteride on the Risk of Prostate Cancer. New England Journal of Medicine. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, et al. The Influence of Finasteride on the Development of Prostate Cancer. New England Journal of Medicine. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 5.Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P, Pazdur R. The Risks and Benefits of 5α-Reductase Inhibitors for Prostate-Cancer Prevention. New England Journal of Medicine. 2011;365:97–9. doi: 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh JT, Chen SC, Yu HJ, Chang HC. Finasteride upregulates expression of androgen receptor in hyperplastic prostate and LNCaP cells: Implications for chemoprevention of prostate cancer. Prostate. 2011;71:1115–21. doi: 10.1002/pros.21325. [DOI] [PubMed] [Google Scholar]
- 7.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 8.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Hara M, Kiefer D, Farrell K, Kemper K. A review of 12 commonly used medicinal herbs. Arch Fam Med. 1998;7:523–36. doi: 10.1001/archfami.7.6.523. [DOI] [PubMed] [Google Scholar]
- 13.Qi LW, Wang CZ, Yuan CS. American Ginseng: Potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–54. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Musende AG, Eberding A, Jia W, Ramsay E, Bally MB, Guns ET. Rh2 or its aglycone aPPD in combination with docetaxel for treatment of prostate cancer. Prostate. 2010;70:1437–47. doi: 10.1002/pros.21179. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Wang H, Rayburn ER, Zhao Y, Hill DL, Zhang R. 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. Br J Cancer. 2008;98:792–802. doi: 10.1038/sj.bjc.6604227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON, Sawyers CL. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–8. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 17.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LWK. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: Role of bone stromal cells. Int J Cancer. 1994;57:406–12. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Qi Y, Ge Y, Duplessis T, Rowan BG, Ip C, Cheng H, Rennie PS, Horikawa I, Lustig AJ, Yu Q, Zhang H, et al. Telomerase as an important target of androgen signaling blockade for prostate cancer treatment. Mol Cancer Ther. 2010;9:2016–25. doi: 10.1158/1535-7163.MCT-09-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 20.Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci USA. 1996;93:5517–21. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y, Zhang H, Gao AC, Marshall JR, Ip C. Androgen receptor signaling intensity is a key factor in determining the sensitivity of prostate cancer cells to selenium inhibition of growth and cancer-specific biomarkers. Mol Cancer Ther. 2005;4:1047–55. doi: 10.1158/1535-7163.MCT-05-0124. [DOI] [PubMed] [Google Scholar]
- 22.Dong Y, Lee SO, Zhang H, Marshall J, Gao AC, Ip C. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64:19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- 23.Dong Y, Zhang H, Hawthorn L, Ganther HE, Ip C. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Res. 2003;63:52–9. [PubMed] [Google Scholar]
- 24.Langley E, Zhou ZX, Wilson EM. Evidence for an anti-parallel orientation of the ligand-activated human androgen receptor dimer. J Biol Chem. 1995;270:29983–90. doi: 10.1074/jbc.270.50.29983. [DOI] [PubMed] [Google Scholar]
- 25.Ting HJ, Yeh S, Nishimura K, Chang C. Supervillin associates with androgen receptor and modulates its transcriptional activity. Proc Natl Acad Sci USA. 2002;99:661–6. doi: 10.1073/pnas.022469899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gleave ME, Hsieh JT, Wu HC, von Eschenbach AC, Chung LW. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Res. 1992;52:1598–605. [PubMed] [Google Scholar]
- 27.Kim D, Gregory CW, Smith GJ, Mohler JL. Immunohistochemical quantitation of androgen receptor expression using color video image analysis. Cytometry. 1999;35:2–10. doi: 10.1002/(sici)1097-0320(19990101)35:1<2::aid-cyto2>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E. Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry. 1992;31:2393–9. doi: 10.1021/bi00123a026. [DOI] [PubMed] [Google Scholar]
- 29.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–48. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin HK, Hu YC, Lee DK, Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol Endocrinol. 2004;18:2409–23. doi: 10.1210/me.2004-0117. [DOI] [PubMed] [Google Scholar]
- 31.Xu LL, Shi Y, Petrovics G, Sun C, Makarem M, Zhang W, Sesterhenn IA, McLeod DG, Sun L, Moul JW, Srivastava S. PMEPA1, an androgen-regulated NEDD4-binding protein, exhibits cell growth inhibitory function and decreased expression during prostate cancer progression. Cancer Res. 2003;63:4299–304. [PubMed] [Google Scholar]
- 32.Cha TL, Qiu L, Chen CT, Wen Y, Hung MC. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005;65:2287–95. doi: 10.1158/0008-5472.CAN-04-3250. [DOI] [PubMed] [Google Scholar]
- 33.Chiu FL, Lin JK. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate. 2008;68:61–71. doi: 10.1002/pros.20690. [DOI] [PubMed] [Google Scholar]
- 34.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 35.Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell Stress Chaperones. 2002;7:55–64. doi: 10.1379/1466-1268(2002)007<0055:eogoar>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou ZX, Lane MV, Kemppainen JA, French FS, Wilson EM. Specificity of ligand-dependent androgen receptor stabilization: receptor domain interactions influence ligand dissociation and receptor stability. Molecular Endocrinology. 1995;9:208–18. doi: 10.1210/mend.9.2.7776971. [DOI] [PubMed] [Google Scholar]
- 37.Wolf DA, Herzinger T, Hermeking H, Blaschke D, Horz W. Transcriptional and posttranscriptional regulation of human androgen receptor expression by androgen. Mol Endocrinol. 1993;7:924–36. doi: 10.1210/mend.7.7.8413317. [DOI] [PubMed] [Google Scholar]
- 38.Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, Pretlow TG, Kung HJ. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002;62:6606–14. [PubMed] [Google Scholar]
- 39.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proceedings of the National Academy of Sciences. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis JN, Wojno KJ, Daignault S, Hofer MD, Kuefer R, Rubin MA, Day ML. Elevated E2F1 inhibits transcription of the androgen receptor in metastatic hormone-resistant prostate cancer. Cancer Res. 2006;66:11897–906. doi: 10.1158/0008-5472.CAN-06-2497. [DOI] [PubMed] [Google Scholar]
- 41.Lindzey J, Grossmann M, Kumar MV, Tindall DJ. Regulation of the 5′-flanking region of the mouse androgen receptor gene by cAMP and androgen. Mol Endocrinol. 1993;7:1530–40. doi: 10.1210/mend.7.12.7511785. [DOI] [PubMed] [Google Scholar]
- 42.Mizokami A, Yeh SY, Chang C. Identification of 3′,5′-cyclic adenosine monophosphate response element and other cis-acting elements in the human androgen receptor gene promoter. Mol Endocrinol. 1994;8:77–88. doi: 10.1210/mend.8.1.8152432. [DOI] [PubMed] [Google Scholar]
- 43.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, Molife LR, Hunt J, Messiou C, Parker C, Dearnaley D, Swennenhuis JF, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 Inhibition with Abiraterone in Castration-Resistant Prostate Cancer: Induction of Steroidogenesis and Androgen Receptor Splice Variants. Clinical Cancer Research. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mashima T, Okabe S, Seimiya H. Pharmacological targeting of constitutively active truncated androgen receptor by nigericin and suppression of hormone-refractory prostate cancer cell growth. Mol Pharmacol. 2010;78:846–54. doi: 10.1124/mol.110.064790. [DOI] [PubMed] [Google Scholar]
- 47.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, Watt K, Tam T, Yang YC, Banuelos CA, Williams DE, McEwan IJ, et al. Regression of Castrate-Recurrent Prostate Cancer by a Small-Molecule Inhibitor of the Amino-Terminus Domain of the Androgen Receptor. Cancer Cell. 2010;17:535–46. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Yamashita S, Lai KP, Chuang KL, Xu D, Miyamoto H, Tochigi T, Pang ST, Li L, Arai Y, Kung HJ, Yeh S, Chang C. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14:74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Cao B, Liu X, Fu X, Xiong Z, Chen L, Sartor O, Dong Y, Zhang H. Berberine Suppresses Androgen Receptor Signaling in Prostate Cancer. Molecular Cancer Therapeutics. 2011;10:1346–56. doi: 10.1158/1535-7163.MCT-10-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narizhneva NV, Tararova ND, Ryabokon P, Shyshynova I, Prokvolit A, Komarov PG, Purmal AA, Gudkov AV, Gurova KV. Small molecule screening reveals a transcription-independent pro-survival function of androgen receptor in castration-resistant prostate cancer. Cell Cycle. 2009;8:4155–67. doi: 10.4161/cc.8.24.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.